Research Article Open Access
Leptin and Adiponectin Levels in Idiopathic Pulmonary Fibrosis: Association with Hypoxia
| Foteini Malli1, Panagiotis Georgoulias2, Varvara Valotassiou2, Fotini Bardaka1, Irene Tsilioni1,3, Konstantinos I Gourgoulianis1 and Zoe Daniil1,* | |
| 1Respiratory Medicine Department, University of Thessaly, School of Medicine, Larissa, Greece | |
| 2Nuclear Medicine Department, University of Thessaly, Larissa, Greece | |
| 3Molecular Immunopharmacology and Drug Discovery Laboratory, Department of Integrative Physiology and Pathobiology, Tufts University School of Medicine, Boston, MA, USA | |
| Corresponding Author : | Zoe Daniil University Hospital of Larissa Respiratory Medicine Department Mezourlo (Biopolis), 41110, Larissa, Greece Tel: +30 241 3502898 Fax: +30 241 3501563 E-mail: zdaniil@med.uth.gr |
| Received: Octomber 25, 2015;Accepted: December 1, 2015;Published: December 5, 2015 | |
| Citation: Malli F, Georgoulias P, Valotassiou V, Bardaka F, Tsilioni I, et al. (2015) Leptin and Adiponectin Levels in Idiopathic Pulmonary Fibrosis: Association with Hypoxia. J Obes Weight Loss Ther 5:284. doi:10.4172/2165-7904.1000284 |
|
| Copyright:© 2015 Malli F, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited | |
| Related article at Pubmed, Scholar Google | |
Visit for more related articles at Journal of Obesity & Weight Loss Therapy
Abstract
Background: Studies in the literature suggest a regulatory role of leptin and adiponectin in the development of fibrosis in various organs. However, their role in idiopathic pulmonary fibrosis (IPF) has not been examined in the past. The study aimed to assess the levels of leptin and adiponectin in IPF patients and 22 controls. Methods: Leptin and adiponectin were measured in serum while leptin was assessed in broncholaveloar lavage fluid and exhaled breath condensate of IPF patients. The association of the adipokines levels with markers of disease severity was also addressed. Results: Leptin levels adjusted for BMI and leptin to adiponectin (L/A) ratio in male patients with PaO2 <65 were significantly reduced as compared to male patients with PaO2 ≥65 mmHg. L/A ratio was positively associated with PaO2. Additionally, L/A ratio were positively associated with Saint George Respiratory Questionnaire components. Interestingly, exhaled breath condensate and bronchoalveolar lavage fluid leptin were not associated with corresponding serum leptin levels. Discussion: Overall our findings suggest a possible role of leptin and adiponectin in the severity and/or pathogenesis of IPF. Further studies are required in order to clarify the mechanisms and physiological relevance of our observations.
| Keywords |
| Idiopathic pulmonary fibrosis; Leptin; Adiponectin; Hypoxia; Bronchoalveolar lavage Fluid; Exhaled breath condensate |
| Introduction |
| Idiopathic pulmonary fibrosis (IPF) is a devastating form of fibrosing interstitial pneumonia that is associated with a poor survival [1]. The aetiology of the disease remains unknown contributing to the lack of effective treatment. IPF pathogenesis is considered to be multifactorial and several mechanisms have been proposed to play a role. Recent investigations suggest that an unknown injury leads to transforming growth factor-β (TGF-β) activation and alveolar basement disruption resulting in excess collagen deposition and development of IPF [2]. |
| Leptin is a protein that is mainly produced by adipose tissue, however it is secreted in lower amounts by various other tissues [3]. Recent investigations have identified the lung as a leptin responsive and producing organ, while extensive research has been published concerning leptins role in the respiratory system. Beyond its role in the regulation of food intake and body composition, leptin exerts proinflammatory properties. According to experimental data, increased leptin levels enhance liver inflammation and fibrogenesis. In fact, leptin is essential for the induction of TGF-β in the context of chronic liver injury that ultimately leads to liver fibrosis [4]. In this respect, one could argue that leptin may enhance lung fibrogenesis. However literature lacks data concerning leptins role in pulmonary fibrosis. |
