Case Report Open Access
Leber��?s (plus?) Hereditary Optic Neuropathy: A Case Report
Arife CA1*, Cansu S2 and Ufuk E1
1Department of Neurology, Istanbul Education and Research Hospital, Istanbul, Turkey
2Istanbul Education and Research Hospital, Istanbul, Turkey
- *Corresponding Author:
- Arife CA
Department of Neurology
Istanbul Education and Research Hospital
Istanbul, Turkey
Tel: 905338141817
E-mail: cimenatalar@yahoo.com.tr
Received Date: November 16, 2016; Accepted Date: December 3, 2016; Published Date: December 10, 2016
Citation: Arife CA, Cansu S, Ufuk E (2016) Leber’s (Plus?) Hereditary Optic Neuropathy: A Case Report. J Pediatr Neurol Disord 2: 109. doi: 10.4172/2572-5203.1000109
Copyright: © 2016 Arife CA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Pediatric Neurological Disorders
Abstract
Leber's hereditary optic neuropathy (LHON)-plus is a maternally inherited genetic disorder of young males and characterized by severe progressive vision loss with other neurological and systemic symptoms. Here we present a young male with subacute progressive vision loss and Parkinsonism symptoms like right arm rigidity and endocrine abnormalities like hypoparathyroidism as a probable LHON-plus case.
Introduction
Leber’s hereditary optic neuropathy (LHON) is an important cause of progressive painless visual loss among young male patients [1]. It is characterized by bilateral subacute loss of central vision owing to focal degeneration of the retinal ganglion cell layer and optic nerve [2]. Recently new case reports of subacute visual failure and additional prominent neurological features like dystonia, myoclonic jerks, ataxia, multiple sclerosis like symptoms are described and defined as LHONPLUS cases [3,4]. Here we present a case with subacute bilateral vision loss accompanied by different neurological examination and cranial magnetic resonance imagining (MRI) findings as a probable LHONplus case.
Case Report
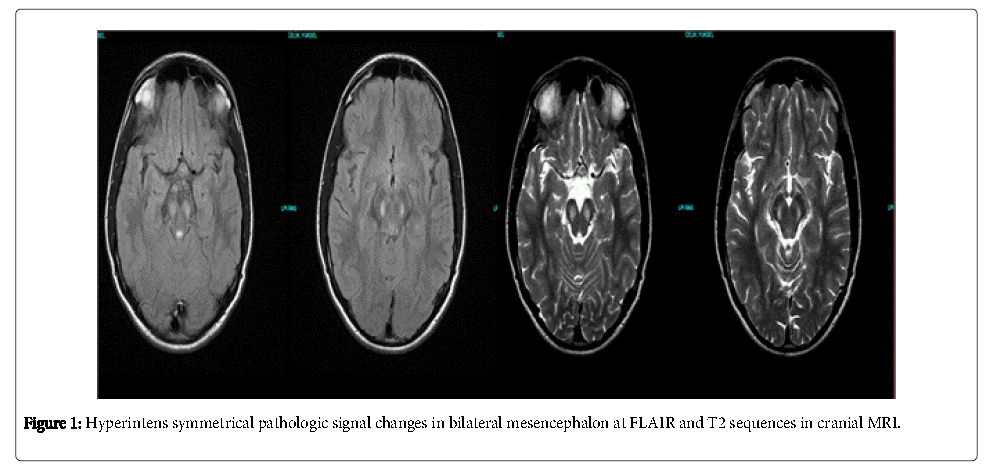
17 years old male patient applied to an outpatient opthalmology clinic with bilateral progressive painless blurred vision starting one month ago. There was bilateral optic disc pallor in fundoscopy and his visual accurate was diminished to counting fingers from one meter. The patient was diagnosed as optic neuropathy and referred to our clinic by opthalmologist for neurological assessment. His personal background was unremarkable. His uncle had a history of bilateral vision loss at childhood. His general medical examination was normal. There was apathy, bilateral subacute progressive visual loss and rigidity at his right arm in his neurological examination. There was bilateral optic atropy, the pupils were reactive to light and there was an afferent pupillary defect. The orbital MRI results were normal. Ä°n the cranial MRI there were bilateral symmetrical hyperintens lesions in T2 and FLAIR and hypointens lesions without contrast enchancement in T1 sequances at mesencephalon (Figure 1). Ä°n visual evoked potantials (VEP) there were bilateral loss of P100 responses. The organic aminoacid levels for detecting mitocondrial cytopathies in urine were in normal ranges. Laboratory results of parathormone was 3.2 pikogram (pg)/millileter(ml) (normal ranges are 10-65 pg/ml) and 25– hidroxyl vitamin D: 7.8 ng/ml (normal ranges: >30 ng/ml). Ä°n cerebrospinal fluid (CSF) examination the protein levels were slightly elevated, the IgG index, lactic acid and piruvic acid levels were significantly high. The CSF lactat/piruvate ratio was normal. Serum copper level (70 microgram/desyleter), ceruloplasmin level (20.8 mg/dl) and 24 hour-urine copper levels (3.0 micrograms per day) were performed for differential diagnosis of Wilson disease and all results were normal. The electroencephalograpy (EEG) examination showed generalized bioelectric disorganisation. Maculary optic coherans tomography and fundus angiography results were normal. We planned genetic testing for mtDNA analysis for confirmation but the patient declined.
