Research Article Open Access
Leaf Photosynthesis and Plant Competitive Success in a Mixed-grass Prairie: With Reference to Exotic Grasses Invasion
Dong X1,2*, Patton J2, Gu L3, Wang J4 and Patton B21Texas A&M AgriLife Research and Extension Center, Uvalde, Texas-78801, USA
2North Dakota State University, Central Grasslands Research Extension Center, Streeter, N.D. 58483, USA
3Environmental Sciences Division, Oak Ridge National Laboratory, Oak Ridge, Tennessee-37831, USA
4Chinese Research Academy of Environmental Sciences, No. 8 Dayangfang, Beiyuan, Chaoyang District, Beijing- 100012, China
- *Corresponding Author:
- Xuejun Dong
Assistant Professor of Crop Physiology
Texas A&M AgriLifer Research and Extension Center, Uvalde, Texas-78801, USA
Tel: 830-278-9151
E-mail: xuejun.dong@ag.tamu.edu
Received date: October 19, 2014; Accepted date: November 10, 2014; Published date: November 26, 2014
Citation: Dong X, Patton J, Gu L, Wang J, Patton B (2014) Leaf Photosynthesis and Plant Competitive Success in a Mixed-grass Prairie: With Reference to Exotic Grasses Invasion. J Ecosys Ecograph 4:152. doi:10.4172/2157-7625.1000152
Copyright: 2014 Dong X, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Ecosystem & Ecography
Abstract
The widespread invasion of exotic cool-season grasses in mixed-grass rangeland is diminishing the hope of bringing back the natural native plant communities. However, ecophysiological mechanisms explaining the relative competitiveness of these invasive grasses over the native species generally are lacking. We used experimental data collected in south-central North Dakota, USA to address this issue. Photosynthetic potential was obtained from the net assimilation (A) vs. internal CO2 (Ci) response curves from plants grown in a greenhouse. Plant success was defined as the average frequency measured over 25 years (1988 to 2012) on overflow range sites across five levels of grazing intensity. Also, estimated leaf area index of individual species under field conditions was used to indicate plant success. The correlation between photosynthetic potential based on A/Ci curves and plant frequency was negative. The correlation between leaf photosynthesis and plant success (defined as leaf area within a unit land area) was also negative, although statistically weak. These results suggest that the two cool-season grasses, Poa pratensis and Bromus inermis, do not rely on superior leaf-level photosynthesis for competitive success. Instead, some other traits, such as early and late-season growth, may be more important for them to gain dominance in the mixed-grass prairie. We propose that the negative photosynthesis-frequency relation as observed in this study results from a strong competition for limited soil nutrients in the mixed-grass prairie. It has implications for the stability and productivity of the grassland under various human disruptions influencing the soil nutrient status.
Keywords
Bromus inermis; Invasive species; Leaf area index; Photosynthetic potential; Poa pratensis; Principal component analysis; Rangeland management
Introduction
Worldwide, invasive plants pose profound impacts on plant communities and ecosystem function [1]. In the mixed-grass prairie of North America, the widespread invasion of exotic cool-season grasses, such as Poa pratensis L. and Bromus inermis Leyss., is diminishing the hope of bringing back the natural native plant communities [2-3]. A best management strategy for these rangelands, based on several plant community studies [4-6], is perhaps to utilize these cool-season exotics while reducing their cover and increasing diversity. However, knowledge of the ecophysiological mechanisms that explain the relative competitiveness of these cool-season invasive grasses over the native species generally is lacking [7].
Photosynthesis is the basis of plant growth and production. Although plant productivity is strongly associated with integrated seasonal photosynthetic activity [8], it is difficult to quantify seasonal photosynthesis simultaneously for many plant species. Leaf-level instantaneous photosynthesis is frequently measured [9-11], despite the difficulty of obtaining consistent results contrasting the native and invasive species [12,13]. Leaf-level photosynthesis was positively related to plant success in the C4 grass-dominated tallgrass prairie [14]. Whether this similar broad comparison exists in the highly invaded mixed-grass rangelands has important management implications because cool-season exotic grasses have already dominated these rangelands both in terms of herbage production [15] and cover [4]. This paper compares leaf-level photosynthesis of 26 plant species cooccurring in the mixed-grass prairie near Streeter, N.D. USA. The highlight is on the correlations between photosynthetic potential and plant success, with special reference to exotic invasive grasses Poa pratensis and Bromus inermis.