| Adiponectin, that is the most abundant gene product of adipose tissue, demonstrates anti-inflammatory properties. Several studies have shown that adiponectin antagonizes leptins profibrotic properties [5]. Adiponectin inhibits the TGF-β induced expression of profibrogenic cytokines (e.g. CTGF) [6] while adiponectin knock-out mice exhibit induced renal interstitial fibrosis that is ameliorated by adiponectin replacement [7]. The latter observations mirror the hypothesis that adiponectin may have a protective effect in lung fibrosis however there are no reports in the literature examining this effect. |
| We therefore hypothesized that leptin and adiponectin may be involved in the pathobiology of IPF. In this respect we assessed the concentrations of leptin and adiponectin in serum, exhaled breath condensate (EBC) and bronchoalveolar lavage fluid (BALF) and we sought to investigate their association with clinical indices of disease severity. |
| Methods |
| Subjects |
| Patients were recruited sequentially from the respiratory outpatient clinic of the University Hospital of Larissa, Greece, between June 2008- June 2010. Controls were recruited during the same period from healthy volunteers. All patients fulfilled the standard criteria [8] for the diagnosis of IPF. The diagnosis was revised according to the new criteria [9] that were published during the preparation of the manuscript. All patients were classified as definite IPF. Control group included subjects who were matched in terms of age and sex with patients and their medical history was free of lung or other known disease, were free of smoking and they received no drugs during the previous period while they had normal pulmonary function tests (PFTs). Subjects with conditions known to affect adipokines levels such as the use of corticosteroids and/or thiazolidinediones, liver and renal insufficiency, autoimmune diseases or signs of infection in 6 weeks prior to inclusion were excluded from the study. None of the patients received corticosteroid and/or immunosuppressive treatment for IPF. Ethics committee approval and written informed consent from all subjects were obtained. |
| At baseline all participants underwent clinical examination, PFTs and arterial blood gas analysis for evaluation of disease severity. Arterial blood gas analysis was performed with a commercially available blood gas analyzer (model 1630; Instrumentation Laboratories, Milan Italy); alveolar to arterial (A-a) gradient was calculated by the formula: P(A-a)O2=(713xFiO2-1,25xPCO2)-. PFTs were performed by a technician who was blinded to patients medical history (Bodyplethysmograph, Master-Screen Body, Viasys Healthcare, Germany). Health related quality of life was assessed with Saint Georges Respiratory Questionnaire. |
| Venous blood was collected in the early morning (between 06:00 and 07:00 A.M) after an overnight fast. Blood was centrifuged at 1500g for 10 min at 4°C and serum was collected and frozen at -80°C until measurements. |
| Body composition |
| All participants were subjected to anthropometric measurements. Body height was determined to the nearest 0.5 cm with subjects standing barefoot. Body weight was assessed to the nearest 0.1 kg with subjects wearing light clothing and no shoes. Body mass index was calculated as Kg/m2. Body composition was estimated with the use of bioelectric impedance analysis (BIA) with a commercially available body analyzer (BIA 101 System Analyser, Akern, Florence, Italy) according to current recommendations. |
| Serum leptin and adiponectin assay |
| Total serum leptin levels were measured using human RIA (Radioimunoassay) diagnostic kits (KIPMR44), produced by DIASource Europe SA (Belgium), while serum adiponectin levels were determined using a human adiponectin RIA kit (LINCO Research, USA). The sensitivity of leptin assay is 0.1 ng/ml, with a calibrators’ range of 0-64 ng/ml and intra- and inter-assay variation less than 4.8% and 5.3%, respectively. Adiponectin kit has a sensitivity of 1.0 ng/ml and an assay range of 1-200 ng/ml, while the cross-reactivity with hibernation specific protein C1q is less than 0.01%. The intra- and inter-assay variation is less than 6.21% and 9.25%, respectively. All RIA kits (leptin, adiponectin) are calibrated according to valid international standards. The radiotracer used in all kits is 125Iodine (I-125, half-life T1/2 60 days, 35.5 keV gamma radiation, 27-32 keV x-rays, no beta radiation). All sample assays were performed duplicated, included in the same run for each biological parameter. If the difference between duplicate results of a sample was more than 5%, the sample assay was repeated, while the in-run coefficients of variation were 3.5% for leptin and 3.8% for adiponectin. An automatic gamma counter (type: Cobra II/5010, company: Packard, USA) was used, to count the radioactivity and calculate the results. |
