Lemierre Syndrome: A Case Report Shows the Radiological Diagnosis of Multi-System Septic Lesions
Received: 04-Jul-2022 / Manuscript No. roa-22-69338 / Editor assigned: 06-Jul-2022 / PreQC No. roa-22-69338 (PQ) / Reviewed: 20-Jul-2022 / QC No. roa-22-69338 / Revised: 22-Jul-2022 / Manuscript No. roa-22-69338 (R) / Published Date: 29-Jul-2022 DOI: 10.4172/2167-7964.1000392
Abstract
Lemierre Disease is a condition characterised by thrombophlebitis lesions following oropharyngeal infection which results in variable systemic sepsis features. This is a case of 31 years old man who’s been admitted after showing signs of upper airway infection and systemic deterioration that have driven a series of radiological imaging to show multiple solid organ septic lesions, abscesses and IJV thrombosis with bilateral pleural effusion. He had a positive blood culture and this case report describes the approach of this diagnosis using the radiological means and sheds light on the concepts of management of such a rare condition.
Keywords: Lemierre syndrome; Thrombophlebitis; Oropharyngitis; Internal jugular thrombosis
Introduction
Lemierre syndrome (LS) is characterised by septic thrombophlebitis of the internal jugular vein after oropharyngitis, with septic embolisation to the lungs or other organs. The syndrome primarily affects young adults and can potentially be fatal. It has an incidence of 3.6 million people per year, with mortality of around 5%, when diagnosed, therefore it’s known within the medical fields as the forgotten disease because of its rarity [1]. The premise of management is based on antibiotics which would be tailored according to the causative organism, but rarely surgical intervention may be needed.
Case Report
In this case report, we describe a healthy 31 yrs old male admitted on 25th of October 2021 after having a week history of sore throat and intermittent fever followed by left sided chest pain, nausea and poor appetite with element of asthenia, neck pain and progressive dyspnea in response to moderate force. Since the previous day he reported that he had performed his routine daily physical activities with no complaints prior to this occurrence.
His general state of health was generally showing systemic deterioration. On physical examination he had tachypnea with a respiratory rate of 30 breaths per minute. Oxygen saturation of 80% in room air, tachycardia at 112 beats per minute and he had signs of clinical dehydration and perspiration. On chest auscultation, there was notable diffuse reduction of vesicular breathing but cardiac sounds were rhythmic and normal sounding and there were no murmurs. His calves were free from swelling on abdomen and pelvis he had tender upper abdomen but no signs of peritonitis.
Laboratory tests of samples taken on 25th Oct 2021 revealed leukocytosis at 20.2 x10^9/L with neutrophilia of 17.7, while C-reactive protein (CRP) was elevated at 200 mg/L with platelet count of 122 x10^9/L, AKI stage 1 and ALT of 20.
The first blood culture sample was positive showing both Gemella morbillorum, Fusobacterium necrophorum, HIV, Mycobacterium and tonsil swabs were negative.
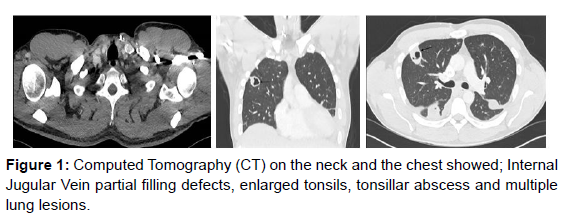
Initially, we commenced antibiotics for pneumonia as a provisional diagnosis but marked deterioration with hypoxia prompted CT neck and thorax. This revealed that on the neck (Figure1), the tonsils, particularly the left one, were enlarged and the upper airways is narrowed, but remains patent. Also, there was a 1.6 cm diameter left tonsillar abscess and partial thrombosis of the left internal jugular vein. On the chest (Figure1), there were two large cavitating lung lesions on the right side with a 3.4cm lesion in the anterior right upper lobe and another 4 cm cavitating lesion in the right lower lobe posteriorly. Furthermore, there was bilateral pleural effusions and gross cardiomegaly, cardiothoracic ratio 68%, but no pericardial effusions (Figure1).
These extensive findings and the abdominal examination have prompted an abdominal CT scan to explore the possibility of further features of systemic sepsis. CT abdomen and pelvis showed (Figure 1), Hepatosplenomegaly (spleen measures up to 16 cm). There were also two hypo- dense, ill-defined lesions in the right liver lobe. The larger of the two lesions was underneath the right hemidiaphragm in liver segment 8 and measures 5.4 cm. The other remaining solid organs were unremarkable.
