Research Article Open Access
Levels of Interleukin -10 in Gingival Crevicular Fluid and its Role in the Initiation and Progression of Gingivitis to Periodontitis
Angel Fenol1*, Remya K Sasidharan2 and Sajitha Krishnan2
1Department of Periodontics, Amrita School of Dentistry, Kochi, Kerala, India
2Department of Biochemistry, Amrita Institute of Medical Sciences, Kochi, Kerala, India
- *Corresponding Author:
- Dr. Angel Fenol
Department of Periodontics
Amrita School of Dentistry
Kochi 682041, Kerala, India
Tel: 00919037326910
E-mail: angelfenol@aims.amrita.edu
Received Date: April 03, 2014; Accepted Date: April 29, 2014; Published Date: May 07, 2014
Citation: Feno Al, Sasidharan RK, Krishnan S (2014) Levels of Interleukin -10 in Gingival Crevicular Fluid and its Role in the Initiation and Progression of Gingivitis to Periodontitis. J Oral Hyg Health 2:135. doi: 10.4172/2332-0702.1000135
Copyright: © 2014 Fenol A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Oral Hygiene & Health
Abstract
Background: Virulence of microorganisms contribute to initiation and progression of periodontal disease but genetic contribution to pathogenesis of periodontitis and specific genetic markers are found to be strong indicators of susceptibility to periodontitis in adults. The purpose of this study was to determine whether interindividual variation in interleukin-10 levels in patients with similar levels of oral hygiene has a role in preventing progression of gingivitis to periodontitis.
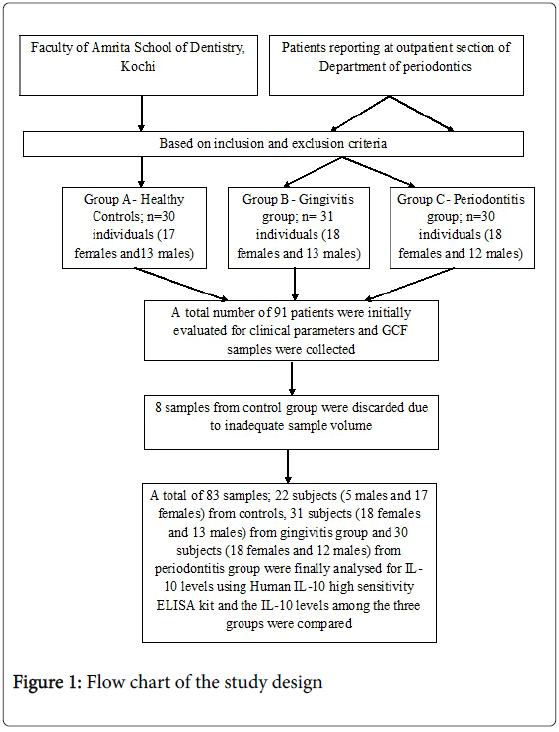
Methods: In the present study, 91 subjects were divided into three groups based on GI (Gingival Index), OHI-S (Oral Hygiene Index-Simples), PPD (Probing pocket depth) and CAL (Clinical attachment level) into healthy, gingivitis and periodontitis groups. GCF (Gingival Crevicular Fluid) samples were collected from each patient using micropipette and IL-10 (interleukin -10) levels were analyzed using ELISA.
Results: The highest mean concentration of IL-10 was obtained for gingivitis group (1128.19 ± 532.90 mg/ml) and lowest for control group (648.96 ± 505.75). The mean IL-10 level of periodontitis (956.22 ± 475.49) group was found to be intermediate between the other two groups. There was statistically significant difference in IL-10 levels between three groups (p<0.05). On pair wise comparison, there was statistically significant difference in IL-10 levels between control and gingivitis group (p<0.05) and control and periodontitis group (p<0.05). But difference in IL-10 levels between gingivitis and periodontitis group did not reach statistically significance (p>0.05).
Conclusions: The results suggest that IL-10 levels are higher in patients with gingivitis than whose condition progressed to periodontitis. In control group, IL-10 level was least suggesting role of bacterial factors in initiating IL-10 response. The data suggests the protective role of anti-inflammatory cytokine, IL-10 in limiting progression of gingivitis to periodontitis.
Keywords
Periodontitis; Gingival crevicular; Inflammation
Introduction
Periodontitis is defined as an inflammatory disease of the supporting tissues of the teeth caused by specific microorganisms resulting in progressive destruction of periodontal ligament and alveolar bone with pocket formation, recession or both. The damage resulting from periodontal disease manifests in variable destruction of tooth supporting bone [1].
Periodontal disease is initiated and sustained by microbial plaque that accumulates in gingival crevicular region and induces an inflammatory response [2]. This inflammation may progress in many individuals to the chronic destructive inflammatory condition termed periodontitis [3]. Although microbial, environmental, behavioral and systemic disease factors are reported to influence risk for moderate to severe periodontitis, there are strong evidences to support that, host immune responses to the microbial challenge, is also associated with different clinical forms of periodontitis [4].
