Research Article Open Access
Left Ventricular Non-Compaction: Mid-myocardial Distribution of Late Gadolinium Enhancement in Compacted Segments
Szemraj-Rogucka Z* and Majos A
Department of Radiology and Diagnostic Imaging, Central Clinical Hospital Medical, University of Lodz, Lodz, Poland
- *Corresponding Author:
- Zofia Szemraj-Rogucka
Department of Radiology and Diagnostic Imaging
Central Clinical Hospital Medical
University of Lodz
Lodz, Poland
Tel: 48512074680
E-mail: szemraj.zofia@gmail.com
Received Date: January 07, 2017; Accepted Date: January 07, 2017; Published Date: January 25, 2017
Citation: Szemraj-Rogucka Z, Majos A (2017) Left Ventricular Non-Compaction: Mid-myocardial Distribution of Late Gadolinium Enhancement in Compacted Segments. OMICS J Radiol 6:246. doi: 10.4172/2167-7964.1000246
Copyright: © 2017 Szemraj-Rogucka Z, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Radiology
Abstract
Background: Cardiovascular Magnetic Resonance (CMR) with Late Gadolinium Enhancement (LGE) is a proven method for detecting myocardial fibrosis. Previous CMR studies described the distribution of LGE in patients with LVNC; however, it still remains unclear. The purpose of the study was to describe the distribution of LGE in patients meeting cardiovascular magnetic resonance criteria for Left Ventricular Non-Compaction (LVNC). Methods: We retrospectively enrolled 15 patients adult patients (11 males and 4 females; mean age, 42 ± 13 years) considered to meet standard CMR criteria for LVNC. For each patient, cine and contrast-enhanced CMR images were analyzed to evaluate LV systolic function and the prevalence and ex-tent of LGE. The presence or absence of LGE was qualitatively determined for each left ventricular myocardial segment. Results: The mean NC/C ratio was 4.6 ± 0.9. The areas of non-compaction were most commonly observed at the apex, the anterior and the lateral walls, mainly on their apical and mid-cavity segments. LGE was present in 11 of the 15 patients (73%). LGE was observed most frequently in the ventricular septum commonly on basal and mid-cavity segments. The distribution of LGE was midmyocardial (n=42; 67%), subepicardial (n=11; 18%), transmural (n=6; 10%) and subendocardial (n=3; 5%), in total of 62 LGE (+) left ventricular segments. No association was found between LGE and non-compaction at left ventricular segmental level (phi coefficient 0,021; p= 0.191). Conclusion: LGE was most often observed in the ventricular septum with mid-myocardial distribution. Distribution of LGE in patients with LVNC is observed in both non-compacted and compacted segments with prevalence of compacted zones. This maintenance the concept that LVNC is a diffuse process including both non-compacted and morphologically normal segments.
Keywords
Left Ventricular Non-Compaction; Late Gadolinium Enhancement
Abbreviations
LVNC: Left Ventricular Non-Compaction; CMR: Cardiovascular Magnetic Resonance; LV: Left Ventricular; LGE: Late Gadolinium Enhancement; NC/C: Non-Compacted-to-Compacted; EDV: End-Diastolic Volume; ESV: End-Systolic Volume; LVEF: Left Ventricular Ejection Fraction; NYHA: New York Heart Association; VT: Ventricular Tachycardia; VF: Ventricular Fibrillation
Introduction
Left Ventricular Non-Compaction (LVNC) is an uncommon cardiac abnormality characterized by multiple prominent ventricular trabeculations and deep intratrabecular recesses [1,2]. It can occur as an isolated disorder or in combination with other congenital cardiac diseases or neuromuscular conditions [3-5]. Left ventricular non-compaction has a broad spectrum of clinical manifestations that range from asymptomatic status, progressive dysfunction, arrhythmias and heart failure that have been found to be associated with myocardial fibrosis [6,7]. Cardiovascular Magnetic Resonance (CMR) with Late Gadolinium Enhancement (LGE) is a proven method for detecting myocardial fibrosis [8]. Previous cardiac magnetic resonance imaging studies described the distribution of LGE in patients with isolated LVNC [7,9-11] however, it still remains unclear. The purpose of this retrospective study is to describe the distribution of LGE in patients meeting cardiovascular magnetic resonance criteria for LVNC.
