Lessons from the Study of Covalent Dimers of the Alzheimer’s Disease-Associate Amyloid β-Protein
Received: 22-Feb-2018 / Accepted Date: 13-Mar-2018 / Published Date: 21-Mar-2018 DOI: 10.4172/2168-9652.1000229
Alzheimer’s disease (AD) is a brain disorder that first manifests in the form of intermittent memory problems and then progresses to dementia and ultimately death. Although the precise cause of AD remains obscure evidence from multiple sources indicate that the amyloid β-protein (Aβ) plays a key role [1]. Aβ comprises a family of proteins with a common core of ~30 amino acids. These peptides are amphipatic in nature and are prone to self-associate and certain aggregates of Aβ are toxic to nerve cells. Aβ of various sequences, but most particularly those that extend C-terminal to alanine 42, are found in the tell-tale amyloid plaques which litter the brains of individuals who die with AD. Several mutations within the Aβ sequence cause early onset AD and are believed to increase the formation of toxic Aβ assemblies. However, such mutations are very rare and most cases of AD occur in individuals with the normal Aβ sequence. Why wild type Aβ folds to form toxic assemblies is unclear. One possibility is that certain post-translational modifications of Aβ may arise in some individuals and that these lead to sufficiently high levels of toxic Aβ assemblies so as to precipitate sporadic AD. In this regard studies of material extracted from AD brain suggest that Aβ with a size (~7-8 kDa) and properties consistent with a covalent dimer maybe particularly important [2].