A complex network of pro and anti-inflammatory cytokines act in inflamed periodontal tissues. Among other cytokines, interleukin-10 (IL-10) is an important multifunctional cytokine. An increase or decrease in IL-10 levels caused by host genetic differences is critical for the individual control of balance between inflammatory, humoral and microbial challenges [5]. It is an anti-inflammatory cytokine, produced by T – helper 2 cells (Th2), macrophages and B cells, which inhibit the synthesis of pro-inflammatory cytokines such as interleukin 1 (IL 1), interleukin 2 (IL 2), interleukin 6 (IL 6), interleukin 8 (IL 8), tumor necrosis factor – α (TNF α) and interferon γ (IFNγ). IL-10 suppresses the production of metalloproteinases, while increasing the synthesis of tissue inhibitors of metalloproteinases in macrophages [6]. Moreover, it stimulates production of osteoprotegrin, which consequently inhibits bone resorption by preventing RANK-RANKL engagement. IL 10 can be a protective cytokine in periodontal disease and regulates pro- inflammatory cytokines, including those implicated in alveolar bone loss. Individuals who are high producers of IL 10 might be more protected against chronic periodontitis due to the anti- inflammatory role of IL 10. Therefore, a genetically determined increase of anti-inflammatory cytokine; IL 10, would down regulate the immune response against periodontopathogenic bacteria [7].
The various biomarkers that are expressed during the inflammatoryprocess in periodontitis are released in gingival crevicular fluid (GCF) serving as an important diagnostic marker. Moreover, collection of GCF is a non -invasive or minimally invasive procedure [8].
Periodontal inflammation may involve both an increase in inflammatory stimulators like IL-1 and a decrease in inflammatory inhibitors like IL-10 and such a double impact may be the underlying factor in severe progressive changes inherent to periodontitis [9]. It is however, not clear how this balance works in different stages of periodontitis and whether there is indeed such a multi-dimensional inflammatory response that may point to the involvement of various cell types in the progression of inflammation. Few studies have investigated the effect of these cytokines, especially IL-10, in patients with aggressive periodontitis [10]. Limited data also exist related to simultaneous changes in “pro inflammatory” versus “anti-inflammatory” pathways in GCF in chronic periodontitis patients [11].
Since IL-10 plays a protective role in disease progression, its levels can be a regulatory factor in limiting the progression of gingivitis to periodontitis even in the presence of local factors. Hence IL-10 levels will have to be higher in patients with gingivitis and lower in patients with periodontitis. The role of anti-inflammatory cytokines like IL-10 in limiting the progression and maintaining a balance in inflammatory conditions like gingivitis and periodontitis needs further investigation.
Thus, the aim of the present study was to evaluate the levels of IL-10 in GCF in healthy controls, subjects with gingivitis and those with periodontitis and to compare its levels in the three study groups.
Materials and Methods
Patient population
The study group consisted of 91 subjects, who were between 35 and 65 years of age. Subjects in gingivits and periodontitis groups were selected from outpatient section of department of periodontics and control group from the faculty of Amrita School of Dentistry, Kochi. Subjects included in the study should have a minimum of 20 teeth. Subjects with history of habits like usage of tobacco or consumption of alcohol, chronic inflammatory disorders of skin and oral mucosa, known systemic illness like diabetes mellitus, hypertension or other cardiovascular disorders, bleeding disorders, hepatitis or human immunodeficiency virus (HIV) infection and immunosuppressive chemotherapy, those on antibiotics or anti-inflammatory drugs within the last 6 months, who underwent professional cleaning / periodontal treatment within the last 6 months or diagnosed as aggressive periodontitis and pregnant and lactating women were excluded from the study. Patients were recruited from a period of May 2012 to June 2013. The study was explained to all the subjects before participation and a written informed consent was obtained from those who agreed to participate voluntarily. Ethical approval for the study was obtained from the ethical committee of the Amrita School of Dentistry, Amrita Vishwa Vidhyapeetam University, Kochi, Kerala, India.
The sample size determined for the study was 26 subjects per group to provide a power of 80% and a confidence interval of 95% [12]. As the inadequacy of GCF sample volume for the ELISA test was anticipated we decided to include a minimum of 30 subjects per group in the study.
