| Ana Domingos* | |
| Laboratory of Molecular Genetics, Rockefeller University, 1230 York Avenue, New York, NY 10065, USA | |
| Corresponding Author : | Ana Domingos, Ph.D Laboratory of Molecular Genetics Rockefeller University, 1230 York Avenue 305 New York, NY 10065, USA E-mail: dominan@rockefeller.edu |
| Received March 25, 2013; Accepted April 22, 2013; Published April 24, 2013 | |
| Citation:Domingos A (2013) Lean on Leptin or Lean for Sugar. J Obes Wt Loss Ther 3:166. doi:10.4172/2165-7904.1000166 | |
| Copyright: © 2013 Domingos A. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Journal of Obesity & Weight Loss Therapy
Dieting has a significant impact on the reward value of food. New pre-clinical studies using optogenetics show that leptin regulates the reward value of sugar, suggesting that leptin reconstitution during weight loss may curb the reward value of sugar.
| Keywords |
| Sugar; Food choice; Food Reward; Leptin; Obesity; Weight loss; Optogenetics; Neuroeconomics |
| Introduction |
| Obesity is an example of phenotypic diversity and exists since preneolithic times, but it is not until recently that it became a global public health concern. The epidemic nature of obesity is well documented, and equally affects societies across contrasting levels of socioeconomic development [1]. According to the most recent data of the World Health Organization, Saudia Arabia has the highest prevalence of obesity (35.2%), followed by Egypt (34.6%), South Africa (33.5%), Mexico (32.8%), United States of America (31.8%), and Venezuela (30.8%) [1]. The link between obesity and maladaptive eating behavior is well accepted, and most therapies for obesity involve voluntary modifications of eating behavior. Some of those modifications involve not only eating less, as well as forceful decisions about what foods to eat. Many clinical and preclinical studies have addressed the quantitative aspect of eating behavior, ie, mechanisms underlying the decision of how much to eat. Such studies have identified hormones, neuropeptides and neural circuits that, directly or indirectly, control hunger ratings or the volume of daily food intake [2-4]. However, very few studies have addressed the qualitative aspect of eating behavior, i.e. mechanisms underlying the decision of what to eat. People eat what they like, but why are certain foods more liked than others? This is a fundamental question that still has no clear answer. Humans and animals generally like sugar. In fact, both humans and mice prefer to consume sugar to artificial sweeteners [5-7]. Such preference owes to a post-ingestive rewarding effect that sugar has, but artificial sweeteners do not [5,8,9]. Sugar intake quickly increases blood glucose, which leads to dopaminergic activation independently of taste perception, the so-called post-ingestive rewarding effect [8-11]. Artificial sweeteners don’t raise blood glucose, lack a post-ingestive rewarding effect, and are thus less preferred. The post-ingestive rewarding effect of sugar does not depend on calorie content, as isocaloric bolus of the I-amino acid l-serine, which is also sweet, does not evoke a post-ingestive rewarding effect [8-11]. The mechanisms by which sugar induces a post-ingestive rewarding effect are still unknown. The hypothesis that a nutrient sensing neural circuit conveys the post-ingestive rewarding effect of sugar, and its reward value still awaits testing. Here we review studies probing the role of leptin on the reward value of food. |
| Weight Loss Versus Food Reward |
| Dieting is widely prescribed as a therapy for obese individuals, but long-term compliance is difficult, partly as a result of the high reward value of highly nutritive food items such as sugars. Clinical studies in dieting subjects have shown that caloric restriction (dieting) has a significant impact on food hedonics – the pleasure derived from eating food [12-14]. Colloquially, weight loss leads one to like even more the foods that ought not to be eaten. The biological mechanisms underlying this altered behavior were only recently experimentally addressed in animal models, and brought new insights into the role of leptin in eating behavior [5]. Leptin is an adipose tissue hormone that functions as an afferent signal in a negative feedback loop that maintains homeostatic control of adipose tissue mass [2-4]. Serum leptin levels are proportional to the amount of adipose tissue, such that weight loss leads to lower circulating leptin levels [2-4]. Leptin is conserved across most species of vertebrates, and its deficiency results in extreme obesity and insatiable hunger, in humans and animals [2-4]. When leptin deficient patients are asked to rate how much they like food, high ratings are prevalent before leptin treatment [12]. After leptin treatment, liking ratings are significantly lower. Leptin replacement therapy reduces these ratings even before weight loss is achieved [13]. These changes in human behavior correlate with changes in brain activity, particularly, in reward-related areas [14]. Whether leptin regulates the reward value of food in leptin non-deficient obese patients still awaits clinical testing. Notwithstanding, the pre-clinical rodent studies discussed below, point to the idea that leptin reconstitution during weight loss can curb the reward value of sugar [5]. |
| Measuring Food Reward in Humans and Animals |
| In humans, food hedonics is measured with subjective rating scales for liking, such as the Likert ordinal scale, which provides a numeric rating based on an intensity scale. These rating tests are generally used by behavioral economists for determining customer preferences and perception. Assays of liking in animals are limited by the fact that they cannot report their ratings. Current assays of liking in animals use subjective measures of orofacial expressions, similar to those used in human infants [15]. Human and non-human primates have vision as their dominant sensory modality and hence have evolved higher order visual functions that subserve unconscious communication [16,17]. Those higher order visual functions include being able to express and interpret orofacial expressions [16,17]. Rodents, however, are nocturnal animals with exquisite olfactory and auditory function but poor high order vision. Rodent assays based on orofacial expressions predict that the neuromodulator dopamine does not play a role in the hedonic value of sugar [15]. However, in humans, antidopaminergic neuroleptic drugs commonly have anhedonia as a side effect [18,19]. The fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM5) describes anhedonia as a “lack of pleasure” and uses weight loss as one of the criteria of diagnosis [18,19]. Indeed, many individuals diagnosed with anhedonia describe a lack of enjoyment for food [18,19]. In addition, rodent assays based on orofacial expressions have not been reported to be able to distinguish between artificial and natural sweeteners, although mice prefer, like humans, to natural to artificial sweeteners [5-7]. Such preference also depends on dopamine induction, as described above [5,8-12]. Some theories of the valuation of food divide “wanting” and “liking” into separate, but somewhat interconnected dimensions of reward [15]. Measures of “wanting”, can be quantified by progressive ratio (PR) schedules of reinforcement [20]. In a PR task, the subject is required to trigger an increasing number of operant responses for each successive reward. The number of responses triggered to obtain the last reward (‘‘break point”) serves as an index of the willingness to work for food [20]. This operant procedure can be used in humans and animals [14,15,20]. However this approach is contingent upon a subject’s motivational state to work, which limits its utility for assessing the pleasurable value of food. Indeed, studies using this assay have found discrepancies among food intake and subjective and operant measures of food value possibly reflecting differential recruitment of neural circuits that control the “motivation to work”, instead of the “motivation to eat”. In addition, PR assays performed in humans in a laboratory setting cannot predict whether a patient is likely to relapse or comply with a diet [21]. In other words, subjects may have little motivation to work for food but still eat in excess if palatable food is offered for free. |
| Optogenetic Metric for the Reward Value Of Sugar |
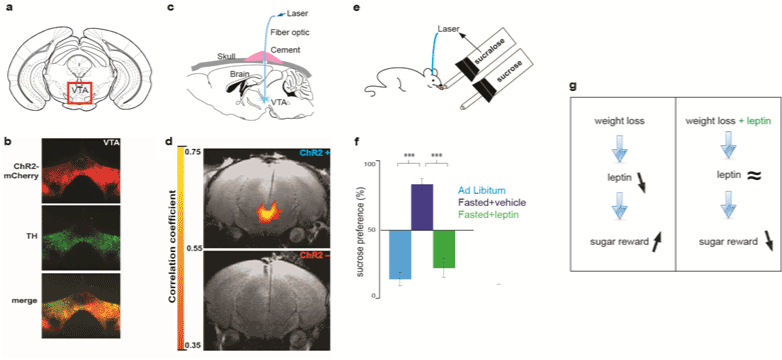