Methods
Patient population
The retrospective review covered morphological cine magnetic resonance imaging findings of ventricular non-compaction in 15 patients between 2012 and 2016. The diagnosis of isolated LV was based on the presence of the following cardiac MRI and clinical criteria [12]: (a) Appearance of two distinct myocardial layers; (b) Marked trabeculation and deep intertrabecular recesses within the non-compacted layer; (c) End-diastolic ratio of Non- Compacted-to- Compacted (NC:C) myocardium >2.3:1, and (d) Absence of other associated congenital or acquired heart disease. Resting electrocardiogram (ECG) and 24-h Holter monitoring were performed in each patient prior to the CMR examination. In addition, the New York Heart Association (NYHA) functional class was recorded for each patient. Invasive coronary angiography was performed in selected patients, according to clinical state. Clinical information was obtained from the medical database.
CMR image acquisition and analysis
All CMR exams were performed on Magnetom Avanto 1.5T scanner (Siemens, Erlanger, Germany) using a 32-channel cardiological coil. Retrospective electrocardiographic gated cine images were acquired using true fast imaging with steady-state free precession sequence in three long-axis views (LV two-chamber and four-chamber long-axis, and LV outflow tract) and short-axis views encompassing the entire LV from base to apex. After baseline imaging, an injection of gadolinium at a dose of 0.2 mg/kg (Gadovist, Bayer Health Care) was administered. Ten minutes later, the LGE images were obtained by using an ECG-triggered, segmented inversion-recovery gradient-echo pulse sequence at the three long-axis and standard short-axis views covering the whole LV.
All CMR data were analyzed using Argus post-processing software (Siemens Medical Systems). LV ejection fraction and ventricular volumes were measured on the SAX cine images. The presence or absence of non-compaction and LGE was qualitatively assessed using the AHA 17 segment model [13]. The ratio of non-compacted to compacted (NC/C) myocardium was measured for each involved myocardial segment in diastole, on short axis slices, and the maximum ratio was then used for analysis. As previously demonstrated, the assessment of NC/C ratio of the apex (segment 17) was excluded; non-compaction was defined as a ratio of non-compacted to compacted myocardium >2.3 [12].
The presence of LGE was determined for each LV myocardial segment by reviewing all short and long axis contrast-enhanced images, with a particular focus on images with elevated signal intensity. Patterns of LGE were visually classified as subendocardial, subepicardial, midmyocardial, or transmural (LGE occupying ≥75% of LV wall thickness). The LGE score was then summed.
Statistical analysis
Continuous variables are expressed as the mean ± SD, and nominal variables as numbers and percentages. Differences in continuous variables between the two groups were assessed using the Student’s t-test or the Mann–Whitney U-test. The chi-square test or Fisher exact tests were computed for non-continuous variables. The phi coefficient was computed to assess the association between the presence of non-compaction and LGE at the LV segmental level. A statistical analysis was performed using the SPSS 14.0 (SPSS) statistical package. For all calculations, P<0.05 was considered significant.
Results
Clinical characteristics
The clinical characteristics of the study group are summarized in Table 1. Fifteen patients were considered to meet the criteria for LVNC, as assessed by CMR. The mean age was 42 ± 13 years (range, 25–64 years), and 11 patients (73%) were male. Most of the patients (67%) were seriously symptomatic (NYHA III/IV). Five patients (33%) presented with documented ventricular arrhythmias and one patient (7%) presented with aborted sudden death. Four patients (27%) had signs of systemic emboli. There were no significant differences in sex, body surface area (BSA), thrombo-embolic events and cardiovascular risk factors (smoking, hypertension, and diabetes) in LGE(−) LVNC patients and LGE(+) LVNC patients. All LGE(+) LVNC patients and 50% of LGE(−) LVNC patients showed abnormal ECG. Significant differences were found in the NYHA functional class and age between LGE(−) and LGE(+) LVNC patient groups.