Clinical measurements
Clinical parameters were evaluated for all teeth, except third molars. Probing pocket depth (PPD) and clinical attachment levels (CAL) of teeth were assessed using a Williams graduated probe at six sites (mesio-buccal, mid-buccal, disto-buccal, mesio-lingual, mid-lingual, or disto-lingual) and the maximum was recorded and IL-10 was collected from that site. Subjects of Group A (healthy controls) had a probing depth of ≤3 mm without any clinical attachment loss and with gingival index (GI) <1 while the subjects of Group B (gingivitis) had probing depth of ≤3 mm without any clinical attachment loss and gingival index ≥1 and <2 and those in Group C (periodontitis) had five or more teeth with probing depth of ≥5 mm and clinical attachment loss of ≥3 mm with radiographic evidence of bone loss and gingival index ≥2. Both groups B and C had comparable oral hygiene index- simplified score (OHI-S).
Site selection and GCF collection
All clinical and radiological examinations, group allocation and sampling site selection were performed by a single trained examiner (A.F.). The samples were collected on the next day by a second examiner (R.K.S). This was done to prevent contamination of GCF with blood, which was associated with probing of inflamed sites. Only one site per subject was selected as sampling site. In healthy group, to ensure an adequate volume, GCF was pooled from multiple sites with no inflammatory signs. In gingivitis patients, site with greatest clinical signs of inflammation, i.e. redness, bleeding on probing and oedema in the absence of CAL, was selected. In chronic periodontitis patients, sites with ≥ 3mm CAL were identifed using a Williams graduated periodontal probe, and site showing greatest clinical signs of inflammation and highest CAL along with intraoral periapical radiographic confirmation of bone loss using long-cone paralleling technique was selected for sampling. On the sample collection day, after gently drying the area with a blast of air, supragingival plaque was removed using a hand scaler without touching the marginal gingiva. The area was isolated using sterile gauze to prevent contamination by saliva and GCF was collected by placing a microcapillary pipette at the entrance of the gingival sulcus, gently touching the marginal gingiva. From each test site,an attempt was made to collect a standardized volume of 1μl using the calibration on the white colour-coded 0.2-2 μL calibrated volumetric microcapillary pipette‡ with an extracrevicular approach (without stimulating the gingiva). Each sample collection was allotted a maximum of 10 minutes and some test sites in healthy group which did not express any volume of GCF within the allotted time, were excluded from the study. Furthermore, micropipettes which were suspected of being contaminated with blood and saliva were excluded from the study. The GCF collected was immediately transferred to eppendorf tubes (0.5 ml), centrifuged at 3000 rpm for 10 minutes and stored at –80°C until the time of assay.
[‡ -Thermo Scientific, Finnpipette F2- 0.2-2 μl]
Analysis of IL-10 in GCF using ELISA
Frozen GCF samples were thawed. The ELISA analyses for IL-10 were performed with commercial test systems§. Total of 83 samples were analyzed for IL-10 levels in GCF using ELISA test. 8 samples from control group were discarded due to inadequate sample volume. Thus a total of 22 subjects (5 males and 17 females) from control group, 31 subjects (18 females and 13 males) from gingivitis group and 30 subjects (18 females and 12 males) from periodontitis group were analyzed for IL-10 levels in GCF.
[§-Diaclone, Human IL-10 high sensitivity ELISA kit, France]
Principle of the method
The IL-10 Kit was a solid phase sandwich Enzyme Linked-Immuno-Sorbent Assay (ELISA). A monoclonal antibody specific for IL-10 had been coated onto the wells of the microtiter strips provided. Samples, including standards of known IL-10 concentrations and unknowns were pipetted into these wells. During the first incubation, the IL-10 antigen and a biotinylated monoclonal antibodyspecific for IL-10 were simultaneously incubated.
After washing, enzyme (streptavidin-peroxydase) was added. All unbound enzyme was removed by washing and first amplification step was performed by adding Biotine-Tyramine reagent. Under action of horse radish peroxide (HRP), a biotinepolymerisation reaction occurs in the region of the HRP linked to the detection antibody. After washing second amplification step was performed and polymerized biotine was revealed by a new streptavidin-HRP step. Finally after washing, substrate was added. The intensity of this coloured product is directly proportional to concentration of IL-10 present in samples.
Data analysis
All data analysis was done using a software program (IBM SPSS version 20). Mean and median of age, PPD, CAL, GI and OHI-S and IL-10 levels were calculated for each group. Intergroup comparison of IL-10 levels, clinical and demographic parameters were done using ANOVA for normally distributed variables and Kruskal-Wallis test for comparison of variables not following normal distribution. For pairwise comparison, Tukey HSD or Mann- Whitney U test was used. Chi-square testwas used for finding the association with gender. The correlation between clinical parameters and IL-10 was done using Spearman’s rank correlation coefficient. The results were considered to be statistically significant if p<0.05.