| As an alternative to the existent rodent assays of oro facial expression and PR schedules of reinforcement, we recently used optogenetics to implement a new assay formmeasuring the reward value of sugar. Optogenetics is a newly developed technology allowing for remote control of neuronal activity using light [22]. It relies on the expression of Chanelrodopsin (ChR2) a light-sensitive cation channel isolated from deep sea algae chlamydomonas reinhardtii [20]. Recombinant ChR2 can be expressed in dopaminergic neurons in the ventral tegmental area (VTA) by genetically modified viral vectors (Figures 1a and 1b) [6,22-24]. Deep brain light stimulation of DA neurons can be achieved by the implantation of an optical fiber that conducts blue light from a laser source (Figures 1a and 1b) [6,23,24]. Light stimulation of DA neurons can be assessed by functional magnetic imaging (Figures 1c and 1d) [6,25,26]. Animals with an implanted optical fiber are placed in a chamber containing contact lickometers that record each lick on sippers dispensing liquid [6]. One of those sippers is coupled to the laser source such that lick detection triggers a laser pulse to activate DA neurons through the optical fiber. This is a self-stimulating configuration, such that licking behavior triggers light stimulation of DA neurons. This setup is configured such that, over a 10-min interval, mice have a choice of two sippers. One of the sippers contains sugar (sucrose) whereas the other sipper contains an artificial sweetener (sucralose) (Figure 1e). Ingestion of sucralose triggers the laser and induces optogenetic stimulation of DA neurons, making sucralose+laser a reference stimulus with a high reward value (Figure 1f, ad libitum). Noteworthy, this effect is not due to optogenetic stimulation of DA neurons per se, as ingestion of sugar is still preferred to water coupled to optogenetic stimulation of DA neurons [6]. A choice behavior indicates the relative value of each option, hence, changes in preference relative to the high value reference stimulus reflect changes in the reward value of sugar [6,26-29]. This new optogenetic assay is based on widely used neuroeconomic approaches to behavior, which can be applied to humans and any animals endowed of decision making behaviors (choosing) [26-29]. Using this assay, we have shown that the natural preference for sucrose over sucralose can be inverted if ingestion of sucralose is supplemented by a proxy post-ingestive rewarding effect in the form of optogenetic activation of DA neurons (Figure 1f, ad libitum) [6,18,19]. This gain of function experiment demonstrates that the lack of preference for artificial sweeteners does not solely derive from sensory transduction in taste bubs. |
| Leptin Regulates the Reward Value of Sugar |
| Using the aforementioned optogenetic assay, we tested the effects of leptin on the reward value of sugar. Animals that have been fasted to lose weight do prefer sucrose over sucralose+laser (Figure 1f). However, if the same fasted animals are treated with leptin, sugar is not as preferred again (Figure 1f). Hence leptin suppresses the reward value of sugar in fasted mice that have lost weight. (Figures 1f and 1g) [6]. The suppression of the reward value of sugar by leptin is accompanied by a suppression of the post-ingestive rewarding effect of sugar [6]. The mechanism by which leptin regulates the reward value of sugar remains to be elucidated. We hypothesize that leptin acts on a nutrient sensing neural circuit in the brain that conveys the reward value of sugar, and its post-ingestive rewarding effect. This neural circuit has yet to be identified, and the aforementioned hypothesis awaits testing. |
| Conclusion |
| These rodent studies show that leptin levels modulate the reward value of food not only in leptin deficient obese humans, but also in leptin non-deficient mice [5,6]. In addition, these studies point to the idea that leptin reconstitution during weight loss can curb the reward value of sugar (Figure 1g). Wether leptin regulates the reward value of food in leptin non-deficient obese patients still awaits clinical testing. |
| Acknowledgements |
| We thank the The Rockefeller University, Picower Foundation, the Klarman Family Foundation for Eating Disorders, the Calouste Gulbenkian Foundation, the Portuguese Foundation for Science and Technology for supporting this work, and Werner Haas for reading and commenting the manuscript |
 |
| Figure 1 |
Make the best use of Scientific Research and information from our 700 + peer reviewed, Open Access Journals