| Determination of leptin and adiponectin in EBC and BALF |
| EBC was collected using a commercially available condenser (EcoScreen, Viasys, Hoechberg, Germany) within 30 minutes after venous blood was obtained according to current guidelines [10-12]. EBC was collected in sterile plastic tubes and pH was immediately assessed. Stable pH was achieved after deaeration with an inert gas (argon, 350 ml/minute for 10 minutes) and was measured using a commercially available pH meter (Model 3510, Jenway, Essex, UK). |
| Twenty three patients underwent fiberoptic bronchoscopy and BAL as previously described [13]. BALF was filtered through nylon gauge in order to remove mucus and was centrifuged at 400g for 10 minutes. The supernatant was collected and frozen at -80°C until measurements. Venous blood was obtained 15 minutes prior to bronchoscopy, centrifuged at 1500g for 10 min at 4°C and serum was collected and frozen at -80°C until measurements. |
| Leptin levels in EBC, BALF and the corresponding serum sample were measured with a commercially available enzyme immunosorbent assay kit (R&D Systems, Mineapolis, USA). |
| Statistical analysis |
| Data are presented as mean ± SD unless otherwise indicated. Normal distribution was assessed by the Kolmogorov-Smirnov test. Comparison between patients and controls was performed with the use of Student’s t test or Mann-Whitney U-test according to variable distribution. Univariate correlations were performed by Pearson’s correlation coefficient for variables that were normally distributed or by Spearman’s correlation coefficient for variables that were not normally distributed. Leptin was logarithmically transformed by calculating its natural logarithm (LN leptin) for use in correlation and regression analysis. Analysis of covariance was used in order to adjust adipokines levels for anthropometric measurements. A p value of <0.05 was considered to be statistically significant. Analysis was performed using the SPSS 16 statistical package (SPSS Chicago, IL). |
| Results |
| Study group |
| The study population consisted of 37 IPF patients (33 males, 68.8 ± 8.25 years) and 22 controls (11 males, 67.9 ± 7.35 years). The clinical characteristics of the study population are shown in Table 1. Patients and controls did not differ in terms of age and BMI. Lung function results are summarized in Table 2. |
| Leptin and adiponectin association with IPF |
| Leptin and adiponectin levels of the study population are presented in Table 3. Leptin has a strong gender-related dimorphism and thus we stratified the two study groups according to gender. Serum leptin presented no significant differences in male patients when compared to controls (9.67 ± 6.12 vs. 9.35 ± 9.77 ng/ml, respectively) and in female patients when compared to controls (31.45 ± 19.17 vs. 12.08 ± 3.05 ng/ml, respectively). Circulating leptin correlated significantly with BMI in patients and controls (p=0.008 and p=0.018, respectively). In the overall group, IPF patients, when compared to controls, did not exhibit significant differences in adiponectin levels (10.41 ± 5.04 vs. 10.12 ± 6.35 mg/ml respectively, p>0.05). We did not observe any significant correlations between adiponectin levels and BMI in neither group. Additionally, we did not observe significant differences in L/A ratio between the study groups. |
| Comparisons of serum adipokines levels were performed after adjustment for BMI. Serum leptin did not differ significantly in male patients vs. male controls and female patients vs. female controls. Similarly, adiponectin levels did not differ significantly after adjustment for anthropometric measurements. |
| Leptin and adiponectin association with IPF severity |
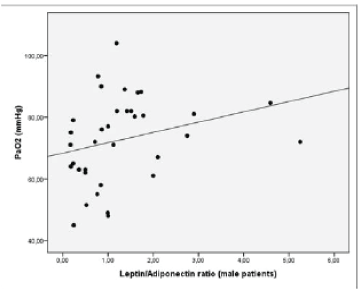
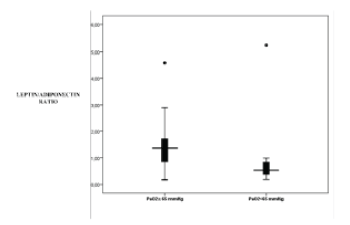
