Leiomyomatosis Peritonealis Disseminate Following Laparoscopic Myomectomy with Power Morcellation
Received: 02-Dec-2017 / Accepted Date: 07-Dec-2017 / Published Date: 11-Dec-2017 DOI: 10.4172/2161-0681.1000329
Abstract
Background: Leiomyomatosis peritonealis disseminate (LPD) is a rare benign entity, but its histological origin remains unclear.
Cases: An unusual DPL case which was diagnosed at 4 years after her initial laparoscopic uterine myomectomy with power morcellation, and recurred 4 years later. The findings during the exploratory laparotomy mimicked peritoneal carcinomatosis, which was characteristic of innumerous nodules throughout the peritoneal surface ranging from several millimeters to cenitimeters in diameters. She was treated with omentectomy and debulking of numerous large tumors. Gonadotropin releasing hormone analogue was given for 6 months after her initial laparotomy. Histopathological examination confirmed a consistent morphology of cellular leiomyoma in the uterine and LPD tumors.
Conclusion: Our case suggested an emerging origin of LPD, iatrogenic implantation following laparoscopic procedures with power morcellation.
Teaching points: LPD following laparoscopic myomectomy with power morcellation can mimic carcinomatosis clinically. Particular caution should be exerted on power morcellation when the leiomyoma had an unusual appearance.
Keywords: Myomectomy; Morcellation; Omentectomy; Debulking
Introduction
Leiomyomatosis peritonealis disseminate (LPD) is defined as “a rare, benign, proliferative lesion, forming multiple nodules of smooth muscle within the peritoneal cavity” by the recent WHO tumor classifications of the female genital tract. Some LPD cases mimic peritoneal carcinomatosis by radiologists and surgeons due to the presence of numerous nodules throughout the peritoneal cavity [1,2]. The etiology and pathophysiology is not well elucidated, but it is proposed that LPD is originated from the differentiation of submesothelial, multi-potential mesenchymal cells [3]. In the past two or three decades, the wide application of laparoscopic uterine myomectomy and hysterectomy with power morcellation has posed a serious problem of iatrogenic implants of a benign leiomyoma, thereof, it is suggested that some LPD may be secondary to the morcellationassociated implants of the uterine leiomyoma rather than de novo peritoneal metaplasia [4]. In this study, we reported an unusual recurrent LPD case mimicking carcinomatosis grossly after laparoscopic myomectomy with power morcellation.
Case Report
The 24-years old woman, G0P0, presented with a pelvic mass for more than two months. She admitted to our hospital in November, 2009. Preoperative B-ultrasonography revealed that a mass in the posterior uterine wall measured 8.4 × 7.3 × 9.0 cm3. Her clinical and familial history is unremarkable. She underwent laparoscopic myomectomy with power morcellation in our hospital. The intraoperative findings included a leiomyomatous mass of 9.0 × 9.0 × 8.0 cm3 in the uterine fundus, which had an indistinct boundary with the surrounding myometrium, a yellow cut surface and a soft texture. The final histopathological diagnosis was cellular leiomyoma with a mitotic figure of 0-1/10HPFs. She recovered quickly from her surgery.
The patient remained well thereafter until she incidentally found a mass in her upper abdomen in October, 2013. At that time, Ultrasonography showed multiple uterine leiomyomas, bilateral ovarian hypoechoic masses and multiple pelvic nodules. Magnetic resonance image (MRI) demonstrated that there were numerous nodules throughout the abdomen and pelvis with abdominal hydrops. The nodules involved the bilateral adnexa, uterus, mesentery, uterovesical pouch, and Douglas pouch. An initial impression of peritoneal carcinomatosis was proposed by the radiologist. However, serum levels of CEA and CA199 were within the normal range while serum CA125 was slightly elevated (81.0 u/mL).