Results
With respect to gender, there was no statistically significant difference between the three groups (p>0.05) (Table 1). 77.3% of controls, 58.1% of gingivitis and 60.0% of periodontitis subjects were females. There was statistically significant difference in age between the three groups (p<0.001). When pairwise comparison was done, there was statistically significant difference in age between control and gingivitis groups (p<0.001) and control and periodontitis group (p<0.001). But the difference in age between gingivitis and periodontitis group was not statistically significant (p=0.347) (Table 2).
| Gender | Category | p value | |||||
|---|---|---|---|---|---|---|---|
| Control | Gingivitis | Periodontitis | 0.307 | ||||
| n | % | N | % | n | % | ||
| Male | 5 | 22.7 | 13 | 41.9 | 12 | 40.0 | |
| Female | 17 | 77.3 | 18 | 58.1 | 18 | 60.0 | |
| Total | 22 | 100.0 | 31 | 100.0 | 30 | 100.0 | |
Table 1: Comparison of gender between healthy controls, subjects with gingivitis and subjects with periodontitis.
| Group A (n=22) | Age | GI | PPD | CAL (mm) | OHI-S | IL-10 (pg/ml) | |
| Mean ±SD† | 37.32±2.90 | 0.37±0.12 | 2.09±0.24 | 0.00±0.00 | 0.95±0.22 | 648.96±505.75 | |
| Median | 36.00 | 0.39 | 2.20 | 0.00 | 0.90 | 498.10 | |
| Range (min, max) | (35,46) | (0.13,0.60) | (1.5,2.3) | - | (0.5, 1.2) | (80.6,1628.0) | |
| Group B (n=31) | Mean ±SD | 44.61±7.14 | 1.35±0.12 | 2.43±0.12 | 0.00±0.00 | 2.70±0.53 | 1128.19±532.90 |
| Median | 45.00 | 1.40 | 2.40 | 0 | 2.60 | 1255.80 | |
| Range (min, max) | (35,61) | (1.03,1.50) | (2.3,2.7) | - | (1.7, 4.4) | (151.6,1817.6) | |
| Group C (n=30) | Mean ±SD | 46.13±7.00 | 2.24±0.25 | 5.51±0.48 | 5.55±0.47 | 2.82±0.49 | 956.22±475.49 |
| Median | 45.50 | 2.20 | 5.51 | 5.52 | 2.7 | 864.40 | |
| Range (min, max) | (37,61) | (1.70,2.70) | (4.6,6.8) | (5,7) | (2.2, 3.8) | (260.0,1728.0) |
Table 2: Descriptive statistics of the study groups showing mean, standard deviation, median and range for age, GI, PPD, CAL, OHI-S and IL-10 levels.
In order to analyze whether the difference in age between groups had a confounding effect on IL-10 levels, correlation of IL-10 levels of three groups were done with age and it was found that there was no statistically significant correlation between IL-10 levels of control, gingivitis and periodontitis groups with age (p>0.05) (Table 3).
| Groups | Spearman’s rank correlation coefficient | ||||
| IL-10 and Age | IL-10 and GI | IL-10 and PPD | IL-10 and CAL | IL-10 and OHI-S | |
| Group A | 0.093 | -0.424* | 0.299 | - | -0.171 |
| Group B | 0.108 | -0.153 | 0.092 | - | -0.081 |
| Group C | -0.300 | -0.005 | -0.222 | -0.137 | 0.324 |
Table 3: Spearman’s rank correlation coefficient
GI and PPD showed statistically significant difference between three groups and on pair wise comparison showed significant difference between each group. The comparison of OHI-S between healthy controls, subjects with gingivitis and subjects with periodontitis, showed that, there was statistically significant difference in oral hygiene index between three groups (p<0.001).When pair wise comparison was done, there was statistically significant difference in OHI-S between control and gingivitis groups (p<0.001) and control and periodontitis group (p<0.001). But there was no statistically significant difference in OHI-S between gingivitis and periodontitis group (p=0.568) (Table 2).This confirmed that the gingivitis and periodontitis groups had comparable oral hygiene.
IL-10 was detected in all evaluated GCF samples (sensitivity <1.30 pg/ml). The GCF IL-10 levels showed difference between each group, with the gingivitis group having the highest and the control group with the lowest. The periodontitis group had a value between that of gingivitis group and control group (Table 2). There was statistically significant difference in IL-10 levels between the three groups (p<0.05). When pair wise comparison was done, there was statistically significant difference in IL-10 levels between control and gingivitis groups (p<0.05) and control and periodontitis group (p<0.05). But the difference in IL-10 levels between gingivitis and periodontitis groups did not reach statistical significance (p>0.05) (Table 4).
| Groups | p value | Groups compared | p value |
| Group A | 0.004* | Group A and Group B | 0.002* |
| Group B | Group A and Group C | 0.015* | |
| Group C | Group B and Group C | 0.162 |
Table 4: Comparison of IL-10 levels among the groups using Kruskal- Wallis test and Mann-Whitney U test.