Materials and Methods
Field site and plant frequency data collection
The field site is located at the Central Grasslands Research Extension Center – NDSU near Streeter, N.D. USA. The climate is of the continental type with January mean temperature of -17ºC and August mean temperature of 20ºC. Mean annual precipitation is 458 mm, 70 percent of which occurs from May to September. The vegetation is dominated by grasses, followed by forbs (20 percent aboveground production) and shrubs (two percent) [16]. The rangeland had not been plowed for at least 50 to 60 years. Starting in 1988, a long-term cattle grazing intensity study was initiated on a land area of 156 ha, which was subdivided into 12 equal-size pastures stocked at four levels of season-long grazing (light, moderate, heavy and extremely heavy grazing) with yearling beef cattle. The light treatment left 65 percent of forage produced in an ‘‘average year’’ remaining at the end of the grazing season, moderate treatment left 50 percent, heavy treatment left 35 percent, and extreme treatment left 20 percent [15]. Frequency of occurrence of all plant species was monitored each year since 1988 in fifty 25 cm×25 cm frames along a 50 m transect on each site. Species frequency was defined as the number of frames in which the species occurred divided by the total number of frames used [17]. Data collected from the overflow range site and all grazing intensities combined [15] were used in this study because plants growing on this range site presumably experienced less resource stress and thus the measured frequency would reflect the species’ intrinsic characteristics.
Photosynthesis/intercellular CO2 curves measured in greenhouse
Seeds of 12 species, both native and introduced, were collected during the summer and fall of 2011 on native prairies of the Central Grasslands Research Extension Center near Streeter, N.D. On 20 Apr 2012, seeds were sown in plastic pots (7.6 cm×7.6 cm×7.6 cm) in replicates of four, using a local prairie soil of the Towner-Barnes complex (black loamy fine sand) that was sifted before use. Root cuttings of two shrubs and transplants of 12 more species were planted in pots in May and June, 2012. Pots were placed in a greenhouse and watered as needed. The plant species measured were all C3 species and included five grasses, one sedge, 18 forbs, and two shrubs (Table 1). The photosynthesis/intercellular CO2 (A/Ci) curve measurements were made using a LI-COR 6400 Portable Photosynthesis System (LICOR Bioscience, Lincoln, N.E. USA) from 7 Jul through 13 Aug 2012, with four replicates of each species. The chamber condition was set as: photosynthetic photon flux density (PPFD) 900 μmol m-2s-1; leaf temperature 28ºC; relative humidity 26 to 29 mmol H2O mol-1; and flow rate 300 mol s-1. The reference CO2 was varied in 15 or 16 steps from 0 to 1500 ppm. The A/Ci curves were fitted to the FvCB model according to [18] and implemented through leafweb.ornl.gov. The A/Ci parameters were scaled to the standard values at 25ºC and reported together with a global scale of datasets of A/Ci curves [19]. This paper provides additional analysis of this data set from North Dakota, focusing on the linkage between photosynthesis and plant competitive success.