| Total patients (n=15) | LGE (+) (n=11) | LGE (-) (n=4) | P-value | |
|---|---|---|---|---|
| Age, years | 42 ± 13 | 46 ± 12.3 | 30 ± 5.9 | 0.036 |
| Sex, male n (%) | 11/15 (73) | 9/11 (82) | 2/4 (50) | 0.517 |
| Body surface area (m2) | 1.8 ± 0.3 | 1.7 ± 0.2 | 1.8 ± 0.4 | 0.348 |
| NYHA functional class III/IV (%) | 10/15 (67) | 10/11 (91) | 1/4 (25) | 0.033 |
| Thrombo-embolic events, n (%) | 4/15 (27) | 4/11 (36) | 0 | 0.517 |
| Abnormal ECG n (%) | 13/15 (87) | 11/11 (100) | 2/4 (50) | 0.057 |
| VT/VF n (%) | 5/15 (33) | 4/11 (36) | 1/4 (25) | 1 |
| Smoker n (%) | 3 (20) | 1 (9) | 2/4 (50) | 0.154 |
| Diabetes n (%) | 1 (7) | 1 (9) | 0 | 1 |
| Hypertension n (%) | 1 (7) | 1 (9) | 0 | 1 |
| Data are expressed as mean ± SD, and n (%). VT/VF: Ventricular Tachycardia/Ventricular Fibrillation; NYHA: New York Heart Association. | ||||
Table 1: Clinical characteristics.
CMR imaging findings
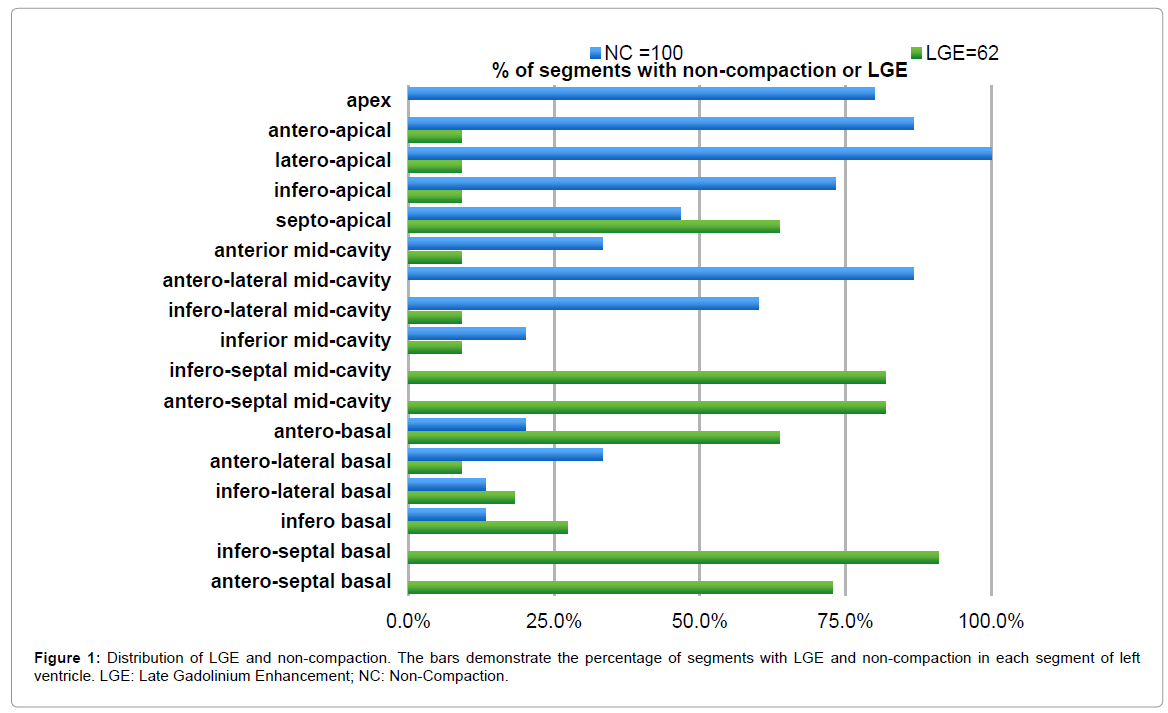
The CMR characteristics of patients with LVNC are listed in Table 2. The mean LVEF and EDV were 33 ± 18.4% and 202 ± 131.7 ml, respectively. Non-compaction was present in 372 LV segments. Among the segments analyzed in our study, 100 (39%) were considered to fulfill the definition of non-compaction. The mean number of non-compacted segments per patient was 6.6 ± 1.6 and the mean NC/C ratio was 4.6 ± 0.9. The areas of non-compaction were most commonly observed at the apex, the anterior and the lateral walls, mainly on their apical and mid-cavity segments (Figure 1).