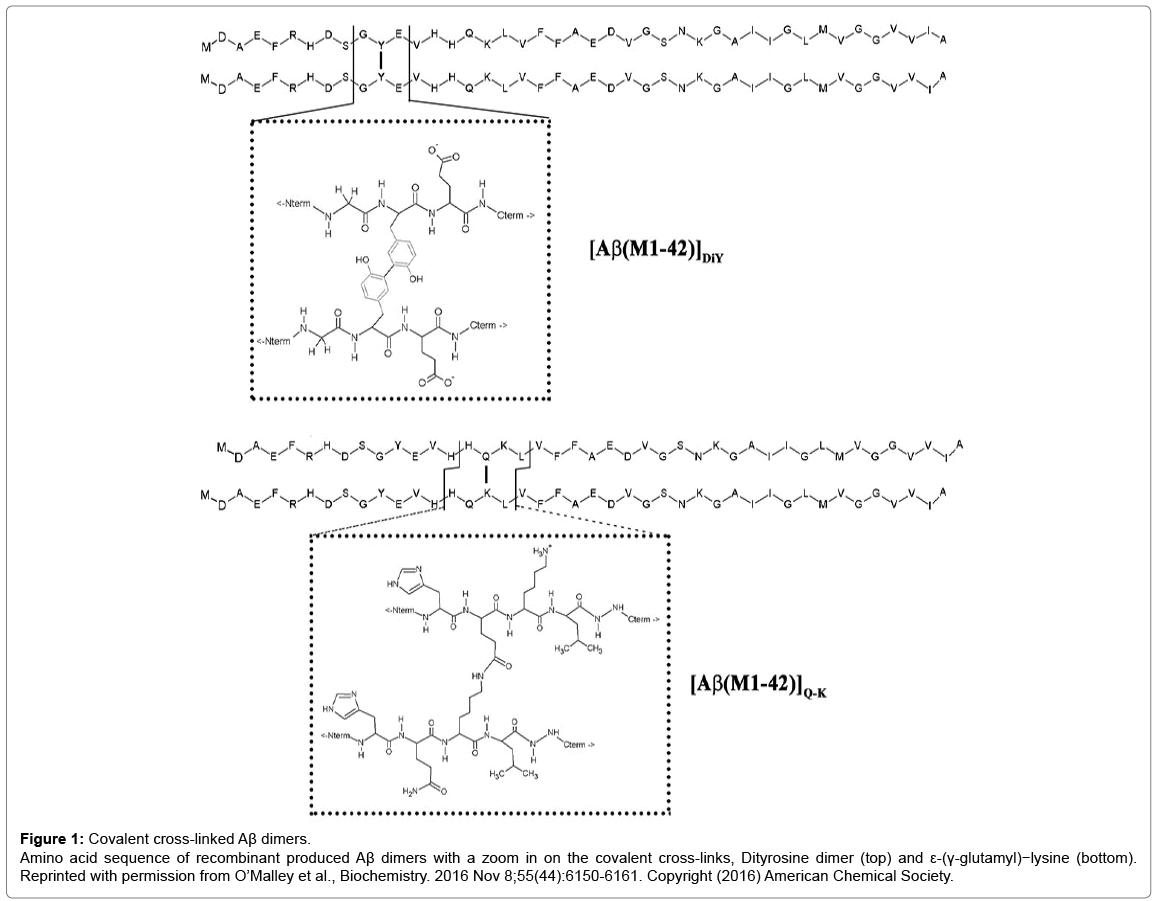
In nature, there are only two reactions that could yield covalent Aβ dimers. One mechanism involves the phenolic coupling of tyrosine residues and the other the formation of an isopeptide bond between Gln15 and Lys16 (Figure 1). Oxidative stress can cause formation of dityrosine (DiY) cross-linking of proteins and there is evidence of increased levels of DiY in AD [3] and in vitro, Aβ can readily be induced to form DiY cross-linked dimers. Cross-linking of Aβ can also be achieved by the enzyme catalyzed formation of isopeptide bonds between Gln15 and Lys16 (Figure 1). In vivo, ε-(γ-glutamyl)-lysine bonds (Q-K) are formed by the action of the enzyme transglutaminase. Q-K formation is known to be increased in AD and synthetic Aβ can serve as a substrate for TG leading to production of isopeptide crosslinked dimers [4]. Surprisingly, until our study the aggregation of Aβ42 Q-K and DiY dimers was not investigated.
Figure 1: Covalent cross-linked Aβ dimers.
Amino acid sequence of recombinant produced Aβ dimers with a zoom in on the covalent cross-links, Dityrosine dimer (top) and ε-(γ-glutamyl)−lysine (bottom). Reprinted with permission from O’Malley et al., Biochemistry. 2016 Nov 8;55(44):6150-6161. Copyright (2016) American Chemical Society.