As a result the presence of subacute progressive bilateral optic neuropathy with left arm rigidity, apathy, positive family history, the MRI findings, and exclusion of other reasons of optic neuropathy lead us to LHON-plus diagnosis.
Discussion
LHON is a maternally inherited disorder characterized by acute or subacute visual loss leading to a central scotoma [5]. Ä°t is the most common cause of blindness in young males [6]. Over 95% of LHON cases are primarily the result of one of three mitochondrial DNA point mutations [7]. First in 1988 at the 1178/ND4, then in the following years the 14484/ND6 and 3460/ND1 mitochondrial DNA mutations were defined [2,3]. These three mutations involve genes encoding complex I subunits of the respiratory chain [2]. Ä°n 5-10% of the cases different types of mutations were defined [4]. Central visual loss is the only symptom of the disease in majority of the LHON cases, but recently new cases are defined in which optic neuropathy is with different neurological symptoms like dystonia, parkinsonism, cerebellar ataxia and myoclonus [2-4]. These cases are called LHONplus. Among these abnormalities multiple sclerosis (MS) like symptoms, spinal cord disease, brainstem and basal ganglia involvement, Charcot-Marie-Tooth and skeleton abnormalities, progressive hearing neuropathy can be mentioned [8]. Ä°n Nikoskelainen et al. study 46 LHON patients were included and 59% had different types of additional neurologic symptoms like tremor, parkinsonism, dystonia, epilepsia, migraine, dementia, polyneuropathy, MS like disease, brainstem involvement, toracal kifosis and pes cavus [8]. Funalot, et al. reported a three patient series of LHON symptoms accompanied by a Leigh-like encephalopathy [9]. All these patients had symmetrical bilateral brainstem lesions like LHON in their cranial MRI and these lesions significantly regressed at the follow-up [10]. Also different cases with bilateral vision loss and MS like lesions in the white matter (LHON-MS) and ‘dystonic syndrome with pediatric onset ‘with striatal necrosis sites in cranial MRI where 14459 mtDNA mutation is positive, are described. None of these cases had brainstem lesions [8,11]. Other neurological symptoms accompany to LHON are summarized at Table 1.
| Age/Gender | Neurologic Symptoms | MRI findings | Mutation | |
|---|---|---|---|---|
| Funakawa I,1995 | Mother and son | Cerebellar ataxia, dysarthria |
Cerebellar atrophy |
Mitochondrial DNA 11778 mutation |
| Murakami T,1996 |
Cerebellar ataxia |
Cerebellar atrophy |
Mitochondrial DNA 11778 mutation |
|
| Shoffner JM | Dystonia | Bilateral basal ganglia lesions | ND6 subunit komplex mitochondrial DNA LDYT144459A point mutation |
|
| Parry-Jones 2008 | 32/female | Periventricular white matter spinal cord | Mitochondrial DNA,T14484C mutation |
|
| B ceranic, 2004 | 45/female, 59/male | Hearing loss | Mitochondrial DNA 11778 mutation | |
| W kuker, 2007 | 36/female | Spastic paraparesis Hypoestesia | Bilateral periventricular white matter involvement | G3460A Mutation |
| W leuzzi, 1992 | 20/female 11/male | Dystonia hypokinesia, bradykinesia myoclonic jerks hypokinesia bradykinesia | bilateral putaminal right n.caudatus head involvement bilateral putaminal involvement | mitochondrial DNA 11778 mutation |
Table 1: Neurological symptoms accompany to LHON.