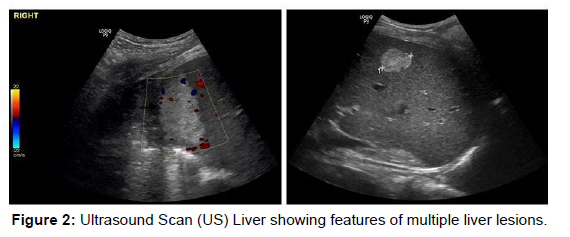
A liver ultrasound scan was arranged to specify the location and the nature of the lesions and explore the possibility of radiological interventions contributing to the management. The scan showed (Figure 2): three well defined hyper echogenic identifiable lesions the largest measures 5.6 cm in diameter high at the dome of the liver. The second more inferiorly in the right lobe measures 2.7 cm and a third tiny hyper-echoic lesion measuring 1.2 cm. These lesions showed no fluid component and were not suitable for drainage (Figure 2).
The diagnosis of Lemierre syndrome was made promoting bilateral chest drains that were indicated for the progressive bilateral pleural effusion and a long course of intravenous antibiotics were started based on the sensitivity. Liver lesions were monitored with repeated imaging.
Throughout his clinical course, the patient remained in a standard ward and he was medicated throughout his stay with analgesics, nonsteroidal, anti-inflammatories and antibiotics including a combination of alternating penicillin, ceftriaxone and metronidazole.
Over the period of his hospital stay the patient’s laboratory parameters have improved to the point that, on Nov 11th, he had leukocytes count of 6.7 and CRP had fallen to 4.0 mg/L. Relief from pain was achieved the patient no longer exhibited dyspnea and his pulse oximetry reading was 95% in room air.
The microbiology advice was at least 4-6 weeks of IV antibiotics. The patient was suitable for discharge home with antibiotics to be continued by the outpatient antimicrobial treatment system. He was to continue on oral anticoagulation for 3 months for the partial Internal Jugular Vein thrombosis.
Discussion
In 1936 André Lemierre, described this syndrome as a combined anaerobic and septic bacterial infections preceding tonsillar involvement [2]. Lemierre’s presentation was predicated on systemic septicemia caused by Fusobacterium necrophorum describing a progression form of suppurative tonsillar and peritonsillar infection through thrombophlebitis of the internal jugular vein, to septic emboli to distant sites such as the lungs. The most common site of infection accounting for more than 85% is the palatine tonsils. Upper respiratory tract infections such as mastoiditis, parotitis, sinusitis, otitis and infections of the skin or subcutaneous tissues can also be the primary site of infection.
Clinical manifestations would include fever from 39 to 41ºC from 4 to 5 days after the onset of pharyngitis [3]. Oedema and pain parallel to the sternocleidomastoid muscle indicate involvement of the para-pharyngeal space. Pain and stiffness of the neck and cervical lymphadenopathy may also occur. Respiratory symptoms are present in the majority of cases. But, atypical presentations can also occur. Haematogenous propagation of bacteria accounts for pulmonary involvement in up to 97% of cases of the syndrome where signs of pleuritic pain and pleural rub can be present. A chest X-ray typically shows opacities and potentially pleural effusions.
Early diagnosis is vital to prevent sepsis and death; but it is very often delayed because of the indolent course and because the syndrome is not well-known. Definitive diagnosis can be made with CT scan, Echocardiography, or duplex scanning of the cervical region. The most useful of these for diagnosis is CT with contrast, which will reveal soft tissues swelling and filling defects or even the thrombus itself in the internal jugular vein lumen.
The first line of treatment for LS is intravenous antimicrobial therapy [4], with coverage for anaerobic microbes. Response to antibiotics is variable and may take from 8 to 12 days for the fever to resolve.
Rarely Surgical ligature and excision of the internal jugular vein might be indicated in persistent cases of embolism or drainage of abscesses or pulmonary empyema. The role of anticoagulation is yet to have a randomized trial to support its use.
Conclusion
In view of the potential mortality of LS, it is essential to recognise this syndrome as early as possible, primarily after presentations suggestive of pulmonary embolism subsequent to bacteraemia of the upper airways, so that the treatment could be of most effect.
References
- Harper LK, Pflug K, Raggio B, April D, Milburn JM (2016) Clinical Images: Lemierre Syndrome: The Forgotten Disease? Ochsner J 16: 7-9.
- Zhao A, Samannodi M, Tahir M, Bensman S, Hocko M (2017) Lemierre's syndrome: Case report and brief literature review. IDCases 10: 15-17.
- Bahall M, Giddings S, Bahall K (2017) Lemierre's syndrome: forgotten, but not absent. BMJ Case Rep 2017: bcr2017221203.
- Veras RO, Barasuol LL, de Lira CP, Klostermann FC, Müller LS, et al. (2018) Lemierre syndrome: case report. J Vasc Bras 17: 337-340.
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Citation: Khater T, Ramadan O, Abdelhamid AT (2022) Lemierre Syndrome: A Case Report Shows the Radiological Diagnosis of Multi-System Septic Lesions. OMICS J Radiol 11: 392. DOI: 10.4172/2167-7964.1000392
Copyright: © 2022 Khater T, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Open Access Journals
Article Tools
Article Usage
- Total views: 2653
- [From(publication date): 0-2022 - Mar 29, 2025]
- Breakdown by view type
- HTML page views: 2225
- PDF downloads: 428