Intragroup comparison of periodontitis group with PPD≤5mm and PPD >5 mm and CAL ≤ 5 mm and CAL>5 mm were done. It was found that there was no statistically significant difference in IL-10 levels in periodontitis subjects with PPD≤5mm and PPD>5 mm (p>0.05) and CAL ≤ 5 mm and CAL>5 mm (p>0.05) (Table 5).
| Periodontitis Group | n | IL-10 (Mean ± SD) | p value |
|---|---|---|---|
| PPD≤5mm | 7 | 1000.63±520.66 | 0.787 |
| PPD>5mm | 23 | 942.704±472.53 | |
| CAL≤5mm | 4 | 856.40± 589.91 | 0.625 |
| CAL>5mm | 26 | 971.58±467.62 |
Table 5: Intragroup comparison of IL-10 levels in Group C based on severity of periodontitis
Gingival index and IL-10 levels showed a negative borderline correlation in healthy controls, using Spearman’s rank correlation coefficient. No other correlation was found between IL-10 levels and any other clinical parameters in any of the three groups (Table 3).
Discussion
Periodontal disease results from interaction of host defense mechanisms with plaque microorganisms. The immune response is mediated by release of cytokines involved in inflammatory reactions which are characteristic of periodontitis [13]. Recent evidence suggests that cytokines form complex networks and their functions are reciprocally regulated [14].
IL-10 is a pleiotropic cytokine known for its immunosuppressive properties. It has a dual role; it plays a major role in suppressing immune and inflammatory responses as well as B cell activation [14] and has been implicated in the suppression of tissue destruction [11]. It also enhances the production of the IL-1 receptor antagonist (IL-1ra) in polymorphonuclear leukocytes stimulated with lipopolysaccharide (LPS) and suppresses the production of metalloproteinases [6]. Therefore it was suggested that IL-10 might have an important regulatory role in limiting the duration and extent of the inflammatory response [15].
The primary objective of the study was to evaluate the levels of IL-10 in GCF of healthy controls, subjects with gingivitis and subjects with periodontitis and to compare the values between each group so as to determine if IL-10 has a role in limiting the progression of disease from gingivitis to periodontitis. The GCF samples from three groups were collected. The GCF IL-10 levels were analyzed for all the three groups using a commercially available high sensitivity ELISA kit. To the best of our knowledge, the present study is the first of its kind to determine and compare the levels of IL-10 in GCF in subjects without clinical signs of gingival inflammation, those with gingivitis and those with periodontitis.
IL-10 was detected in all evaluated GCF samples (sensitivity <1.30 pg/ml). The GCF IL-10 levels showed difference between each group, with the gingivitis group having the highest and the control group with the lowest. The periodontitis group had a value between that of gingivitis group and control group (Table 4). The value of IL-10 in the control group was significantly lower than in gingivitis group, may be because the stimulation required for the production of IL-10 was not sufficient enough to initiate a positive response. Monocytes and macrophages secrete IL-10 after activation with various endogenous as well as exogenous mediators such as bacterial LPS [via activation of toll-like receptor 4, TNF Receptor Associated Factor 3, Nuclear Factor Kappa B p65/p50, and Extracellular Signal Regulated Kinases] and catecholamines (via activation of protein kinase A and cAMP Response Element Binding Protein-1/ Activating Transcription Factor-1) by inducing of IL-10 gene transcription [16]. In this study we have been able to find that the control group had the lowest levels of IL-10 when compared to gingivitis and periodontitis group. In control group the local factors were very minimal and the gingival index score was < 1 which means that there was no clinically detectable gingivitis. In this condition, there would not have been sufficient stimuli for an increase in the levels of IL-10. This is in accordance with the study by Gamonal et al., [13], which stated that IL-10 levels were undetectable in GCF of healthy controls. de Waal-Malefyt et al., [17], demonstrated that human monocytes activated by LPS were able to produce high levels of IL-10, in a dose dependent fashion.
The gingivitis group had a higher IL-10 level than control group. Gingivitis was detected by the presence of bleeding and an absence of clinical attachment loss. Presence of bleeding suggests an onset of inflammation. Local factors were present and OHI-S scores were comparable to that of periodontitis. Thus this bacterial load could have initiated the inflammation and hence led to the upregulation of IL-10.
In our present study, the periodontitis group had a higher level of IL-10 than controls. When compared to the control group, there was IL-10 production because of the severity of the inflammatory process going on. A similar result was observed by Stein and Hendrix, [18], who suggested that gingival mononuclear cells extracted from adult periodontitis patients constitutively produced more IL-10 than gingival mononuclear cells derived from non-inflamed tissues.
Both the gingivitis group and the periodontitis group had comparable oral hygiene as assessed by OHI-S. The result that IL-10 levels are higher in gingivitis group than periodontitis group with comparable OHI-S index reinforces the anti-inflammatory role of this cytokine, in preventing the progression of the disease and maintaining a stable condition. The low levels of IL-10 in periodontitis group may be responsible for preventing the progression of inflammatory process from gingivitis to periodontitis.