Using highly pure recombinantly produced Aβ42 [5] and enzymatic cross-linking we generated DiY and Q-K dimers and studied their aggregation kinetics and the nature of the aggregates they formed. Aggregation kinetics were monitored using 3 different techniques: Thioflavin T (ThT) fluorescence, analytical size exclusion chromatography (aSEC), and quasielastic light scattering (QLS). ThT is a benzothiazole dye known to bind to a variety of β-sheet-rich aggregates such that binding causes a red shift in the excitation spectrum of ThT. The assay we employed provides a continuous readout of changes in ThT fluorescence and thus the formation of ThT-positive aggregates.
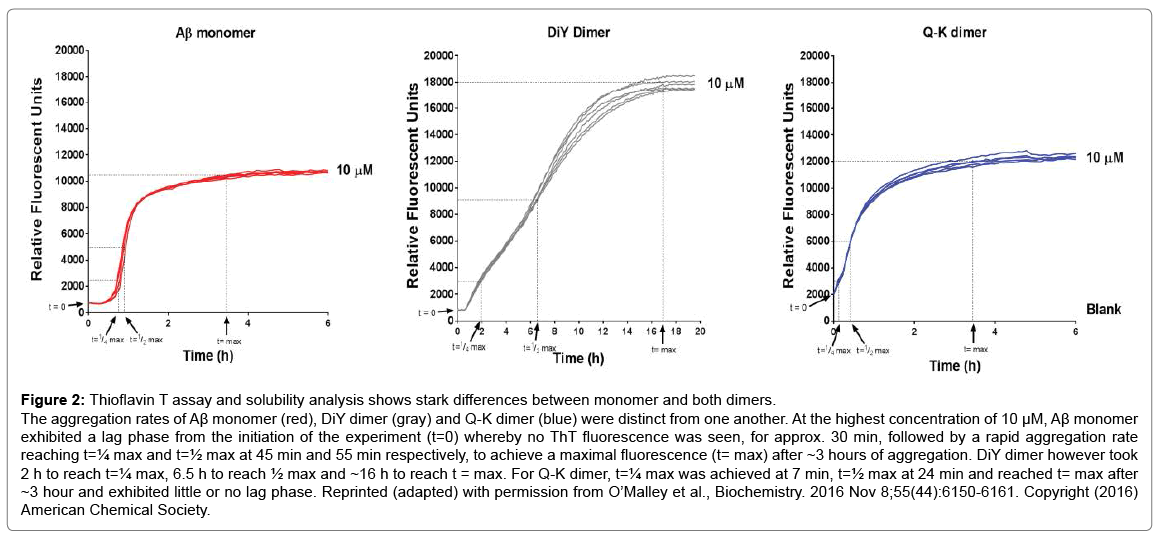
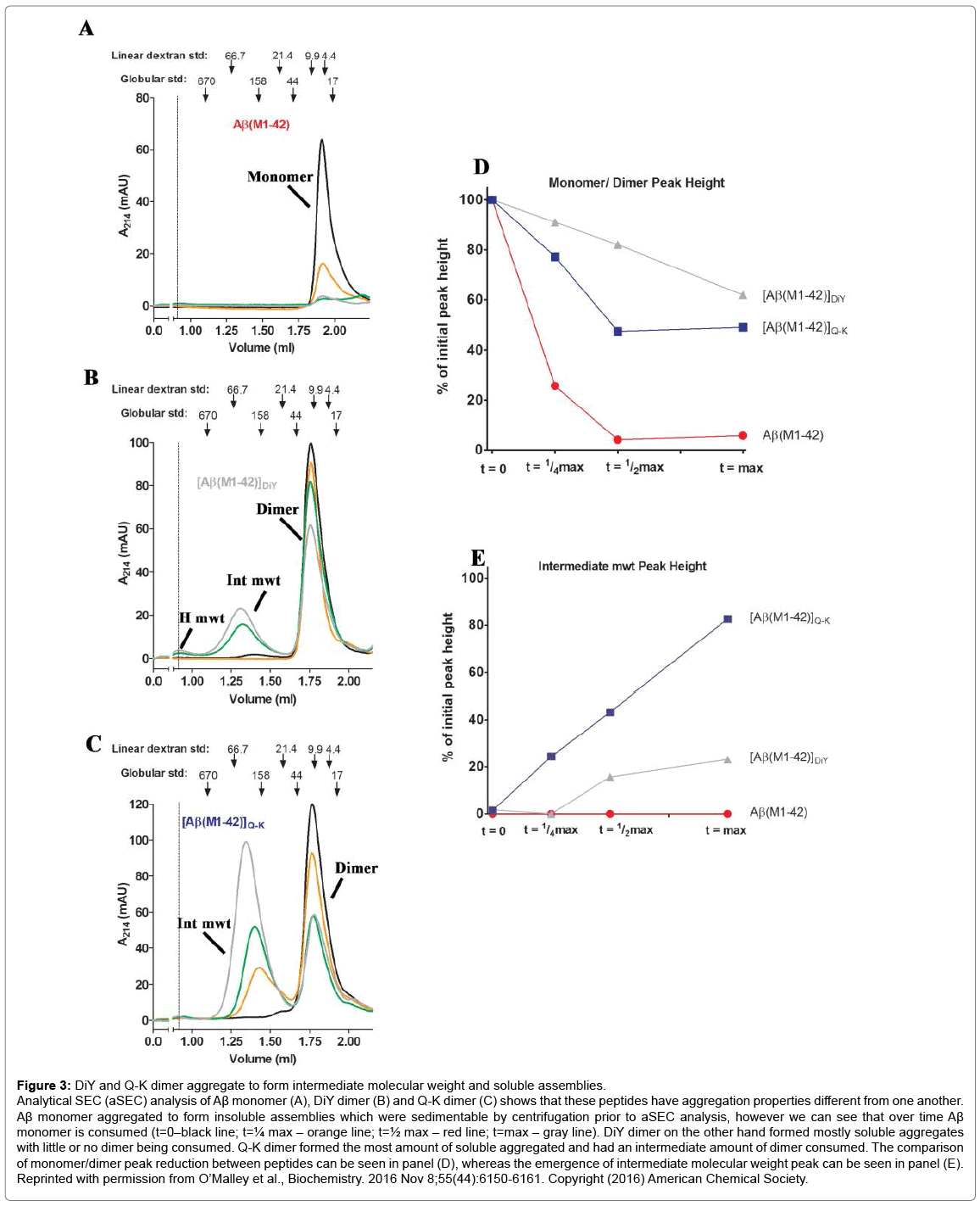
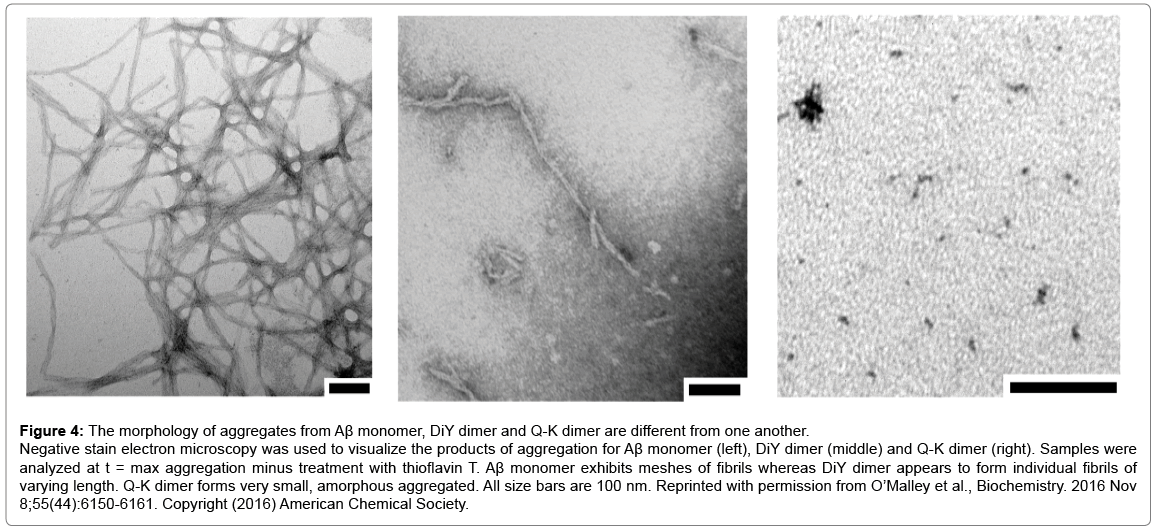
QLS is a non-invasive method that was used to measure the average diffusion coefficient of particles in solution and therefore provides an estimate of the average hydrodynamic radius (RH) of protein aggregates. aSEC separates soluble aggregates based on size and complements QLS. Four distinct phases of the aggregation process were identified by the ThT assay: (1) t=0, the earliest measurable time point after dimers or monomers were first isolated and before incubation at 37°C; (2) t=max, the interval at which ThT fluorescence reached its maximal plateau value, (3) t=¼max, an interval ¼ that of t=max; and (4) t=½max, an interval ½ that of t=max (Figure 2). At each of these 4 time points samples were analyzed by QLS and aSEC. Thus, we were able to define the kinetics of ThT-positive aggregates and estimate the size