Exploratory laparotomy found that innumerous tumor implants were throughout the abdomen and pelvis, resembling to peritoneal carcinomatosis. Specifically, the uterine serosa was full of hundreds of nodules with a diameter ranging from 0.2 cm to 2.0 cm. The left and right ovary harbored 3 tumors (0.5-2.0 cm in the diameters) and one (4.0 cm), respectively. There were innumerous tumors in the omentum, intestinal mesentery, descending colon, sigmoid colon, rectum, the right lateral pelvis, the left posterior peritoneum, the abdominal excision, uterovesical pouch, and Douglas pouch. The diameters of the tumors varied from a diameter of a few millimeters (miliary seeds) to 6 cm. The tumors distributed at the surface of the pelvic and abdominal organs except that the tumors in the descending and sigmoid colon had a pushing growth towards the outer myometrium proper. Partial omentectomy and debulking of large tumors (>1 cm in the diameter) were performed to preserve her fertility. More than 30 tumors were removed from the peritoneal and pelvic cavity. The miliary tumorlets remained untreated. The pathological diagnosis this time was leiomyomatosis peritonealis disseminate (LPD), cellular variant with low mitotic figures (0-1/10HPF). After her second surgery, the patient was treated by gonadotropin releasing hormone analogues (GnRH-A) for 6 months.
The patient had the excision of the mass in the abdominal wall in June 2016 at a local hospital. The pathological diagnosis was “leiomyoma”. She received 4 cycles of artificial reproduction at the beginning of 2017 in our hospital, but failed to conceive a baby. A uterine mass was found by B-ultrasonography during this interval. It gradually enlarged in the next two months. The patient had to undergo another abdominal surgery in June 2017. The exploratory laparotomy found an enlarged uterus with two leiomyomas (6.0 and 0.5 in the diameters). The omentum contained numerous tumors which ranged 0.1-0.5 cm in diameters. Numerous nodules with varying sizes were also present on the surface peritoneum of the pelvis, uterovesical peritoneal reflection, Douglas pouch, right recessus hepatorenalis and intestinal mesentery. The removal of large tumors (>60) in the pelvis and peritoneum, uterine myomectomy, and omentectomy were carried out. The final pathological diagnosis was LPD.
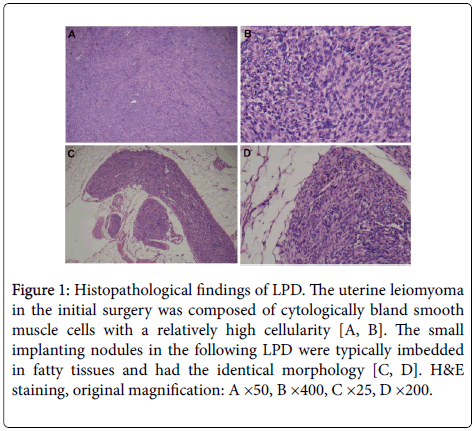
Histopathological examination showed that the uterine leiomyomas and LPD tumors from her surgeries had the identical morphology. The tumors were composed of predominant bland smooth muscle cells with a relatively high cellularity (Figures 1A and 1B). Mitotic figures were uncommon throughout the tumors (<1/10 HPFs). There was no atypia and tumor cell necrosis in these tumors. The small implanting nodules were imbedded in fatty tissues (Figures 1C and 1D) or attached on the surface of organs. Most nodules were well circumscribed and some had an infiltrative board. Müllerian glands were present in a few disseminated tumors in the Douglas pouch and ovary. Immunohistochemical staining showed that the tumor cells were mostly Desmin +ve, smooth muscle actin +ve, estrogen receptor +ve, progesterone receptor +ve, CD34 -ve, CD117 -ve and focal CD10 +ve (<10%). The Ki67 index was very low (<1%).
Figure 1: Histopathological findings of LPD. The uterine leiomyoma in the initial surgery was composed of cytologically bland smooth muscle cells with a relatively high cellularity (A, B). The small implanting nodules in the following LPD were typically imbedded in fatty tissues and had the identical morphology (C, D). H&E staining, original magnification: A ×50, B ×400, C ×25, D ×200.