Study by Gemell and Seymour [19], found a low percentage of gingival IL-10 + CD8 cells extracted from adult periodontitis lesions (CAL>5mm) compared to group with CAL < 4mm. This shows that patients with more severe periodontitis have lower levels of IL-10 as compared with less severe groups. Yamazaki et al., [14], suggested that in gingival tissues, the expression of IL-10 in periodontitis group (CAL 4.8±1.5mm) was found to be lower than that from control group with CAL 2.0± 0.3mm. Gorska et al., [20] did not observe vigorous production of cytokines, IL-4 and IL-10 nor their higher frequency in tissue supernatants obtained from patients with chronic periodontitis, while they were present most often in biopsies collected from normal tissues of patients undergoing orthodontic treatment. As patients undergoing orthodontic treatment will have some amount of gingival inflammation and cannot be considered as having a clinically healthy gingiva, the control group in this study falls under the category of gingivitis in our study. Passoja et al., . [21], in a study found that serum IL-10 levels of control subjects were higher than subjects with periodontitis, where control subjects had minimally inflamed periodontal tissues. Gemell and Seymour in 1998 [19], suggested that the presence of a high percentage of IL-10 + T cells might indicate a stable lesion, while a low percentage of IL-10 + T cells might indicate a progressive lesion. IL-10 is thought to play a role in periodontitis, especially by downregulation of the production of monocyte derived proinflammatory cytokines and stimulating protective antibody production [22].
The result of the present study showed that patients with gingivitis had greater levels of IL-10 as compared to those with periodontitis, but the result did not reach statistical significance. This may probably be due to the fact that only a small percentage of sites that have been assigned the clinical diagnosis of periodontitis actually progressed from gingivitis in a given period of time. Moreover, it is impossible from cross-sectional studies to identify progressing lesions and only longitudinal studies can detect sites that have progressed, by documenting increases in clinical attachment loss measurements [23].
On analysis of IL-10 levels in patients with comparable OHI-S scores, elevated levels were found in subjects with gingivitis when compared to those with periodontitis. Although virulence of the microorganisms contribute to the initiation and progression of the disease, genetic contribution [24,25] to the pathogenesis of periodontitis, as established by previous studies [26] and specific genetic markers are found to be strong indicators of susceptibility to periodontitis in adults. Thus the possible explanation for this variation in the progression of periodontal disease can be attributed to the genetic polymorphisms in IL-10 gene as shown in previous studies by Scarel-Caminaga et al. [7]; Sumer et al., [4] and Cullinan et al.[27] and the inter- individual variation in IL-10 levels as investigated by Yamamoto et al. [28]. It seems possible that thegenetic polymorphism in the IL-10 gene might be useful as a marker to diagnose susceptibility to chronic periodontitis [4].
It was also observed that, in periodontitis group IL-10 levels did not vary with variation in the severity of periodontitis. The reason for which may possibly be due to the fewer number of samples in the group with PPD ≤ 5 mm (7 subjects) and with CAL ≤ 5 mm (4 subjects) when compared to that in group with PPD >5 mm (23 subjects) and CAL >5 mm (26 subjects). In our study the inclusion criteria for periodonitis patients was CAL ≥ 3 mm, hence the PPD and CAL in periodontitis group had a narrow range of 4.6 mm to 6.84 mm and 4.65 mm to 6.84 mm respectively. A broader range of PPD or CAL for the division of groups would have yielded a statistically significant difference (Table 5).
We did not plan any intervention because, even though inflammation may initiate IL-10 production, the levels sufficient enough to arrest the progression of the disease is genetically determined and not modified by treatment. This was earlier shown by Goutoudi et al. [11] in a study where he found that IL-10 total amounts were similar in non-diseased and diseased sites of the periodontal patients, while IL-10 total amounts remained almost unchanged during the 32-week period of follow up. Therefore, it might be speculated that IL-10 levels could be systematically regulated by a certain ‘type’ of immunologic response, while tissue inflammation and periodontal destruction seemed rather unlikely to significantly influence its levels [13]. Toker et al., [29] in a study couldn’t find any difference in the IL-10 levels between baseline and 6 weeks. Yamamoto et al. [28] suggested that IL-10 levels could be due to certain “high” producer patients, or to a specific Th1/Th2 response. Fu et al. [30] measured GCF levels of IFN-γ, IL-10 and IL-17 at baseline, week 8, week 16 and week 24 after mechanical removal of dental plaque and found that IL-17 declined to control levels, while IFN-γ and IL-10 levels remained unchanged.