| Total patients (n=15) | LGE (+) (n=11) | LGE (-) (n=4) | P-value | |
|---|---|---|---|---|
| NC segments per patient | 6.6 ± 1.6 | 6.8± 1.8 | 6.25 ± 0.8 | 0.581 |
| NC/C ratio | 4.64 ± 0.9 | 4.76 ± 1.2 | 4.60 ± 0.8 | 0.644 |
| LV end-diastolic diameter (mm) | 65.8 ± 9.2 | 69 ± 8.7 | 57 ± 1.8 | 0.002 |
| LVEDV (ml) | 281.6 ± 132 | 314.3 ± 139.8 | 192 ± 23.5 | 0.177 |
| LVESV (ml) | 202 ± 131.7 | 242.5 ± 130.5 | 90.2 ± 34.4 | 0.026 |
| LVEF (%) | 33 ± 18.4 | 25.4 ± 13.8 | 53.5 ± 12.9 | 0.006 |
| Data are expressed as mean ± SD, and n (%). NC/C: Non-Compacted/Compacted Ratio; LV: Left Ventricular; EDV: End-Diastolic Volume; ESV: End-Systolic Volume; EF: Ejection Fraction; LGE: Late Gadolinium Enhancement. | ||||
Table 2: Cardiovascular magnetic resonance characteristics.
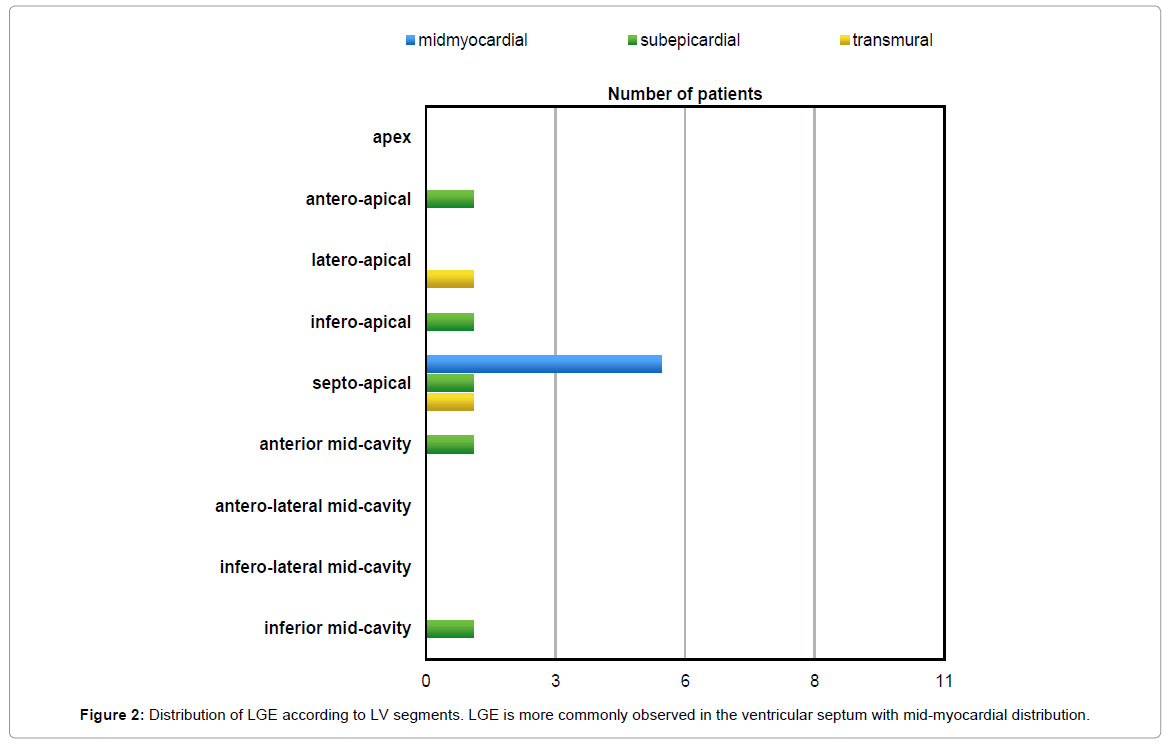
LGE was present in 11 of the 15 patients (73%). In total, sixty-two (24%) LV segments presented LGE, most (84%) of which were located in the compacted segments. LGE was observed most frequently in the ventricular septum commonly on basal and mid-cavity segments (Figure 1). The distribution of LGE was midmyocardial (n=42; 67%), subepicardial (n=11; 18%), transmural (n=6; 10%) and subendocardial (n=3; 5%), in total of 62 LGE (+) left ventricular segments (Figure 2). What is significant, no association was found between late gadolinium enhancement and non-compaction at left ventricular segmental level (phi coefficient 0,021; p=0.191).
