Leaky Gut and Post-Prandial, Redox and Inflammatory Markers in HRT Treated Menopausal Females: Benefits by a Pharma-Grade Fermented Papaya Preparation (FPP-ORI)
Received: 22-Apr-2020 / Accepted Date: 20-May-2020 / Published Date: 27-May-2020 DOI: 10.4172/2167-065X.1000193
Abstract
The aim of the present study was to test an evidence-based functional food (FPP-ORI, Osato Research Institute, Gifu, Japan) on redox and immune system modulatory efficiency together with gut permeability in HRT-treated postmenopause women. The study population consisted of 74 postmenopausal women who were on hormone replacement therapy (HRT) divided in two groups, matched as for age, duration of menopause diagnosis, BMI, dietary intake and physical activity and supplemented as follows: Group A unsupplemented HRT-treated women and group B HRT-treated women supplemented with FPP 4.5 g 2 times a day. Treatments were maintained for 6 months. A further Group C consisted of 25 untreated menopausal women who served as control. A high-fat test meal was given to both groups as breakfast each observation day (entry, at 3rd and 6th months) afterwards. At these timing blood samples were taken for the following tests: erythrocytes redox parameters, oxidized low-density lipoproteins/ β2-glycoprotein I (oxLDL/b2GPI), interleukin 6 (IL6) and lypopolysaccharide (LPS). As compared to group C, group A did not any significant change of MDA and redox parameters concentration. However, in subnot jects with BMI<24, HRT treatment was associated to a significant beneficial variation of MDA but of the other redox parameters (GPx, SOD1 and CAT). FPP supplementation yielded a significant improvement of all redox parameters at 6 months (p<0.01) irrespective of the BMI. Plasma oxLDL/b2GPI in group A was abnormally high (2 h and 4 h values vs. baseline, p<0.05). Supplementation with FPP showed a significant normalization of this parameter (p<0.05 vs. group A after challenge meal). LPS and IL-6 showed a significant time-course increase in group A. However,this phenomena were improved in group B (p<0.01). Taken overall the above evidence-based FPP nutraceutical intervention may provide a valid opportunity within a wider health-promoting and disease-preventing, namely neuro-protective, strategy in menopausal women.
Keywords: HRT; Menopause; Redox dysfunction; FPP-ORI; MDA; Leaky gut; Interleukin-6
Introduction
Menopause is likely to be associated to an impaired redox balance due to a concomitant decrease of antioxidant capacity and enhanced oxidative stress phenomena [1,2]. This redox imbalance is then affecting various target organs at different degree [3] and unsuspectingly unrelated to cardiovascular risk marker, for instance [4]. The decline of circulating estrogen is advocated for as one of the causative factor for such derangement, especially as for cardiovascular health [5] and very recently it has been suggested that a novel membrane-bound receptor, i.e. the G Protein-Coupled Estrogen Receptor, which mediates non-genomic actions of 17β-estradiol, may play a key pathogenetic role [6].
Thus, among the known cardiovascular and, partially, bone health benefits of HRT, an improvement of redox balance has also been attributed to this intervention [7-10]. Nonetheless, this data is not univocal and contradictory reports have appeared as well. Indeed, while some works show that HRT is increasing oxidative stress reduction-related PON1 activity together with a decline of autoantibody titers against oxidized apolipoprotein B in LDL and ox- LDL [10], other data suggest a lack of consistency of HRT affecting these parameters [11]. In particular, HRT would lead to an increase in TAC without an equivalent change in serum levels of oxLDL/β2GPI complexes [11] or the beneficial antioxidant effect of HRT would be nullified by concomitant metabolic disease [12]. Although using as HRT an outdated conjugated equine hormone preparation, an Indian study even showed that this treatment increased DNA oxidation with an increased nitrite level as well as a decreased GSH level as compared with controls [13]. However, also an earlier Italian study randomized to a sequential oral and transdermal estradiol regimen (2 mg oral micronized 17beta-estradiol/day or 1.5 mg 17beta-estradiol gel/day, showed that the levels of 8-epi PGF2alpha, one of the most reliable pathways to assess oxidative stress status in vivo, did not change [14]. Moreover, also an American clinical work added that HRT would not significantly modify the circulating levels of antioxidant vitamins (beta-carotene, vitamin C, vitamin E/triglycerides [15]. These controversial negative data are also somehow reconciling with experimental findings dating back 2014 where estrogen and combined HRT did not substantially differ in opposing LDL oxidation in vitro or systemic oxidative stress in vivo [16], To make this field more intriguing, Pei et al. has been recently shown that a high fat unbalanced meal would alter gut permeability and trigger LPS-related inflammatory changes in premenopausal women [17] and obese menopausal women too [18], This may open a new investigational/ interventional scenario considering that leaky gut, LPS and inflammatory cytokines are all being interconnected one another. This biochemical milieu has been reported to be associated, among others, to neurodegenerative disease by inducing memory impairment as model for Alzheimer’s Disease (AD) [19] but also suggested in the clinical ground [20,21] where some mechanisms have been already tracked down [22], It is not clear however if HRT per se’ could exert the above biochemical protection and whether the addition of functional food would be of any benefit.