We finally considered the patient as LHON-plus with progressive bilateral vision loss, right arm rigidity and apathy in neurological examination and bilateral, symmetrical pathological signal changes at mesencephalon in cranial MRI .We searched the literature and could not find any other case report with similar MRI findings. The patient had an uncle with the same vision loss history but we could not perform genetic study to other family members. Our patient also had primary hypo-parathyroidisim which supports the mitochondrial disease diagnosis. There are some case reports of mitochondrial disease in literature where hypo-parathyroidism is presented as the initial symptom [12,13].
As a result in a male patient with subacute progressive optic neuropathy the presence of other neurological symptoms can suggest a LHON-plus phenotype. Clinicians must be aware of these ‘plus' phenotypes to ensure early diagnosis, and patients in this high-risk group are best served by a multidisciplinary team minimize the functional consequences of these systemic complications.
References
- Teive HAG, Troiano AR, Raskin S, Werneck LC (2004) Leber's hereditary optic neuropathy--case report and literature review. Sao Paulo Med J 4: 122-276.
- Man YMP, Turnbull DM, Chinnery PF (2002) Leber hereditary optic neuropathy. J Med Genet 39: 162-169.
- Sadun AA, Morgia C, Carelli V (2011) Leber’s Hereditary Optic Neuropathy. Current Treatment Options in Neurology 13: 109-17.
- Nakaso K, Adachi Y, Fusayasu E, Doi K, Imamura K, et al. (2012) Lebers Hereditary Optic Neuropathy with Olivocerebellar Degeneration due to G11778A and T3394C Mutations in the Mitochondrial DNA. J Clin Neurol 8: 230-234.
- Man PYW, Turnbull DM, Chinnery PF (2002) Leber hereditary optic neuropathy. J Med Genet 39: 162-169.
- Jeong-Min H (2000) A Family with Leber’s Hereditary Optic Neuropathy with Mitochondrial 11778/ND4 and 4216/ND1 Mutations. Korean J Opthalmol 14: 45-48.
- Schieving JH, Vries BB, Hol F, Stroink H (2008) [Sudden blindness: consider Leber's hereditary optic neuropathy]. Ned Tijdschr Geneeskd 152: 2313-2316.
- Nikoskelainen EK, Marttila RJ, Huoponen K, Juvonen V (1995) Leber: neurological abnormalities in patients with Leber‘s hereditary optic neuropathy. J Neurol Neurosurg Psychiatry 59: 160-164.
- Benoit F, Pascal R, Alain V, Daniele R, Jean-Paul B, et al. (2002) Leigh-like Encephalopathy Complicating Leber’s Hereditary Optic Neuropathy. Ann Neurol 52: 374-377.
- Saracchi E, Difrancesco JC, Brighina L, Marzorati L, Curtò NA, et al. (2013) A case of Leber hereditary optic neuropathy plus dystonia caused by G14459A mitochondrial mutation. Neurol Sci 34: 407-408.
- Abu-Amero KK, Bosley TM, Bohlega S, McLean D (2005) Complex I respiratory defect in LHON plus dystonia with no mitochondrial DNA mutation. Br J Ophthalmol 89: 1380-1381.
- Carelli V, Ross-Cisneros FN, Sadun AA (2004) Mitochondrial dysfunction as a cause of optic neuropathies. Prog Retin Eye Res 23: 53-89.
- Ashrafzadeh F, Ghaemi N, Akhondian J, Beiraghi Toosi M, Elmi S (2013) Hypoparathyroidism as the first manifestation of kearns-sayre syndrome: a case report. Iran J Child Neurol 7: 53-57.
Relevant Topics
- Behavioral Psychology
- Chiari malformation
- Chronic Traumatic Encephalopathy
- Congenital Brain Defects
- Duchenne Muscular Dystrophy
- Epilepsy and Seizures
- Genetic and Metabolic Disorders
- Genetic Epilepsies
- Headaches and Migraines
- Movement Disorders
- Neonatal encephalopathy
- Neurodevelopmental Disorders
- Neurogenetic Disorders
- Neurological Complications of AIDS
- Neuromuscular Disease
- Pediatric Brain Tumour
- Pediatric Sleep Disorders
- Stroke and Perinatal Injuries
Recommended Journals
Article Tools
Article Usage
- Total views: 14012
- [From(publication date):
December-2016 - Apr 01, 2025] - Breakdown by view type
- HTML page views : 13002
- PDF downloads : 1010