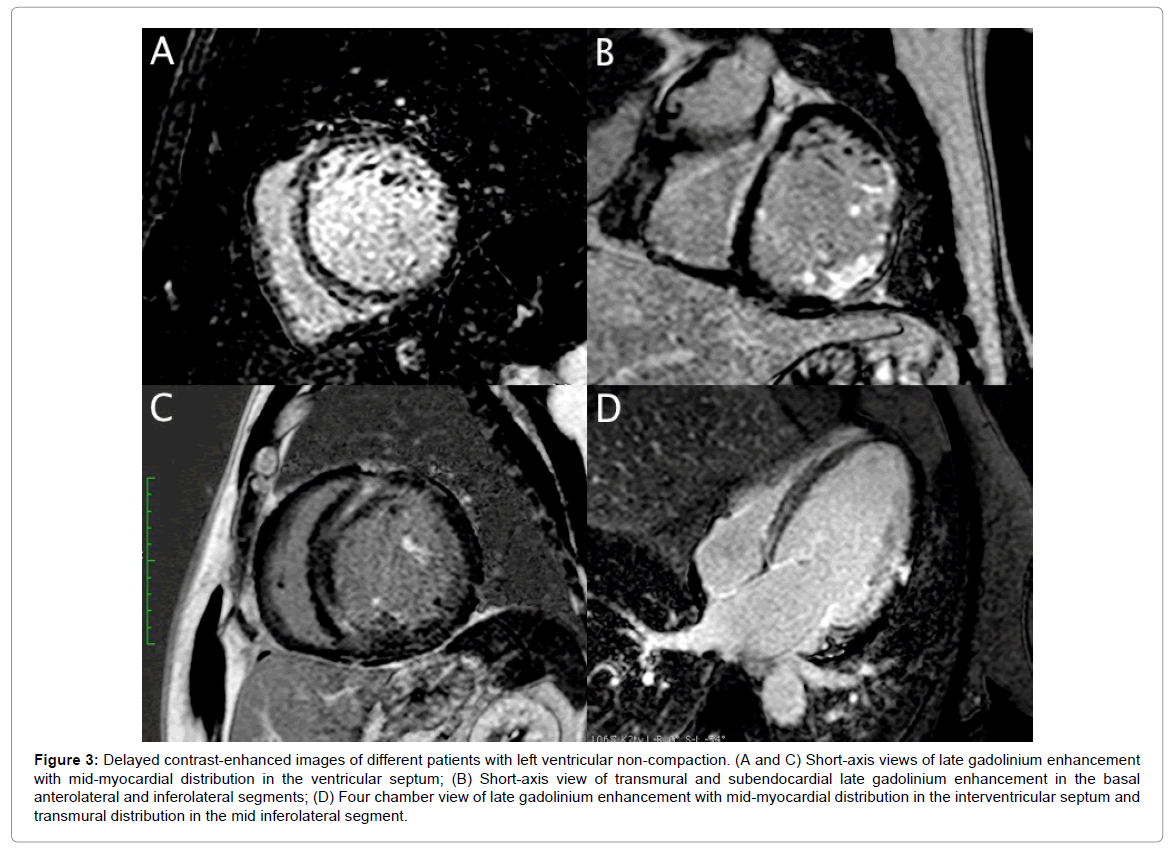
Compared to LGE- patients, LGE+ patients had significantly lower LVEF (25.4 ± 13.8% vs. 53.5 ± 12.9%, p=0.006) and greater LV end-diastolic diameter (69 ± 8.7 vs. 57 ± 1.8 mm, p=0.002) and LVESV (242.5 ± 130.5 vs. 90.2 ± 34.4 ml, p=0.026). There were no significant differences in terms of the number of non-compacted segments and NC/C ratio between LGE+ and LGE- patients. Figure 3 shows examples of contrast-enhanced CMR images of patients with LVNC and different patterns of LGE distribution.
Figure 3: Delayed contrast-enhanced images of different patients with left ventricular non-compaction. (A and C) Short-axis views of late gadolinium enhancement with mid-myocardial distribution in the ventricular septum; (B) Short-axis view of transmural and subendocardial late gadolinium enhancement in the basal anterolateral and inferolateral segments; (D) Four chamber view of late gadolinium enhancement with mid-myocardial distribution in the interventricular septum and transmural distribution in the mid inferolateral segment.
Discussion
This study investigated the distribution of LGE in patients with LVNC and its relation to disease severity.