Discussion
LPD, also known as disseminated peritoneal leiomyomatosis (DPL), is a rare, benign clinical condition with a good prognosis. Approximately 100 cases have been reported in the English literature since it was first described by Wilson and Peale in 1952. LPD mostly affects young women of reproductive age although it can occur in postmenopausal women and in male patients [5]. It was characterized by the random distribution of multiple fibromuscular nodules on the peritoneal surfaces of the pelvic and abdominal cavities. In classical LPD cases with numerous tumors, most of these nodules measured less than 1 centimeter or several millimeters (tumorlet) in the diameter, thereof, mimics peritoneal carcinomatosis during operation. In fact, malignant tumors are frequently suspected by preoperative imaging procedures, such as abdominal ultrasound, CT or MRI [1,2].
The pathogenesis of LPD remains to be elucidated at present. A traditional hypothesis is that LPD might be originated from metaplasia of submesothelial, multi-potential mesenchymal cells [3]. A few cases with concurrent LPD and endometriosis favor the common origin of both entities [3]. A metaplastic origin from the pelvic peritoneum (the secondary Müllerian system) has been proposed for occasional endomteriosis cases. Some evidence emerges to indicate that the initiation and progression of LPD may be associated with hormonal factors, most specifically with high levels of estrogen [3]. In fact, estrogen and progesterone receptors can be demonstrated in tumor cells by immunohistochemical methods. In line with the potential effects of hormonal stimulus, LPD can regress or remains stable after deprivation of such stimulus, for example, after delivery, or abandoning administration of exogenous estrogens and contraceptive pills. Regression of LPD can occur in cases treated by a GnRH agonist whereas the peritoneal nodules may enlarge again when GnRH agonist is discontinued or the patient becomes pregnant. Radical excision of the peritoneal nodules is generally not required for most LPD cases. However, malignant transformation or concurrent leiomyosarcoma in LPD was occasionally reported in the literature [6]. Therefore, sufficient sampling and careful assessment by the pathologists are prerequisite for correct diagnosis under such rare conditions.
In the past several years, the application of power morcellation during laparoscopic uterine myomectomy and hysterectomy has critically posed the concern for potential peritoneal seeding and dissemination of uterine benign and malignant tumor tissue. The “iatrogenic ” peritoneal leiomyomas caused by fragmentation implantation from morcellation have encountered not infrequently by now although the true incidence remains unknown at present. Some cases also provided the potential link between dissemination of morcellated tumor pieces and the development of LPD [4]. LPD is an exceedingly rare, but increasing event following laparoscopic myomectomy, which unequivocally represents for another origin of LPD: iatrogenic implantation. Our patient generated a clinical impression of carcinomatosis due to the presence of innumerous minute nodules throughout the peritoneal and pelvic cavity. This classical pattern is distinct from the iatrogenic LPD cases reported previously, most of which harbored a limited number of tumor implants with a relatively large size [4,7]. We believe that the uterine cellular leiomyoma has a soft texture which can be easily morcellated into droplet-like tiny tissue pieces. These pieces can readily disseminate throughout the peritoneum and result in the appearance of carcinomatosis. As a collar, the use of a morecellator should be particularly meticulous when leiomyomatous nodules had an unusual appearance, such as the oft texture in our case. The tumors had better be morcellated in a contained manner to prevent tumor seeding when morcellation is the only choice. The administration of GnRH-A is an alternative treatment for some common LPD patients [8]. Apparently, our patient did not benefit much from this treatment due to her unusual tumor relapse. The therapeutic effects of endocrine therapy in DPL following morcellation are encouraged to be reassessed in the future when more cases with long follow-up are accumulated. We think that the development of novel endocrine therapy is especially important considering that fertility preservation is a permanent requirement for young women as in the current report. Unlike those with potential peritoneal origin, such iatrogenic DPL should be treated with sufficient surgery as indicated by our case and those reported previously [4,7].