Thus, the aim of the present study was to test a functional food (FPP-ORI, Osato Research Institute, Gifu, Japan) endowed with an established validation as an effective redox modulator [23-25], on leaky gut and post-prandial, redox and inflammatory markers in HRTtreated postmenopause women. As a matter of fact, we have very recently shown that in non-HRT treated menopausal women, FPP was significantly better than an antioxidant-rich food supplementation in terms of protection from oxidative stress and mitochondrial dysfunction [26].
Materials and Methods
The FPP was obtained from Carica papaya L. cultivated in selected non-GMO organically-treated Hawaian crops undergoing a 10 months patented yeast fermentation process in Japan with pharmaceuticalgrade batch-to-batch control also by Electron Spin Resonance at the Osato Research Institute (Gifu, Japan). The final composition of FPP per 100 g is as follows: 90.7 mg carbohydrates, 14 μg copper, 6 mg lysine, 13 mg valine, 37 mg glutamic acid, 2.5 mg calcium, 11 mg glycine, 17 μg vitamin B6, 8 mg proline, 27 mg aspartic acid, 5 mg hystidine, 18 mg leucine, 16.9 mg potassium, 11 mg serine, 9 mg tyrosine, 11 mg phenylalanine, 9 mg isoleucine, 8 mg treonine, 5 mg methionine, 16 mg arginine, 4.6 mg magnesium, 240 μg niacin, 75 μg zinc, 2 μg folic acid and 2 mg tryptophan.
Study design and population: 74 post-menopausal (>1 year since last menstruation) non-smoker women (body mass index 21-29 kg/m2) with stable body weight in the month prior to the trial (±1 kg) who were on hormone replacement therapy (HRT) or bioidentical hormone replacement therapy (BHRT) without any steroids, ongoing food supplements, vegan diet or high intake of dietary fiber or fermented food (>400 g/day) were recruited. Major past or ongoing diseases, together with alcohol, drug abuse of use for the past 3 months of prebiotics, probiotics, either alone or as food enrichment or antibiotics, were considered exclusion criteria.
Subjects were divided in two groups (37 each one), matched as for age, duration of menopause diagnosis, BMI, dietary intake and physical activity (kcal/week), while being instructed to refrain from intense physical exercise. As for dietary intake, the National Institutes of Health Diet History Questionnaire was employed to evaluate diet history (food frequency and portion size) over the past month and at each planned observation visit.
Treatment assignments followed a computer-generated randomization sequences. Supplementation was carried out as follows: Group A was left unsupplemented and group B: was supplemented with FPP 4.5 g 2 times a day, given sublingually in the morning and approximately 2 h after lunch. Treatments were maintained for 6 months. A further separate groip (Group C) consisting of 25 HRT/ BHRT-untreated menopausal women (age 51-57) served as control.
Ethics
The study was conducted prospectively and the protocol was compliant with the World Medical Association Declaration of Helsinki (Ethical Principles for Medical Research Involving Human Subjects).All procedures were approved by an independent Ethical Committee for nonpharmacological research (ReGenera Research Group for Aging Intervention, Milan, Italy). All subjects gave written informed consent.
Medical check-ups were performed between 08:00 h and 09:00 h after an overnight fasting. On the examination day, at 1, 3 and 6 months, blood samples were taken for routine testing by automated standardized procedures (Hitachi 911, using commercial kits).
However, further studies as below indicated, were also carried out 2 h and 4 h after a specific test breakfast meal consumed only once at the entry, after 3 and months. All patients followed written instructions with regard to a standardized breakfast formulated to induce a metabolic burden [27] as follows: total calories (960 kcal) were distributed in three fractions of the diet, such as fats (50% of total energy), carbohydrates (38% of total energy), and protein (12% of total energy). All participants were asked to consume the test meal within 20 min. None of the participants reported any gastrointestinal discomfort during or after each meal.