In agreement with previous studies, we observed that non-compacted segments are most commonly located at the apex, the anterior and the lateral walls and that the septal segments are rarely affected by LVNC [6,11,12,14]. This may be explained by the normal compaction process that goes from base to apex, from epicardium to endocardium, and from the septal to the lateral wall. Different degrees of arrest in this normal embryologic process may explain the typical pattern of distribution of non-compacted segments [12,15].
In our study, LGE was a common finding, present in 11 of the 15 patients (73%). It was observed in both non-compacted and compacted segments with prevalence of compacted zones. This confirms the concept that LVNC is a diffuse process which includes both non-compacted and morphologically normal segments [11,16]. The pathophysiologic mechanisms leading to myocardial fibrosis in patients with LVNC remains unclear.
In our study, CMR imaging revealed subendocardial and transmural distribution of LGE in non-compacted segments and mainly midmyocardial distribution in compacted segments. These data are in agreement with previous CMR studies [6,7,11,14] and myocardial tissue samples [17,18] which confirmed the heterogeneous distribution of myocardial fibrosis in patients with LVNC.
Subendocardial and transmural distribution of LGE in non-compacted segments might be explained by the defects in the coronary microcirculation that have been reported in LVNC patients, which suggests that the vascularisation of the abnormal myocardium is affected [19]. Several pathological examinations revealed necrotic myocytes in the non-compacted area [20,21], and decreased myocardial perfusion was reported in the non-compacted area [22-24]. Thus, defects in the coronary microcirculation might be responsible for ischemic lesions and myocardial fibrosis, despite no evidence of a previous myocardial infarction. In our study, four of the 15 patients underwent invasive coronary angiography, according to the clinical judgment at the time of diagnosis. Only one patient had coronary artery lesions causing a greater than 50% narrowing with no history of myocardial infarction.
As mentioned above LGE was observed most commonly in compacted segments, in the ventricular septum, with midmyocardial distribution. Our findings are consistent with previous LVNC studies [6,7,11] but to the best of our knowledge this is the first study that emphasize midmyocardial distribution of LGE in compacted segments. The mechanisms for midwall fibrosis in non-ischemic cardiomyopathies are related to different processes. They are thought to be the result of a combination of factors such as genetic predisposition, microvascular ischemia, and abnormal modulation of the immune system, permanent adrenergic activation and metabolic dysregulation [25-27]. Furthermore, increased wall stress due to LV enlargement can lead to microvascular ischemia with subsequent myocyte necrosis. Jenni et al. [19] have demonstrated a diminution in coronary flow reserve in both non-compacted and compacted segments of myocardium in LVNC. Maladaptive processes related to increased wall stress caused by progressive LV remodeling may result in focal myocyte necrosis, preferentially affecting the middle, circumferential myocardial layer [28]. LVNC is genetically heterogeneous, presenting in familial and sporadic forms. Different mutations in sarcomere protein genes were identified and there seems to be a shared molecular etiology of different cardiomyopathy phenotypes, including LVNC, hypertrophic and dilated cardiomyopathies [29]. The underlying pathological mechanisms for this familial predisposition to fibrosis may be explained by the fact that a number of defective genes implicated in familial DCM have also been found to code for cytoskeletal proteins, and this could set up a chronic injury–repair scenario resulting in fibrosis [26].
The study also evaluated the relationship between the prevalence of myocardial fibrosis, clinical characteristics and other CMR findings. In agreement with previous studies, we observed that the presence of LGE is related to adverse clinical outcome and reduced ventricular function [6,7,14]. In our study adverse clinical consequences were expressed by NYHA III/IV, abnormal ECG, and ventricular arrhythmias. The results will need to be confirmed by further studies with a larger sample size. What is interesting, our CMR imaging showed LGE in one patient with normal LV systolic function. This data is consistent with Nucifora et al. findings who also observed small amounts of myocardial fibrosis in asymptomatic patients and in patients with preserved LVEF. The authors suggested that cardiac injury in isolated LVNC may begin much earlier than the onset of symptoms and LV systolic dysfunction, and implies a role of LGE as a marker of subclinical disease [6].
Study Limitations
The present study has some limitations. First, it was a retrospective study, with a small sample size because of the relatively rare entity and its single-center nature. Moreover, coronary artery disease was ruled out by invasive coronary angiography only in selected patients, according to clinical indications. Furthermore, we did not undertake family screening. Finally, there were no clinical follow-up data and the relationship between LGE and prognostic information cannot be determined. To sum up, further studies of larger populations, with several CMR examinations and long-term follow-ups, are needed.