of these aggregates. We found that the Q-K dimer aggregated much faster than the monomer (Compare Figure 2C vs. 2A), while the DiY dimer aggregated slower (Compare Figure 2B vs. 2A). Strikingly, aSEC and QLS revealed that monomer rapidly disappeared from solution forming large assemblies that were readily sedimented by low speed centrifugation. In contrast, the Q-K and DiY dimers were consumed at much slower rates than the monomer, and they formed smaller but more strongly ThT positive aggregates (Figure 3). Negative contrast electron microscopy on endstage aggregates confirmed that dimers formed aggregates very different from those formed by monomer (Figure 4). Collectively these results demonstrate that Q-K and DiY dimers have aggregation paths and products which result in the generation of more soluble and diffusible assemblies. Although we did not investigate the biological activity of aggregates formed by Q-K or DiY dimers, it is widely believed that soluble aggregates are more toxic than insoluble aggregates. Hence, it is tempting to speculate that covalent Aβ42 dimers may play an important role in AD. Furthermore, since we show that ThT fluorescence is not specific for fibrils but that certain prefibrillar structures can also bind to ThT, these findings have wider implications for the protein aggregation field and how changes in ThT fluorescence are interpreted.
Figure 2: Thioflavin T assay and solubility analysis shows stark differences between monomer and both dimers.