The present study highlights the role of IL-10 in preventing the progression of gingivitis to periodontitis. When local factors initiate an inflammatory response which leads to production of cytokines, in IL-10 “high” producer patients there will be increased production of IL-10 limiting the progression of the disease. IL-10 is a rather late cytokine being produced after the pro-inflammatory mediators. Thereby, it has a special physiological significance in limiting and preventing an excessive immune response and in limiting collateral damage. Concurrently, it strengthens the “scavenger” functions of the immune system which is important after a confiict with antigens, and it contributes to the peripheral tolerance during antigen persistence [16]. When IL-10 secretion is less, pro inflammatory cytokine production cannot be counteracted leading to progression of periodontal disease.
Hence we can say that IL-10 can down regulate production of pro-inflammatory cytokine levels of IL-1 and TNF production in response to various inflammatory stimuli. This will also decrease alveolar bone resorption as IL-10 has been shown to be potent inhibitor of osteoclast resorption [31]. The limitations of the present study are that it is a cross sectional study and such studies may not always show an association in disease of multifactorial etiology such as periodontitis. Longitudinal studies are more reliable in the interpretation of such an association. Moreover, evaluation of individual cytokines instead of the overall balance of cytokines with opposing or similar functions may influence the accuracy in determining the role of IL-10 in periodontitis. There should have also been a definitive clinical data concerning the actual disease activity (i.e., active bone resorption) at the time of sample collection. Further studies with larger samples and analysis of IL-10 gene polymorphisms are still required for exact determination of the role of IL-10 in initiation and progression of periodontitis.
Conclusion
The results suggest that IL-10 levels are higher in patients with gingivitis than whose condition progressed to periodontitis. In control group, IL-10 level was least suggesting role of bacterial factors in initiating IL-10 response. The data suggests the protective role of anti-inflammatory cytokine, IL-10 in limiting progression of gingivitis to periodontitis.
Acknowledgement
This study was supported by grant from Colgate Palmolive India Private Limited. The authors are grateful to Mr. Sreejith for technical assistance and Mr. Deepak for statistical assistance.
References
- Hinrichs JE, Novak MJ (2012) Classification of diseases and conditions affecting the periodontium. In: Carranza FA (eds.), Carranza’s Clinical Peridontology, (11thedn.), Reed Elsevier India Private Limited, New Delhi, 41.
- Scarel-Caminaga RM, Trevilatto PC, Souza AP, Brito RB, Line SR (2002) Investigation of an IL-2 polymorphism in patients with different levels of chronic periodontitis. J ClinPeriodontol 29: 587-591.
- Kinane DF, Hart TC (2003) Genes and gene polymorphisms associated with periodontal disease. Crit Rev Oral Biol Med 14: 430-449.
- Sumer AP, Kara N, Keles GC, Gunes S, Koprulu H, et al. (2007) Association of interleukin-10 gene polymorphisms with severe generalized chronic periodontitis. J Periodontol 78: 493-497.
- Gonzales JR, Michel J, Diete A, Herrmann JM, Bödeker RH, et al. (2002) Analysis of genetic polymorphisms at the interleukin-10 loci in aggressive and chronic periodontitis. J ClinPeriodontol 29: 816-822.
- Lacraz S, Nicod LP, Chicheportiche R, Welgus HG, Dayer JM (1995) IL-10 inhibits metalloproteinase and stimulates TIMP-1 production in human mononuclear phagocytes. J Clin Invest 96: 2304-2310.
- Scarel-Caminaga RM, Trevilatto PC, Souza AP, Brito RB, Camargo LE, et al. (2004) Interleukin 10 gene promoter polymorphisms are associated with chronic periodontitis. J ClinPeriodontol 31: 443-448.
- Lamster IB (1997) Evaluation of components of gingival crevicular fluid as diagnostic tests. Ann Periodontol 2: 123-137.
- Deschner J, Arnold B, Kage A, Zimmermann B, Kanitz V, et al. (2000) Suppression of interleukin-10 release from human periodontal ligament cells by interleukin-1beta in vitro. Arch Oral Biol 45: 179-183.
- Kamma JJ, Giannopoulou C, Vasdekis VG, Mombelli A (2004) Cytokine profile in gingival crevicular fluid of aggressive periodontitis: influence of smoking and stress. J ClinPeriodontol 31: 894-902.
- Goutoudi P, Diza E, Arvanitidou M (2004) Effect of periodontal therapy on crevicular fluid interleukin-1beta and interleukin-10 levels in chronic periodontitis. J Dent 32: 511-520.
- Pradeep AR, Roopa Y, Swati PP (2008) Interleukin-4, a T-helper 2 cell cytokine, is associated with the remission of periodontal disease. J Periodontal Res 43: 712-716.
- Gamonal J, Acevedo A, Bascones A, Jorge O, Silva A (2000) Levels of interleukin-1 beta, -8, and -10 and RANTES in gingival crevicular fluid and cell populations in adult periodontitis patients and the effect of periodontal treatment. J Periodontol 71: 1535-1545.