Conclusion
LGE was most often observed in the ventricular septum with midmyocardial distribution. Distribution of LGE in patients with LVNC is observed in both non-compacted and compacted segments with prevalence of compacted zones. This maintenance the concept that LVNC is a diffuse process including both non-compacted and morphologically normal segments. The potential clinical usefulness of LGE in patients with LVNC needs to be confirmed by further studies with a larger sample size.
Ethical Approval and Consent to Participate
I confirm that Medical University of Lodz Ethics Committee has approved the study and informed consent was obtained from the patients with LVNC.
Consent for Publication
I confirm that formal written consent to publish all participants’ data was obtained from individuals involved in the study.
Competing Interests
The authors declare that they have no competing interests.
Funding
The Medical University of Lodz, Poland supported this study; research task No. 503/5-087-01/503-51-003.
Authors’ Contribution
ZSZR conceived, designed the study and drafted the manuscript. AM reviewed and edited the manuscript. ZSZR and AM carried out images analyses. ZSZR was responsible for cases selected and analysis. All authors read and approved the final manuscript.
Acknowledgement
We would like to thank Åukasz Moliszewski from Information Systems for his help in extracting data from the clinical CMR database.
Authors’ Information
ZSZR and AM are joint corresponding authors.
References
- Chin TK, Perloff JK, Williams RG, Jue K, Mohrmann R (1990) Isolated noncompaction of left ventricular myocardium: a study of eight cases. Circulation 82: 507-513.
- Towbin JA, Lorts A, Jefferies JL (2015) Left ventricular non-compaction cardiomyopathy. The Lancet 386: 813-825.
- Bagur RH, Lederlin M, Montaudon M, Latrabe V, Corneloup O, et al. (2008) Ebstein anomaly associated with left ventricular noncompaction. Circulation 118: e662-e664.
- Ichida F, Tsubata S, Bowles KR, Haneda N, Uese K, et al. (2001) Novel gene mutations in patients with left ventricular noncompaction or Barth syndrome. Circulation 103: 1256-1263.
- Ichida F, Hamamichi Y, Miyawaki T, Ono Y, Kamiya T, et al. (1999) Clinical features of isolated noncompaction of the ventricular myocardium: long-term clinical course, hemodynamic properties, and genetic background. J Am Coll Cardiol 34: 233-240.
- Nucifora G, Aquaro GD, Pingitore A, Masci PG, Lombardi M (2011) Myocardial fibrosis in isolated left ventricular non-compaction and its relation to disease severity. Eur J Heart Fail 13: 170-176.
- Wan J, Zhao S, Cheng H, Lu M, Jiang S, et al. (2013) Varied distributions of late gadolinium enhancement found among patients meeting cardiovascular magnetic resonance criteria for isolated left ventricular non-compaction. J Cardiovasc Magn Reson 15: 20.
- Mahrholdt H, Wagner A, Judd RM, Sechtem U, Kim RJ (2005) Delayed enhancement cardiovascular magnetic resonance assessment of non-ischaemic cardiomyopathies. Eur Heart J 26: 1461-1474.
- Liu X, Kino A, Francois C, Tuite D, Dill K, et al. (2008) Cardiac magnetic resonance imaging findings in a patient with noncompaction of ventricular myocardium. Clin Imaging 32: 223-226.
- Eitel I, Fuernau G, Walther C, Razek V, Kivelitz D, et al. (2008) Delayed enhancement magnetic resonance imaging in isolated noncompaction of ventricular myocardium. Clin Res Cardiol 97: 277-279.
- Dursun M, Agayev A, Nisli K, Ertugrul T, Onur I, et al. (2010) MR imaging features of ventricular noncompaction: emphasis on distribution and pattern of fibrosis. Eur J Radiol 74: 147-151.
- Petersen SE, Selvanayagam JB, Wiesmann F, Robson MD, Francis JM, et al. (2005) Left ventricular non-compaction: insights from cardiovascular magnetic resonance imaging. J Am Coll Cardiol 46: 101-105.
- Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, et al. (2002) Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart: a statement for healthcare professionals from the cardiac imaging committee of the council on clinical cardiology of the American heart association. Circulation 105: 539-542.
- Lachhab A, Doghmi N, Elfakir Y, Taoussi O, Benyass A, et al. (2012) Insights from magnetic resonance imaging of left ventricular non-compaction in adults of North African descent. Int Arch Med 5: 10.