The aggregation rates of Aβ monomer (red), DiY dimer (gray) and Q-K dimer (blue) were distinct from one another. At the highest concentration of 10 μM, Aβ monomer exhibited a lag phase from the initiation of the experiment (t=0) whereby no ThT fluorescence was seen, for approx. 30 min, followed by a rapid aggregation rate reaching t=¼ max and t=½ max at 45 min and 55 min respectively, to achieve a maximal fluorescence (t= max) after ~3 hours of aggregation. DiY dimer however took 2 h to reach t=¼ max, 6.5 h to reach ½ max and ~16 h to reach t = max. For Q-K dimer, t=¼ max was achieved at 7 min, t=½ max at 24 min and reached t= max after ~3 hour and exhibited little or no lag phase. Reprinted (adapted) with permission from O’Malley et al., Biochemistry. 2016 Nov 8;55(44):6150-6161. Copyright (2016) American Chemical Society.
Figure 3: DiY and Q-K dimer aggregate to form intermediate molecular weight and soluble assemblies.
Analytical SEC (aSEC) analysis of Aβ monomer (A), DiY dimer (B) and Q-K dimer (C) shows that these peptides have aggregation properties different from one another. Aβ monomer aggregated to form insoluble assemblies which were sedimentable by centrifugation prior to aSEC analysis, however we can see that over time Aβ monomer is consumed (t=0–black line; t=¼ max – orange line; t=½ max – red line; t=max – gray line). DiY dimer on the other hand formed mostly soluble aggregates with little or no dimer being consumed. Q-K dimer formed the most amount of soluble aggregated and had an intermediate amount of dimer consumed. The comparison of monomer/dimer peak reduction between peptides can be seen in panel (D), whereas the emergence of intermediate molecular weight peak can be seen in panel (E). Reprinted with permission from O’Malley et al., Biochemistry. 2016 Nov 8;55(44):6150-6161. Copyright (2016) American Chemical Society.
Figure 4: The morphology of aggregates from Aβ monomer, DiY dimer and Q-K dimer are different from one another.
Negative stain electron microscopy was used to visualize the products of aggregation for Aβ monomer (left), DiY dimer (middle) and Q-K dimer (right). Samples were analyzed at t = max aggregation minus treatment with thioflavin T. Aβ monomer exhibits meshes of fibrils whereas DiY dimer appears to form individual fibrils of varying length. Q-K dimer forms very small, amorphous aggregated. All size bars are 100 nm. Reprinted with permission from O’Malley et al., Biochemistry. 2016 Nov 8;55(44):6150-6161. Copyright (2016) American Chemical Society.
References
- Karran E, De Strooper B (2016) The amyloid cascade hypothesis: are we poised for success or failure? J Neurochem 139: 237-252.
- Shankar GM, Li S, Mehta TH, Garcia-Munoz A, Shepardson NE, et al. (2008) Amyloid-beta protein dimers isolated directly from Alzheimer's brains impair synaptic plasticity and memory. Nat Med 14: 837-842.
- Moir RD, Tseitlin KA, Soscia S, Hyman BT, Irizarry MC, et al. (2005) Autoantibodies to redox-modified oligomeric Abeta are attenuated in the plasma of Alzheimer's disease patients. J Biol Chem 280: 17458-17463.
- Hartley DM, Zhao C, Speier AC, Woodard GA, Li S, et al. (2008) Transglutaminase induces protofibril-like amyloid beta-protein assemblies that are protease-resistant and inhibit long-term potentiation, J Biol Chem 283: 16790-16800.
- Walsh DM, Thulin E, Minogue AM, Gustavsson N, Pang E, et al. (2009) A facile method for expression and purification of the Alzheimer's disease-associated amyloid beta-peptide. FEBS J 276: 1266-1281.
Citation: O’Malley TT, Walsh DM (2018) Lessons from the Study of Covalent Dimers of the Alzheimer’s Disease-Associate Amyloid β-Protein. Biochem Physiol 7:229. DOI: 10.4172/2168-9652.1000229
Copyright: © 2018 O’Malley TT, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 4050
- [From(publication date): 0-2018 - Dec 04, 2024]
- Breakdown by view type
- HTML page views: 3353
- PDF downloads: 697