| ABGs L/A ratio in male patients was positively associated with PaO2 (p=0.038, r=0.364) (Figure 1). Leptin levels when adjusted for BMI in male patients with PaO2 <65 were significantly reduced as compared to male patients with PaO2 ≥65 mmHg (0.21 vs. 0.38, p=0.031, respectively) (Figure 2). Additionally, L/A ratio in male IPF patients with PaO2 <65 mmHg was significantly reduced vs. male patients with PaO2 ≥65 mmHg (1.07 vs. 1.45, p=0.045, respectively) (Figure 3). We did not observe a significant relationship of L/A ratio with hypoxia in female patients |
| PFTs |
| We did not observe any significant correlations of leptin, adiponectin or L/A ratio and PFT parameters (i.e. FVC%pred, DLCO %pred , RV%pred, TLC%pred) in male or female IPF patients. |
| Quality of life |
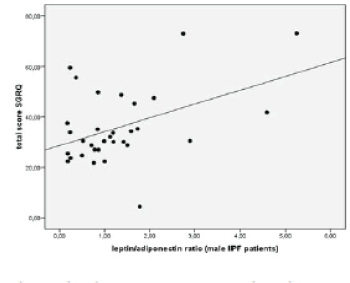
| We observed a significant positive association of L/A ratio with SQRQ total score (p=0.002, r=0.509), activity score (p=0.001, r=0.540) and impacts score (p=0.039, r=0.392). When patients were analyzed according to gender we found that in males there was a positive association of L/A ratio with SGRQ total score (p= 0.011, r=0.442) (Figure 4) and with activity score in SGRQ (p=0.023, r=0.400). |
| Leptin and adiponectin association with EBC and BAL |
| Table 3 presents the characteristics of EBC and BALF analysis of the patients. EBC pH was 6.07 ± 0.52. Leptin levels in EBC were 0.29 ± 0.06 ng/ml. We did not observe any correlation of leptin EBC levels with serum leptin concentrations. Leptin levels in BALF were 0.153 ± 0.07 ng/ml. There were no significant correlations observed between leptin levels in BALF and PFTs or ABGs. We did not observe any correlation of leptin BALF levels with corresponding serum leptin concentrations. |
| Discussion |
| In the present study we assessed the levels of leptin and adiponectin in serum, EBC and BALF in IPF patients and controls. Leptin levels and L/A ratio were reduced in IPF patients with PaO2 <65 mmHg, suggesting that leptin and adiponectin may be associated with disease severity. In accordance to these findings L/A ratio was positively associated with PaO2. Interestingly, EBC and BALF leptin was not associated with serum leptin suggesting that leptin homeostasis in the lung is only partially influenced by systemic leptin production. Collectively our findings underline a potential relationship of leptin and adiponectin with IPF and might provide further insight in the understanding of the pathogenesis of the disease. |
| According to current concepts, leptin and adiponectin play a significant role in the pathogenesis of fibrosis in various tissues. Experimental data suggest that increased leptin and reduced adiponectin may enhance liver inflammation and fibrogenesis [14]. Leptin induces collagen gene expression by liver cells in vitro [15]. On the contrary, adiponectin induces the apoptosis and reduces the proliferation of hepatic stellate cells, the key cells that produce collagen in cirrhosis. Additionally, leptin promotes peritoneal fibrogenesis through synergistic activation of the TGF-β system [16]. Moreover, leptin stimulates type-1 collagen production in renal mesangial cells [17] and induces the expression of TGF-β by glomerular endothelial cells [18]. According to these findings, leptin has profibrogenic properties and on the contrary adiponectin exerts antifibrogenic actions. However, recently published data have shown that leptin may be implicated in bleomycin-induced pulmonary fibrosis, suggesting a significant role of leptin IPF development [19]. To our knowledge there are no data regarding adipokines role in IPF and our study is the first to examine leptin and adiponectin levels in patients with idiopathic pulmonary fibrosis. Further studies are required in order to clarify the adipokines role in IPF. |
| In our study we observed that L/A ratio is reduced in hypoxic IPF patients suggesting a relationship between leptin and adiponectin with disease severity. Leptin and adiponectin dysregulation may be associated with the pathogenetic mechanisms linked to IPF and therefore affect the extent of the disease. We failed to demonstrate significant differences in leptin and adiponectin levels between patients and controls which may be due to the small number of participants included in our study. It is possible that systemic leptin levels may not directly correspond to their intrapulmonary concentration, while the adipokines actions may depend on other factors such as local production or consumption, and/or the number of the leptin and adiponectin receptors which have not been addressed in our study. While the fact that reduced L/A ratio is associated with hypoxia and therefore increased disease severity further studies including a larger cohort should be designed to elucidate the exact role of adipose tissue derived hormones with IPF development. |