Morning samples were withdrawn at 7:00-8:30 a.m. to avoid potential confounding diurnal variation of oxidative stress biomarkers profile [23]. After an overnight fast, venous blood (10 mL) was obtained from an antecubital vein using a monovette blood collection system, inoculated into either non-anticoagulated tube swhich were left to clot in a clean plain tube for 20-30 min at room temperature, and in sodium EDTA-containing tubes. Serum was obtained from 2 mL of blood without anticoagulant,separated by centrifugation at 3000 rpm for 15 min and analyzed within 3 hours. One mL of anticoagulated blood was used for hematologic analysis. The remaining anticoagulated blood was centrifuged at 1500 rpm for 10 min at +4°C and, after prior mixing with 10 μlof 2% butylated hydroxytoluene (to prevent lipid oxidation during the assay, the plasma and leucocytes layer was then removed and the packed erythrocyte sediments were washed three times in cold isotonic saline (0.9%, v/w) and then hemolyzed with a nine-fold volume of phosphate buffer (50 mM, pH 7.4).After addition of butylhydroxytoluol (4 mL per mL), hemolyzed erythrocyte samples were stored at -30°C for <3 months pending measurement of enzymatic activity.Test cuvette containing 1 ml of buffer and 2 ml of haemolysate served as standard.The hemoglobin (Hb) concentration in the hemolysate was measured by the Drabkin method and adjusted to 10 g/dl with distilled water for the estimation of RBC redox parameters, as below specified.
SOD activity estimation in the RBC. A chloroform-ethanol extract was prepared by adding 0.5 ml hemolysate (containing 10 g Hb/dl) to 3-5 ml of ice-cold water, followed by 1 ml of ethanol then 0.6 ml of chloroform and thoroughly mixed. SOD activity in red blood cell lysate was determined by xanthine and xanthine oxidase (Sigma; St. Louis, MO, USA) to generate superoxide radicals reacting with 2-(4- iodophenyl)-3-(4-nitrophenpl)-5phenyltetrazolium chloride to form a red formazan dye. The SOD-1 activity was then measured by the degree of inhibition of this reaction by adding xanthine oxidase at a concentration as to induce a 0.020% change in absorbance per minute at 550 nm (Randox). The activity of SOD was calculated by applying the following formula: Activity of SOD (IU/gHb) = SOD activity (IU)/ Hemoglobin (gr/dl) x 100.
Measurement of glutathione peroxidase activity
GPx (EC 1.6.4.2) activity was measured as follows. Briefly, 0.02 ml of heparinised blood was treated with 0.1 ml of 5 mM GSH, 0.1 ml of 25 mM NaN3, 0.1 ml of 1.25 mM H2O2, and phosphate buffer (pH 7, 0.05 mM,) in a total volume of 2.5 ml at 37°C for 10 min. The enzyme reaction in the tube, which contains NADPH, reduced glutathione, sodium azide, and glutathione reductase, was triggered by adding hydrogen peroxide. The variation in absorbance at 340 nm was checked by spectrophotometry (Randox Kit). The result was displayed as units per gram of hemoglobin (IU/gHb) = GPx activity (IU)/ Hemoglobin (g/dl). All samples were tested in duplicate.
Catalase activity in the erythrocytes lysate required the assay mixture consisted of 1.0 ml phosphate buffer (0.05 M, pH 7.0), 0.975 ml hydrogen peroxide (0.019 M), 0.025 ml of red blood cell hemolysate (10% w/v) in a total volume of 2.0 ml. The quantification of catalase activitywas calculated over a defined time (0 to 60 sec) at 240 nm by measuringspectrophotometrically the rate of H2O2 utilization with the molar extinction coefficient for H2O2 being 43.6 M-1 cm-1 and finally expressed as k/g Hb, (or mU per gHb).One unit of catalase activity was defined as the rate constant of the first-order reaction. The catalase activity was expressed as U/g Hb.
Lipid peroxidation, was measured as malonildialdehyde (MDA), Reaction mixture contained 500 μL of thiobarbituric acid (TBA) (final concentration 0.67%), 0.5 ml trichloracetic acid (TCA) (final concentration 20%) and 100 μL serum. Incubated at 100°C for 30 min; centrifuge at 12,000 rpm for 5 min. The reaction was stopped by cooling the samples under tap water, the MDA-TBA adduct was extracted in n-butanol for plasma samples and the absorbance of the organic layer was read at 532 nm by comparing the absorption to the standard curve of MDA equivalents generated by acid-catalyzed hydrolysis of 1,1,3,3-tetramethoxypropane with molar extinction coefficient 1.56 x 105 M-1 cm-1C. Each group was repeated in triplicate and expressed as nanomoles of MDA micromoles of MDA equivalents per gram of Hb.
Serum concentrations of IL-6
The cytokine concentration was determined by using linear regression analysis over a pre-built standard curve and tested each time in duplicate. Serum samples (200 ul) was pipetted into wells and then bound by the immobilized antibody. The plates were incubated and washed, and the amount of bound enzyme-labeled detection antibody was measured by adding a stabilized chromogenic substrateto the wells. If antibody-enzyme conjugate is present, the substrate will be oxidized, and the color that develops is proportional to the amount of bound IL-6.The plates were then read at the appropriate wavelength with an enzyme-linked immunosorbent assay (ELISA) reader. Dilutional experiments were also performed to accurately determine cytokine levels.