Our case should be discriminated from low grade leiomyosarcoma or stromal sarcoma since the persistent, widely disseminated disease. Careful pathological examination with extensive sampling did not show any evidence of malignancy, such as tumor coagulative necrosis, significant cytological atypia and active mitotic figures. These features opposed the diagnosis of low grade leiomyosarcoma. Lack of vascular invasion, no significant components of small arterioles, involvement of the peritoneal surface, strong expression for smooth muscle markers and very limited CD10 expression did not support the diagnosis of low grade endometrial stromal sarcoma. Above all, the prolonged clinical history (109 months from her first surgery and 49 months from her second surgery) without extra-abdominal spread suggests an indolent clinical course, which is very unlikely for a low grade sarcoma, but is a typical feature for LPD [7].
In conclusion, we here presented an unusual case of carcinomatosis like LPD following laparoscopic uterine myomectomy with power morcellation. The patient had a prolonged clinical history but a favorable prognosis at present although she did not benefit much from the treatment of GnRH-A. Taken together, we believe that a subset of DPL cases are originated from mocellation associated implantation rather than the common metaplastic origin. Such kind of DPL should be emphasized due to the potentially increasing incidence as a result of the wide application of power morcellation in laparoscopic procedures in the recent two or three decades. Great effort should be taken to develop reliable methods for the prevention and intervention on this rare entity.
Source(s) of the Work
The authors’ own work.
Disclosure of Potential Conflicts of Interests
None declared.
Disclosure of Funding
Not applicable.
References
- Talebian Yazdi A, De Smet K, Antic M, Ilsen B (2010) Leiomyomatosis peritonealis disseminata in a 50-year-old woman: imaging findings. JBR-BTR 93: 193-195.
- Papadatos D, Taourel P, Bret PM (1996) CT of leiomyomatosis peritonealis disseminata mimicking peritoneal carcinomatosis. AJR Am J Roentgenol 167: 475-476.
- Kuo T, London SN, Dinh TV (1980) Endometriosis occurring in leiomyomatosis peritonealis disseminata: ultrastructural study and histogenetic consideration. Am J Surg Pathol 4: 197-204.
- Nguyen D, Maheshwary R, Tran C, Rudkin S, Treaster L (2017) Diffuse peritoneal leiomyomatosis status post laparoscopic hysterectomy with power morcellation: A case report with review of literature. Gynecol Oncol Rep 19: 59-61.
- Tavassoli FA, Norris HJ (1982) Peritoneal leiomyomatosis (leiomyomatosis peritonealis disseminata): a clinicopathologic study of 20 cases with ultrastructural observations. Int J Gynecol Pathol 1: 59-74.
- Raspagliesi F, Quattrone P, Grosso G, Cobellis L, Di Re E (1996) Malignant degeneration in leiomyomatosis peritonealis disseminate. Gynecol Oncol 61: 272-274.
- Wang KL, Guo RX, Yuan ZF, Li AJ, Li LX (2017) Clinical analysis of leiomyomatosis peritonealis disseminate after laparoscopic uterine myomectomy in ten cases. Zhonghua Fu Chan Ke Za Zhi 52: 533-538.
- Hales HA, Peterson CM, Jones KP, Quinn JD (2017) Leiomyomatosis peritonealis disseminata treated with a gonadotropin- releasing hormone agonist. Am J Obstet Gynecol 167: 515-516.
Citation: Sun Y, Lan X, Shi H, Wu X, Lu B (2017) Leiomyomatosis Peritonealis Disseminate Following Laparoscopic Myomectomy with Power Morcellation. J Clin Exp Pathol 7: 329. DOI: 10.4172/2161-0681.1000329
Copyright: ©2017 Sun Y, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 4076
- [From(publication date): 0-2017 - Nov 23, 2024]
- Breakdown by view type
- HTML page views: 3416
- PDF downloads: 660