| Studies have previously examined the effect of hypoxia on leptin and adiponectin. Researchers have observed that hypoxia and tissue ischemia upregulate leptin and down-regulate adiponectin expression [20,21]. In fact, hypoxia-inducible factor 1 (HIF-1) is a promoter for the leptin gene in vitro [22]. However the results from in-vivo studies are rather contradictory. Researchers have shown that short-term highaltitude exposure does not result in changes in leptin and adiponectin concentrations [23]. Others have demonstrated that leptin does not significantly differ following 8 weeks of hypoxia [24]. Bruder et al. reported that hypoxia increases adiponectin levels but does not affect leptin concentrations [25]. In the present study we observed that serum leptin is reduced in IPF patients with hypoxia while adiponectin concentrations do not differ in hypoxic vs. non hypoxic patients. One may speculate that experimental conditions cannot identically reproduce in vivo conditions. Additionally, most of in vivo studies have examined the effects of short-term hypoxic exposure in leptin levels and cannot identically mimic the intermittent hypoxia observed in IPF. Clearly, the relationship between leptin and hypoxia needs further investigation. |
| Leptin is synthesized and secreted mainly by white adipose tissue in proportion to fat stores and BMI [26]. Additionally, studies have exhibited that leptin gene is expressed in type II pneumocytes [27] and airway bronchial cells [27,28] in humans, while the lung is a leptin responsive organ since the leptin receptor is expressed by airway cells and peripheral epithelial cells [3]. In our study, leptin was positively associated with BMI and FM, both in IPF patients and controls, suggesting that systemic leptin secretion in IPF is in line with the reported feedback mechanism involved in the regulation of body weight. However, we did not observe any significant correlation of leptin EBC and BALF levels with serum leptin concentrations. Our results may suggest that leptin homeostasis in the lung is only partially influenced by systemic leptin production by white adipose tissue. One potential explanation may be that leptin may be locally consumed within the lung. Clearly, the latter hypothesis needs confirmation by further studies while additional studies are warranted to clarify how the present observations directly impact on the pathobiology of IPF. |
| The SGRQ is a self-administered health related quality of life instrument that contains 50 questions divided into three components, symptoms, activity and impacts. SGRQ was originally designed for use in patients with chronic obstructive pulmonary disease however it has acceptable validity and reliability for use in patients with IPF [29]. We observed that L/A ratio was positively associated with SGRQ components indicating that the adipokines may be associated with health related quality of life in IPF. Experimental data suggest that disturbed function of leptin in the central nervous system may be associated with depression [30]. However our study cannot determine whether our findings reflect a direct action of leptin in the CNS or present an epiphenomenon. The clarification of the above hypotheses are a matter of future studies. |
| Conclusion |
| In conclusion, the present study provides evidence suggesting a possible role of leptin in the severity and/or pathogenesis of IPF. Notably, leptin and L/A ratio was significantly reduced in male IPF patients with hypoxia. Additionally, we did not observe any significant correlation of leptin EBC and BALF levels with serum leptin concentrations, suggesting that lung leptin levels are not directly influenced by systemic leptin production. However, the mechanisms and physiological relevance of the aforementioned findings deserve further investigation. |
References
- Bjoraker JA, Ryu JH, Edwin MK, Myers JL, Tazelaar HD, et al. (1998) Prognostic significance of histopathologic subsets in idiopathic pulmonary fibrosis.Am J RespirCrit Care Med 157: 199-203.