LPS measurement
Plasma concentration of LPS was determined by a Limulus amebocyte extract using a quantitative kinetic chromogenic assay (Lonza, Walkersville, MD, USA). Samples were diluted 10-fold in pyrogen-free materials in glass tubes to avoid plastic tubes adhesion of endotoxin. Then, they were heated to 70°C for 10 min to inactivate endotoxin neutralizing agents that inhibit the activity of endotoxin in the assay. A 1:200 (vol/vol) metallo-modified polyanionic dispersant, was added to samples to optimize the reaction quality. All samples were tested in duplicate while assuring a <10% as intra-assay coefficient of variation.
Oxidized low-density lipoproteins (oxLDL)/β2-glycoprotein I (β2-GPI)
Plasma oxLDL/b2GPI complexes were measured by a sandwich enzyme-linked immunosorbent using polyclonal antibody against human β2-GPIcoated onto 96-microwell plates. Polyclonal anti-human apoprotein B antibody served to intercept oxLDL/b2GPI complexes for its reactivity with b2GPI. Diluted sample (500 μL) containing 10 μmol/L MgCl2 were initially subjected to incubation for 2 h at room temperature and then at 4°C overnight with polyethylene glycol. Between each step, the wells were washed 3 times with PBS containing 0.05% Tween 20. After washing, biotinylated antibody was added to the microwells andincubated for 30 min, followed by Streptavidinhorseradishperoxidase for 30 min. Standard β2-GPI/ox-LDL complexes were also processed in order to obtain the standard curve. Color was developed with tetramethyl benzidine/H2O2 substrate for 30 min and the reaction was stopped by 2M sulfuric acid. Color was developed with tetramethyl benzidine/H2O2 for 30 min and the reaction stopped with 0.36 N sulphuric acid. Optical density was read at a wavelengthof 450 nm with a microplate reader. The oxLDL/b2GPI complex concentration (expressed in U/mL) was calculated against a reference curve built with serial dilutions of a reference serum. Intraand inter-assay coefficients of variation were all <15%.
Statistical analysis
Data are expressed as mean ± SD: The statistical analysis was performed with post-test (multiple comparison test) or one-way ANOVA test with were used if the data were normally distributed. Analysis of variance (ANOVA) and (Tukey test which was used because of the lack of a Gaussian distribution in the results. To test the differences between the endpoint and baseline values, the Wilcoxon test or the paired t-test were conducted if the data were normally distributed. The frequency of results above the sensitivity limit of the system in all groups was compared by Fisher’s exact test.
Results and Discussion