- Yamazaki K, Tabeta K, Nakajima T, Ohsawa Y, Ueki K, et al. (2001) Interleukin-10 gene promoter polymorphism in Japanese patients with adult and early-onset periodontitis. J ClinPeriodontol 28: 828-832.
- Cassatella MA, Meda L, Bonora S, Ceska M, Constantin G (1993) Interleukin 10 (IL-10) inhibits the release of proinflammatory cytokines from human polymorphonuclear leukocytes. Evidence for an autocrine role of tumor necrosis factor and IL-1ß in mediating the production of IL-8 triggered by lipopolysaccharide. J Exp Med 178:2207-2211.
- Sabat R, Grütz G, Warszawska K, Kirsch S, Witte E, et al. (2010) Biology of interleukin-10. Cytokine Growth Factor Rev 21: 331-344.
- de Waal Malefyt R, Abrams J, Bennett B, Figdor CG, de Vries JE (1991) Interleukin 10(IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J Exp Med 174: 1209-1220.
- Stein SH, Hendrix CL (1996) Interleukin 10 promotes anticollagen antibody production in gingival mononuclear cells. J Dent Res 75:158.
- Gemmell E, Seymour GJ (1998) Cytokine profiles of cells extracted from humans with periodontal diseases. J Dent Res 77: 16-26.
- Gorska R, Gregorek H, Kowalski J,Laskus-Perendyk A, Syczewska M, et al.(2003) Relationship between clinical parameters and cytokine profiles in inflamed gingival tissue and serum samples from patients with chronic periodontitis. J ClincPeriodontol 30:1046-1052.
- Passoja A, Puijola I, Knuuttila M, Niemelä O, Karttunen R, et al. (2010) Serum levels of interleukin-10 and tumour necrosis factor-α in chronic periodontitis. J ClinPeriodontol 37: 881-887.
- Rousset F, Garcia E, Defrance T, Péronne C, Vezzio N, et al. (1992) Interleukin 10 is a potent growth and differentiation factor for activated human B lymphocytes. ProcNatlAcadSci U S A 89: 1890-1893.
- Armitage GC (2004) Analysis of gingival crevice fluid and risk of progression of periodontitis. Periodontol 2000 34: 109-119.
- Michalowicz BS, Aeppli D, Virag JG, Klump DG, Hinrichs JE, et al. (1991) Periodontal findings in adult twins. J Periodontol 62: 293-299.
- Michalowicz BS (1994) Genetic and heritable risk factors in periodontal disease. J Periodontol 65: 479-488.
- Kornman KS, Crane A, Wang HY, di Giovine FS, Newman MG, et al. (1997) The interleukin-1 genotype as a severity factor in adult periodontal disease. J ClinPeriodontol 24: 72-77.
- Cullinan MP, Westerman B, Hamlet SM, Palmer JE, Faddy MJ, et al. (2008) Progression of periodontal disease and interleukin-10 gene polymorphism. J Periodontal Res 43: 328-333.
- Yamamoto M, Fujihashi K, Hiroi T, McGhee JR, Van Dyke TE, et al. (1997) Molecular and cellular mechanisms for periodontal diseases: role of Th1 and Th2 type cytokines in induction of mucosal inflammation. J Periodontal Res 32: 115-119.
- Toker H, Poyraz O, Eren K (2008) Effect of periodontal treatment on IL-1beta, IL-1ra, and IL-10 levels in gingival crevicular fluid in patients with aggressive periodontitis. J ClinPeriodontol 35: 507-513.
- Fu QY, Zhang L, Duan L, Qian SY, Pang HX (2013) Correlation of chronic periodontitis in tropical area and IFN-γ, IL-10, IL-17 levels. Asian Pac J Trop Med 6: 489-492.
- Owens JM, Gallagher AC, Chambers TJ (1996) IL-10 modulates formation of osteoclasts in murine hemopoietic cultures. J Immunol 157: 936-940.
Relevant Topics
- Advanced Bleeding Gums
- Advanced Receeding Gums
- Bleeding Gums
- Children’s Oral Health
- Coronal Fracture
- Dental Anestheia and Sedation
- Dental Plaque
- Dental Radiology
- Dentistry and Diabetes
- Fluoride Treatments
- Gum Cancer
- Gum Infection
- Occlusal Splint
- Oral and Maxillofacial Pathology
- Oral Hygiene
- Oral Hygiene Blogs
- Oral Hygiene Case Reports
- Oral Hygiene Practice
- Oral Leukoplakia
- Oral Microbiome
- Oral Rehydration
- Oral Surgery Special Issue
- Orthodontistry
- Periodontal Disease Management
- Periodontistry
- Root Canal Treatment
- Tele-Dentistry
Recommended Journals
Article Tools
Article Usage
- Total views: 16945
- [From(publication date):
July-2014 - Apr 24, 2025] - Breakdown by view type
- HTML page views : 11811
- PDF downloads : 5134