- Coward WR, Saini G, Jenkins G (2010) The pathogenesis of idiopathic pulmonary fibrosis.TherAdvRespir Dis 4: 367-388.
- Malli F, Papaioannou AI, Gourgoulianis KI, Daniil Z (2010) The role of leptin in the respiratory system: an overview.Respir Res 11: 152.
- Leclercq IA, Farrell GC, Schriemer R, Robertson GR (2002) Leptin is essential for the hepatic fibrogenic response to chronic liver injury.J Hepatol 37: 206-213.
- Buechler C, Wanninger J, Neumeier M (2011) Adiponectin, a key adipokine in obesity related liver diseases.World J Gastroenterol 17: 2801-2811.
- Marra F, Aleffi S, Bertolani C, Petrai I, Vizzutti F (2005) Adipokines and liver fibrosis.Eur Rev Med PharmacolSci 9: 279-284.
- Ohashi K, Iwatani H, Kihara S, Nakagawa Y, Komura N, et al. (2007) Exacerbation of albuminuria and renal fibrosis in subtotal renal ablation model of adiponectin-knockout mice.ArteriosclerThrombVascBiol 27: 1910-1917.
- American Thoracic Society. (2000) Idiopathic pulmonary fibrosis: diagnosis and treatment. International consensus statement. American Thoracic Society (ATS), and the European Respiratory Society (ERS). Am J RespirCrit Care Med, 161: 646-664.
- Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, et al. (2011) An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management.Am J RespirCrit Care Med 183: 788-824.
- Joppa P, Petrasova D, Stancak B, Tkacova R (2006) Systemic inflammation in patients with COPD and pulmonary hypertension.Chest 130: 326-333.
- Barst RJ, McGoon M, Torbicki A, Sitbon O, Krowka MJ, et al. (2004) Diagnosis and differential assessment of pulmonary arterial hypertension.J Am CollCardiol 43: 40S-47S.
- Horvath I, Hunt J, Barnes PJ, Alving K, Antczak A, et al (2005) Exhaled breath condensate: methodological recommendations and unresolved questions. EurRespir J 26: 523-548
- Papiris SA, Kollintza A, Karatza M, Manali ED, Sotiropoulou C, et al. (2007) CD8+ T lymphocytes in bronchoalveolar lavage in idiopathic pulmonary fibrosis.J Inflamm (Lond) 4: 14.
- Tsochatzis E, Papatheodoridis GV, Archimandritis AJ (2006) The evolving role of leptin and adiponectin in chronic liver diseases.Am J Gastroenterol 101: 2629-2640.
- Saxena NK, Saliba G, Floyd JJ, Anania FA (2003) Leptin induces increased alpha2(I) collagen gene expression in cultured rat hepatic stellate cells.J Cell Biochem 89: 311-320.
- Yang AH, Huang SW, Chen JY, Lin JK, Chen CY (2007) Leptin augments myofibroblastic conversion and fibrogenic activity of human peritoneal mesothelial cells: a functional implication for peritoneal fibrosis. Nephrol Dial Transplant, 22: 756-762
- Han DC, Isono M, Chen S, Casaretto A, Hong SW, et al. (2001) Leptin stimulates type I collagen production in db/dbmesangial cells: glucose uptake and TGF-beta type II receptor expression.Kidney Int 59: 1315-1323.