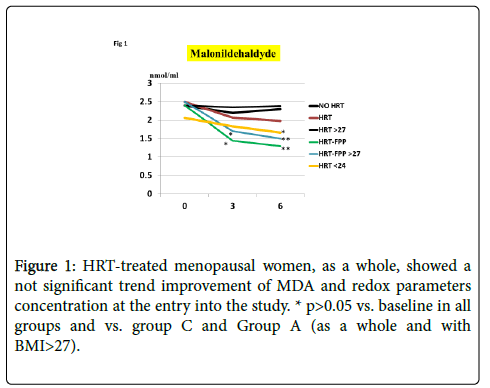
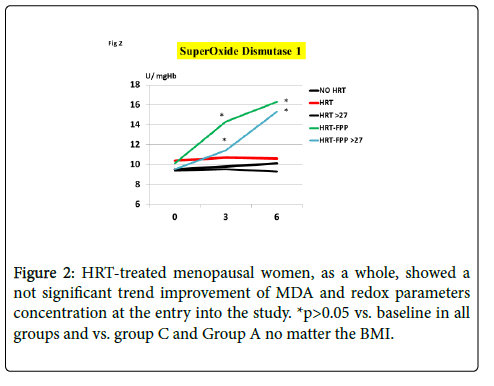
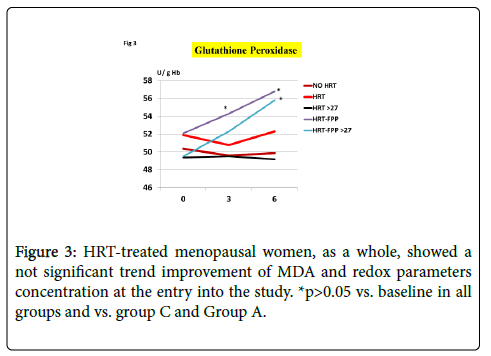
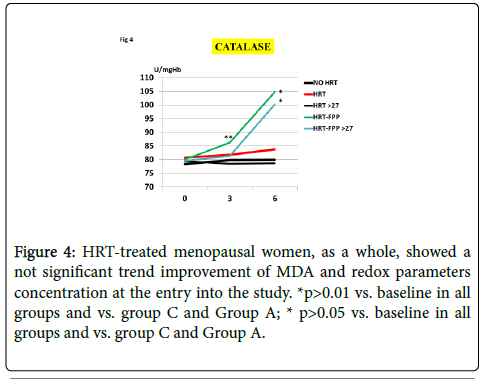
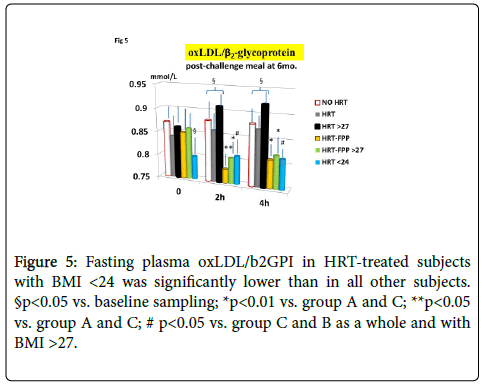
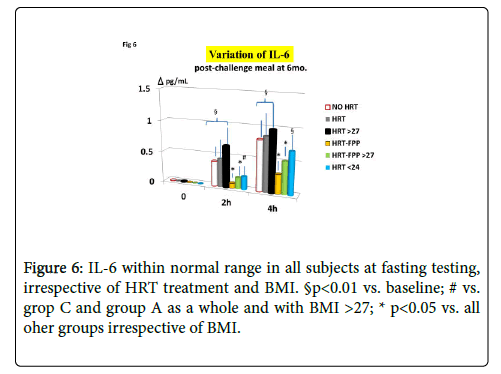
As compared to untreated menopausal control, HRT-treated menopausal women, as a whole, showed a not significant trend improvement of MDA and redox parameters concentration at the entry into the study (p<0.08, Figures 1-4). However, a further analysis, unveiled that in subjects with BMI<24, HRT treatment per sè indeed was associated to a significant beneficial variation (Figure 1, p>0.05 vs. untreated menopause women) but this phenomenon was not observed for the other redox parameters (GPx, SOD1 and CAT, data not shown). On the other hand, HRT-treated women with BMI over 27 showed no change when compared to untreated menopausal women (Figures 1-4). From our prior study [26], it appeared that unsupplemented HRT-treated women showed a significant lower redox parameters as compared to pre-menopausal women and HRT-treated women given routine antioxidants. The present study confirmed similar lower level while FPP supplementation yielded a significant improvement of all redox parameters at 6 months visit (Figures 1-4, p<0.01) irrespective of the BMI and starting already from 3rd month observation for CAT and GPx (p<0.05). Concomitantly, fasting plasma oxLDL/b2GPI in HRT-treated subjects with BMI <24 was significantly lower than in all other subjects (Figure 5, p<0.05). However, this biomarker, except in the subgroup with BMI<24, in the post meal sampling was abnormally high in the unsupplemented groups (2 h and 4 h values vs. baseline, p<0.05) being unaffected by HRT treatment. Supplementation with FPP showed a significant normalization of this parameter (Figure 5, p<0.05 vs. unsupplemented group in all subjects after challenge meal testing). LPS and IL-6 were within normal range in all subjects at fasting testing, irrespective of HRT treatment and BMI (Figures 6 and 7). However, both parameter showed a significant time-course increase in post-prandial testing and at 3- and 6-months observation, respectively (Figures 6 and 7, p<0.01 vs. baseline). However, this phenomenon was partly but significantly mitigated as for IL6 variation and fully prevented as for LPS in FPP-supplemented group either in normal weight and overweight individuals (Figures 6 and 7, p<0.01 vs. unsupplemented group). Interestingly, also HRT-treated women with BMI <24, at 6 h post challenge meal, showed a significant variation increase of IL6 (Figure 6, p<0.05 vs. baseline an vs. FPP-supplemented group as a whole).