- Sedmera D, Pexieder T, Vuillemin M, Thompson RP, Anderson RH (2000) Developmental patterning of the myocardium. Anat Rec 258: 319-337.
- Bellavia D, Michelena HI, Martinez M, Pellikka PA, Bruce CJ, et al. (2010) Speckle myocardial imaging modalities for early detection of myocadial impairment in isolated left ventricular non- compaction. Heart 96: 440-447.
- Pujadas S, Bordes R, Bayes-Genis A (2005) Ventricular non-compaction cardiomyopathy: CMR and pathology findings. Heart 91: 582.
- Ivan D, Flamm SD, Abrams J, Kindo M, Heck K, et al. (2005) Isolated ventricular non-compaction in adults with idiopathic cardiomyopathy: cardiac magnetic resonance and pathologic characterization of the anomaly. J Heart Lung Transplant 24: 781-786.
- Jenni R, Wyss CA, Oechslin EN, Kaufmann PA (2002) Isolated ventricular noncompaction is associated with coronary microcirculatory dysfunction. J Am Coll Cardiol 39: 450-454.
- Ritter M, Oechslin E, Sutsh G, Attenhofer C, Schneider J, et al. (1997) Isolated noncompaction of the myocardium in adults. Mayo Clin Proc 72: 26-31.
- Finsterer J, Stollberger C, Feichtinger H (2002) Histological appearance of left ventricular hypertrabeculation/noncompaction. Cardiology 98: 162-164.
- Junga G, Kneifel S, von Smekal A, Steinert H, Bauersfeld U (1999) Myocardial ischemia in children with isolated ventricular non- compaction. Eur Heart J 20: 910-916.
- Sato Y, Matsumoto N, Matsuo S, Kunimasa T, Yoda S, et al. (2007) Myocardial perfusion abnormality and necrosis in a patient with isolated noncompaction of the ventricular myocardium: evaluation by myocardial perfusion SPECT and magnetic resonance imaging. Int J Cardiol 120: e24-e26.
- Hamamichi Y, Ichida F, Hashimoto I, Uese KHKI, Miyawaki T, et al. (2001) Isolated noncompaction of the ventricular myocardium: ultrafast computed tomography and magnetic resonance imaging. Int J Cardiovasc Imaging 17: 305-314.
- de Leeuw N, Ruiter DJ, Balk AH, de Jonge N, Melchers WJ, et al. (2001) Histopathologic findings in explanted heart tissue from patients with end-stage idiopathic dilated cardiomyopathy. Transpl Int 14: 299-306.
- Ferrari P, Bianchi G (2000) The genomics of cardiovascular disorders: therapeutic implications. Drugs 59: 1025-1042.
- Ryoke T, Gu Y, Mao L, Hongo M, Clark RG, et al. (1999) Progressive cardiac dysfunction and fibrosis in the cardiomyopathic hamster and effects of growth hormone and angiotensin-converting enzyme inhibition. Circulation 100: 1734-1743.
- Choi EY, Choi BW, Kim SA, Rhee SJ, Shim CY, et al. (2009) Patterns of late gadolinium enhancement are associated with ventricular stiffness in patients with advanced non-ischaemic dilated cardiomyopathy. Eur J Heart Fail 11: 573-580.
- Xing Y, Ichida F, Matsuoka T, Isobe T, Ikemoto Y, et al. (2006) Genetic analysis in patients with left ventricular noncompaction and evidence for genetic heterogeneity. Mol Genet Metab 88: 71-77.
Relevant Topics
- Abdominal Radiology
- AI in Radiology
- Breast Imaging
- Cardiovascular Radiology
- Chest Radiology
- Clinical Radiology
- CT Imaging
- Diagnostic Radiology
- Emergency Radiology
- Fluoroscopy Radiology
- General Radiology
- Genitourinary Radiology
- Interventional Radiology Techniques
- Mammography
- Minimal Invasive surgery
- Musculoskeletal Radiology
- Neuroradiology
- Neuroradiology Advances
- Oral and Maxillofacial Radiology
- Radiography
- Radiology Imaging
- Surgical Radiology
- Tele Radiology
- Therapeutic Radiology
Recommended Journals
Article Tools
Article Usage
- Total views: 4657
- [From(publication date):
February-2017 - Jul 15, 2025] - Breakdown by view type
- HTML page views : 3768
- PDF downloads : 889