- Wolf G, Hamann A, Han DC, Helmchen U, Thaiss F, et al (1999) Leptin stimulates proliferation and TGF-beta expression in renal glomerular endothelial cells: potential role in glomerulosclerosis [seecomments]. Kidney Int 56: 860-872
- Jain M, Budinger GR, Lo A, Urich D, Rivera SE, et al (2011) Leptin promotes fibroproliferative acute respiratory distress syndrome by inhibiting peroxisome proliferator-activated receptor-gamma. Am J RespirCrit Care Med 183:1490-1498.
- Tilg H, Moschen AR (2010) Evolution of inflammation in nonalcoholic fatty liver disease: the multiple parallel hits hypothesis.Hepatology 52: 1836-1846.
- Lévy P, Pépin JL, Arnaud C, Tamisier R, Borel JC, et al. (2008) Intermittent hypoxia and sleep-disordered breathing: current concepts and perspectives.EurRespir J 32: 1082-1095.
- Grosfeld A, Andre J, Hauguel-De Mouzon S, Berra E, Pouyssegur J, et al. (2002) Hypoxia-inducible factor 1 transactivates the human leptin gene promoter.J BiolChem 277: 42953-42957.
- Barnholt KE, Hoffman AR, Rock PB, Muza SR, Fulco CS, et al (2006) Endocrine responses to acute and chronic high-altitude exposure (4,300 meters): modulating effects of caloric restriction. Am J PhysiolEndocrinolMetab 290:E1078-1088
- Chaiban JT, Bitar FF, Azar ST (2008) Effect of chronic hypoxia on leptin, insulin, adiponectin, and ghrelin.Metabolism 57: 1019-1022.
- Bruder ED, Hoof JV, Young JB, Raff H (2008) Epidermal growth factor and parathyroid hormone-related peptide mRNA in the mammary gland and their concentrations in milk: effects of postpartum hypoxia in lactating rats.HormMetab Res 40: 446-453.
- Friedman JM, Halaas JL (1998) Leptin and the regulation of body weight in mammals.Nature 395: 763-770.
- Vernooy JH, Drummen NE, van Suylen RJ, Cloots RH, Möller GM, et al. (2009) Enhanced pulmonary leptin expression in patients with severe COPD and asymptomatic smokers.Thorax 64: 26-32.
- Bruno A, Pace E, Chanez P, Gras D, Vachier I, et al. (2009) Leptin and leptin receptor expression in asthma.J Allergy ClinImmunol 124: 230-237, 237.
- Chang JA, Curtis JR, Patrick DL, Raghu G (1999) Assessment of health-related quality of life in patients with interstitial lung disease.Chest 116: 1175-1182.
- Yamada N, Katsuura G, Ochi Y, Ebihara K, Kusakabe T, et al. (2011) Impaired CNS leptin action is implicated in depression associated with obesity.Endocrinology 152: 2634-2643.
Tables and Figures at a glance
| Table 1 | Table 2 | Table 3 |
Figures at a glance
 |
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 | Figure 4 |
Relevant Topics
- Android Obesity
- Anti Obesity Medication
- Bariatric Surgery
- Best Ways to Lose Weight
- Body Mass Index (BMI)
- Child Obesity Statistics
- Comorbidities of Obesity
- Diabetes and Obesity
- Diabetic Diet
- Diet
- Etiology of Obesity
- Exogenous Obesity
- Fat Burning Foods
- Gastric By-pass Surgery
- Genetics of Obesity
- Global Obesity Statistics
- Gynoid Obesity
- Junk Food and Childhood Obesity
- Obesity
- Obesity and Cancer
- Obesity and Nutrition
- Obesity and Sleep Apnea
- Obesity Complications
- Obesity in Pregnancy
- Obesity in United States
- Visceral Obesity
- Weight Loss
- Weight Loss Clinics
- Weight Loss Supplements
- Weight Management Programs
Recommended Journals
Article Tools
Article Usage
- Total views: 10726
- [From(publication date):
December-2015 - Jul 05, 2025] - Breakdown by view type
- HTML page views : 9800
- PDF downloads : 926