Together with other similar literature data (1-3), these findings show that post-menopausal status, even when treated with HRT, is associated to redox system imbalance with decrease of antioxidant enzyme activity and increase of oxidative stress [10,11,14]. Our previous study [26] in HRT-untreated post-menopusal women had also found out that such events did not seem to be related to BMI, family history of late-onset Alzheimer disease. Although our BMI clusters were retrospectively, hence arbitrarily, defined, it seemed that overweight women with BMI over 27, were the one with worst redox status. This is not surprising and till very recently confirmed occurring in pre-menopausal [28] and post-menopausal women [29] as well in adolescent [30]. On the other hand, while normal BMI seemed to protect against increased MDA, irrespective of FPP-ORI supplementation, this healthier group with BMI<24 still maintained a deficient antioxidant anzyme activity along the study period. However we looked at this potential variable, FPP-ORI supplementation proved to be the most significant (positive) modifier of the whole redox status, no matter the BMI. A BMI<24 proved also to partically, but not fully, protect from post-challenge meal IL6 and oxLDL/β2-glycoprotein increase, as typically observed in HRT-treated women (either as a whole and as the subgroup with BMI>27).However, FPP-ORI supplementation yielded the most positive results no matter the BMI, this proving that better body composition and likely healthier life-style may not be enough to counteract the menopausal aging/metabolic changes. Although we lack data, one can indeed envisage that intense physical activity in menopause may require proper antioxidant buffer although a proper active life style has to be promoted [31]. Notwithstanding normal BMI was not a clear-cut parameter associated to the biomarkers we tested, certainly the unsupplemented overweight subgroup showed also the worst IL6, LPS and oxLDL/β2-glycoprotein data. Such higher gut permeability status has been reported in postmenopausal women either overweight [32] or not [33] as well as, very recently, in women in transition menopausal phase [34].This latter inflammatory citokines-LPS cluster has been suggested to be associated to higher risk factor for AD [20-22] with LPS hippocampus concentration up to a 26-fold higher [35], in Parkinson [36] and recently discussed by Asti in an excellent review [37]. The LPS lipid A fraction is the one responsible for the endotoxic effects at even very low doses. In a way, LPS may be regarded as a pathological chaperone. In fact, although we could not deepen the microbiological analysis in the present work, it has been reported that the secreted LPS derived from B. fragilis and E. coli have significant neurotoxic properties by inducing the pro-inflammatory transcription factor NF-kB [38]. We don’t have a defined explanation at the moment on how FPP improved gut permeability. From our prior study in iron-supplemented women [39] we had found out that FPP could significantly reduce the ex-vivo fecal oxidative stress as measured by dimethyl sulfoxidemethanesulfinic acid reaction which can be interpreted as mirroring an inraluminal action. Interestingly, Yurinskaya et al. [40] have published this year their data showing that exogenous heat shock protein 70 (HSP70) was found to reduce the LPS-induced oxidative stress from microglial cells, i.e. the main immune cells of the central nervous system. This may be reconciled with our study dating back 2017 in which we showed that FPP could significantly increase plasma level of HSP70 in healthy elderly and inversely correlating with inflammatory cytokines [41]. The validity of the redox dysfunction/ gut-derived LPS neurodegenerative hypothesis has been confirmed till very recently [42]. On the other hand the World Health Organization reported that more than 60% of postmenopausal women are overweight or obese [43] and we proved that FPP would significantly improve the “inflammaging” status either systemically and at gut level also in those subjects with normal BMI who were not totally exempt of some metabolic abnormalities [44].
Conclusion
Taken overall and given the confirmed high safety profile of FPP even in elderly, the above evidence-based nutraceutical intervention may provide a valid opportunity within a wider health-promoting and disease-preventing strategy in menopausal women.
Conflict of Interests
We declare no conflict of interest. All co-authors have agreed to transfer copyright to the publisher after accepted for publication.
References
- Montoya-Estrada A, Velázquez-Yescas KG, Veruete-Bedolla DB, Ruiz-Herrera JD, Villarreal-Barranca A, et al. (2020) Parameters of Oxidative Stress in Reproductive and Postmenopausal Mexican Women. Int J Environ Res Public Health 17: 1492.
- Taleb-Belkadi O, Chaib H, Zemour L, Fatah A, Chafi B, et al. (2016) Lipid profile, inflammation, and oxidative status in peri- and postmenopausal women. Gynecol Endocrinol 32: 982-985.
- Ferreira MJ, Sanches IC, Jorge L, Llesuy SF, Irigoyen MC, et al. (2020) Ovarian status modulates cardiovascular autonomic control and oxidative stress in target organs. Biol Sex Differ 11: 15.
- Bourgonje AR, Abdulle AE, Al-Rawas AM, Al-Maqbali M, Al-Saleh M, et al. (2020) Systemic Oxidative Stress Is Increased in Postmenopausal Women and Independently Associates with Homocysteine Levels. Int J Mol Sci 21:E314.
- Somani YB, Pawelczyk JA, De Souza MJ, Kris-Etherton PM, Proctor DN (2019) Aging women and their endothelium: probing the relative role of estrogen on vasodilator function. Am J Physiol Heart Circ Physiol 317: 395-404.
- Groban L, Tran QK, Ferrario CM, Sun X, Cheng CP, et al. (2020) Female Heart Health: Is GPER the Missing Link? Front Endocrinol (Lausanne) 10: 919.
- Unfer TC, Figueiredo CG, Zanchi MM, Maurer LH, Kemerich DM, et al. (2015) Estrogen plus progestin increase superoxide dismutase and total antioxidant capacity in postmenopausal women. Climacteric 18: 379-388.
- Gómez CE, Mora SQ (2013) HRT decreases DNA and lipid oxidation in postmenopausal women. Climacteric 16: 104-110.
- Gökkuşu C, Özbek Z, Tata G (2012) Hormone replacement therapy: relation to homocysteine and prooxidant-antioxidant status in healthy postmenopausal women. Arch Gynecol Obstet 285: 733-739.
- Castanho VS, Gidlund M, Nakamura R, de Faria EC (2011) Post-menopausal hormone therapy reduces autoantibodies to oxidized apolipoprotein B100. Gynecol Endocrinol 27: 800-806.
- Darabi M, Anil M, Movahedian AA, Zarean EE, Panjehpour M, et al. (2010) Effect of hormone replacement therapy on total serum anti-oxidant potential and oxidized LDL/ß2-glycoprotein I complexes in postmenopausal women. Endocrine Journal 57: 1029-1034.
- Masding MG, Stears AJ, Burdge GC, Wootton SA, Sandeman DD (2006) The benefits of oestrogens on postprandial lipid metabolism are lost in post-menopausal women with Type 2 diabetes. Diabet Med 23: 768-774.
- Akcay T, Dincer Y, Saygili EI, SeyisoÄŸlu H, ErtunÄŸalp E (2010) Assessment of DNA nucleo base oxidation and antioxidant defense in postmenopausal women under hormone replacement therapy. Indian J Med Sci 64: 17-25.
- Maffei S, Mercuri A, Prontera C, Zucchelli GC, Vassalle C (2009) Vasoactive biomarkers and oxidative stress in healthy recently postmenopausal women treated with hormone replacement therapy. Climacteric 9: 452-458.
- Bureau I, Laporte F, Favier M, Faure H, Fields M, et al. (2002) No antioxidant effect of combined HRT on LDL oxidizability and oxidative stress biomarkers in treated post-menopausal women. J Am Coll Nutr 21: 333-338.
- Rontu R, Solakivi T, Teisala K, Lehtimäki T, Punnonen R, et al. (2004) Impact of long-term hormone replacement therapy on in vivo and in vitro markers of lipid oxidation. Free Radic Res 38: 129-137.
- Pei R, DiMarco DM, Putt KK, Martin DA, Chitchumroonchokchai C, et al. (2018) Premeal Low-Fat Yogurt Consumption Reduces Postprandial Inflammation and Markers of Endotoxin Exposure in Healthy Premenopausal Women in a Randomized Controlled Trial. J Nutr 148: 910-916.
- Szulińska M, Loniewski I, van Hemert S, Sobieska M, Bogdański P (2018) Dose-Dependent Effects of Multispecies Probiotic Supplementation on the Lipopolysaccharide (LPS) Level and Cardiometabolic Profile in Obese Postmenopausal Women: A 12-Week Randomized Clinical Trial. V Nutrients 10:E773.
- Zakaria R, Wan-Yaacob WM, Othman Z, Long I, Ahmad AH, et al. (2017) Lipopolysaccharide-induced memory impairment in rats: a model of Alzheimer's disease. Physiol Res 66: 553-565.
- Brown GC (2019) The endotoxin hypothesis of neurodegeneration. J Neuroinflammation 16: 180.
- Batista CRA, Gomes GF, Candelario-Jalil E, Fiebich BL, de Oliveira ACP (2019) Lipopolysaccharide-Induced Neuroinflammation as a Bridge to Understand Neurodegeneration. Int J Mol Sci 20:E2293.
- Alexandrov P, Zhai Y, Li W, Lukiw W (2019) Lipopolysaccharide-stimulated, NF-kB-, miRNA-146a- and miRNA-155-mediated molecular-genetic communication between the human gastrointestinal tract microbiome and the brain. Folia Neuropathol 57: 211-219.
- Marotta F, Koike K, Lorenzetti A (2010) Regulating redox balance gene expression in healthy individuals by nutraceuticals: a pilot study. Rejuvenation Res 13: 175-178.
- Aruoma OI, Hayashi Y, Marotta F, Mantello P, Rachmilewitz E, et al. (2010) Applications and bioefficacy of the functional food supplement fermented papaya preparation. Toxicology 278: 6-16.
- Somanah J, Bourdon E, Rondeau P, Bahorun T, Aruoma OI (2014) Relationship between fermented papaya preparation supplementation, erythrocyte integrity and antioxidant status in pre-diabetics. Food Chem Toxicol 65: 12-17.
- Marotta F, Marcellino M, Catanzaro R, Campiotti A, Lorenzetti A, et al. (2020) Mitochondrial and redox dysfunction in post-menopause as risk factor of neurodegenerative disease: a pilot study testing the role of a validated Japanese functional food. J Biol Regul Homeost Agents 34: 111-121.
- Tsai WC, Li YH, Lin CC, Chao TH, Chen JH (2004) Effects of oxidative stress on endothelial function after a high-fat meal. Clin Sci (Lond) 106: 315-319.
- Tobore TO (2020) Towards a comprehensive theory of obesity and a healthy diet: The causal role of oxidative stress in food addiction and obesity. Behav Brain Res 384: 112560.
- RodrÃguez-San Nicolás A, Sanchez-RodrÃguez MA, ZacarÃas-Flores M, Correa-Muñoz E, Mendoza-Núñez VM (2020) Relationship between central obesity and oxidative stress in premenopausal versus postmenopausal women. Nutr Hosp.
- Zalewska A, Kossakowska A, Taranta-Janusz K, Zięba S, Fejfer K, et al. (2020) Oxidative Damage in Saliva of Overweight and Obese Adolescents. J Clin Med 9.
- Farinha JB, De Carvalho NR, Steckling FM, De Vargas LDS, Courtes AA, et al. (2015) An active lifestyle induces positive antioxidant enzyme modulation in peripheral blood mononuclear cells of overweight/obese postmenopausal women. Life Sci 121: 152-157.
- SzuliÅ„ska M, Åoniewski I, Skrypnik K, Sobieska M, Korybalska K, et al. (2018) Multispecies Probiotic Supplementation Favorably Affects Vascular Function and Reduces Arterial Stiffness in Obese Postmenopausal Women-A 12-Week Placebo-Controlled and Randomized Clinical Study. Nutrients 10:E1672.
- Hersoug LG, Møller P, Loft S (2018) Role of microbiota-derived lipopolysaccharide in adipose tissue inflammation, adipocyte size and pyroptosis during obesity. Nutr Res Rev 31: 153-163.
- Shieh A, Epeldegui M, Karlamangla AS, Greendale GA (2020) Gut permeability, inflammation, and bone density across the menopause transition. JCI Insight 5: 134092.
- Zhao Y, Jaber V, Lukiw WJ (2017) Secretory Products of the Human GI Tract Microbiome and Their Potential Impact on Alzheimer's Disease (AD): Detection of Lipopolysaccharide (LPS) in AD Hippocampus. Front Cell Infect Microbiol 7: 318.
- de Waal GM, Engelbrecht L, Davis T, de Villiers WJS, Kell DB, et al. (2018) Correlative Light-Electron Microscopy detects lipopolysaccharide and its association with fibrin fibres in Parkinson's Disease, Alzheimer's Disease and Type 2 Diabetes Mellitus. Sci Rep 8: 16798.
- Asti A (2017) Bacterial Lipopolysaccharide (LPS) and Alzheimer’s Disease, In: Infection and Alzheimer’s Disease. J Miklossy (Ed.) IOS Press.
- Luchi M, Morrison DC (2000) Comparable endotoxic properties of lipopolysaccharides are manifest in diverse clinical isolates of gram-negative bacteria. Infect Immun 68: 1899-1904.
- Bertuccelli G, Marotta F, Zerbinati N, Cabeca A, He F, et al. (2014) Iron supplementation in young iron-deficient females causes gastrointestinal redox imbalance: protective effect of a fermented nutraceutical. J Biol Regul Homeost Agents 28: 53-63.
- Yurinskaya MM, Garbuz DG, Evgen'ev MB, Vinokurov MG (2020) Exogenous HSP70 and Signaling Pathways Involved in the Inhibition of LPS-Induced Neurotoxicity of Neuroblastoma Cells. Mol Biol (Mosk) 54: 128-136.
- Marotta F, Koike K, Lorenzetti A, Naito Y, Fayet F, et al. (2007) Nutraceutical strategy in aging: targeting heat shock protein and inflammatory profile through understanding interleukin-6 polymorphism. Ann N Y Acad Sci 1119: 196-202.
- Loffredo L, Ettorre E, Zicari AM, Inghilleri M, Nocella C, et al. (2020) Oxidative Stress and Gut-Derived Lipopolysaccharides in Neurodegenerative Disease: Role of NOX2. Oxid Med Cell Longev 2020: 8630275.
- Polotsky HN, Polotsky AJ (2010) Metabolic implications of menopause. Semin Reprod Med 28: 426-434.
- Mankowski RT, Leeuwenburgh C, Manini TM, Woods AJ, Anton SD (2018) Effects of Fermented Papaya Preparation (FPP) on Safety Outcomes in Older Adults - A Short Report of a Placebo-Controlled Clinical Trial. J Frailty Aging 7: 142-146.
Citation: Lorenzetti A, Marotta F, Kumar N, Sciuto M, Cervi J, et al. (2020) Leaky Gut and Post-Prandial, Redox and Inflammatory Markers in HRT-Treated Menopausal Females: Benefits by a Pharma-Grade Fermented Papaya Preparation (FPP-ORI). Clin Pharmacol Biopharm 9: 193. DOI: 10.4172/2167-065X.1000193
Copyright: © 2020 Lorenzetti A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 2969
- [From(publication date): 0-2020 - Apr 05, 2025]
- Breakdown by view type
- HTML page views: 2204
- PDF downloads: 765