| Scientific name | Abbreviation | Longevity/Functional group | Native status | Common name |
|---|---|---|---|---|
| AchilleamillefoliumL. | Achmil | perennial forb | native | common yarrow |
| Ambrosia psilostachyaDC. | Ambpsi | perennial forb | native | western ragweed |
| AntennarianeglectaGreene | Antneg | perennial forb | native | field pussy-toes |
| Artemisia absinthiumL. | Artabs | perennial forb/ subshrub | introduced | wormwood, absinthium |
| Artemisia frigidaWilld. | Artfri | perennial subshrub | native | fringed sagewort, prairie sagewort |
| Artemisia ludovicianaNutt. | Artlud | perennial forb | native | white sage, cudweed sagewort |
| BromusinermisLeyss. | Broine | perennial grass | introduced | smooth brome |
| CarexinopsL.H. Bailey ssp. heliophila(Mack.) Crins [CarexheliophilaMack.] | Carhel | perennial sedge | native | sun sedge |
| Cirsiumarvense(L.) Scop. | Cirarv | perennial forb | introduced | Canada thistle |
| Cirsiumflodmanii(Rydb.) Arthur | Cirflo | perennial forb | native | Flodman’s thistle |
| Elymusrepens(L.) Gould [AgropyronrepensL.] | Agrrep | perennial grass | introduced | quackgrass |
| GeumtriflorumPursh | Geutri | perennial forb | native | prairie smoke, torch flower |
| Grindelia squarrosa(Pursh) Dunal | Grisqu | biennial/ perennial forb | native | curly-cup gumweed |
| Helianthus pauciflorusNutt. ssp. pauciflorus[Helianthus rigidus(Cass.) Desf.] | Helrig | perennial forb | native | stiff sunflower |
| Melilotusofficinalis(L.) Lam. | Meloff | annual/biennial forb | introduced | yellow sweetclover |
| Nassellaviridula(Trin.) Barkworth [StipaviridulaTrin.] | Stivir | perennial grass | native | green needlegrass |
| Oligoneuronrigidum(L.) var. rigidum[SolidagorigidaL.] | Solrig | perennial forb | native | stiff goldenrod |
| Oxalis strictaL. | Oxastr | annual/ perennial forb | native | yellow wood sorrel,common yellow oxalis |
| Pascopyrumsmithii(Rydb.) Á. Löve [AgropyronsmithiiRydb.] | Agrsmi | perennial grass | native | western wheatgrass |
| PoapratensisL. | Poapra | perennial grass | native/ introduced | Kentucky bluegrass |
| Rosa arkansanaPorter | Rosark | shrub | native | prairie rose |
| SolidagocanadensisL. | Solcan | perennial forb | native | Canada goldenrod |
| SolidagomissouriensisNutt. | Solmis | perennial forb | native | Missouri goldenrod |
| SymphoricarposoccidentalisHook. | Symocc | shrub | native | western snowberry, buckbrush |
| Symphyotrichumericoides(L.) G.L. Nesom var. ericoides [Aster ericoidesL.] | Asteri | perennial forb | native | white aster, heath aster |
| TaraxacumofficinaleF.H. Wigg | Taroff | perennial forb | native/ introduced | common dandelion |
Table 1: List of C3 species used in the photosynthesis-CO2 response curve measurement.
The pots were overwintered by placing them in a shallow trench located on a sloping field on 8 Nov 2012. The tops of the pots were level with the soil surface, with soil around each tray of pots. They then were covered with an eight-inch thick layer of straw fastened down by a wire mesh. The pots were lifted on 5 May 2013, returned to the greenhouse and watered when needed. The photosynthesis survey of the major species was conducted from 11 Jun to 19 Jun 2013 under conditions similar to that for the A/Ci curve measurement survey in 2012. Leaf samples were collected on 25 June 2013 for total nitrogen content analysis. Also, specific leaf area was measured in order to convert leaf nitrogen content from the dry matter to leaf area basis.
Leaf photosynthetic rates measured under field conditions
Leaf photosynthetic rates for a wide range of plant species were measured on 13 days in 2008 (from 18 Jun to 18 Sep), 12 days in 2009 (1 Jun to 24 Sep) and eight days in 2010 (19 May to 21 Sep). The measurements were made within two 10 m×10 m exclosures located in one moderately grazed pasture and one extremely heavy grazing pasture. The vegetation within the exclosure was clipped to mimic the removal of herbage by cattle grazing. Photosynthesis measurements were made on clear or mostly clear days from 9 AM to 12 PM at a leaf temperature of 16.4 to 32.9ºC, PPFD of 500 to 1330 μmol m-2 s-1, and relative humidity of 28.3 to 65.5 percent. Measurements made on days when the volumetric soil water content at 23 cm depth of soil was lower than 0.2 (permanent wilting point in this prairie soil) are not included as we are interested in photosynthesis under less stressful conditions. During each of the three years, 16 to 20 plant species were measured in the exclosures (with each species measured four to 20 times). Grand averages of photosynthetic rates for each species under different grazing pressures were used in further analysis linking photosynthesis with leaf area index.
Leaf area index for individual plant species
A leaf area survey was conducted monthly four times during the growing season, May to September, for three years (2009 through 2011). A 1-m2 frame was placed on two designated plots in the vicinity of the photosynthesis measurements. Four subplots within each frame were delineated and the percent contribution of total leaf area to the whole frame was noted for each. Separately, each subplot was visually surveyed to estimate leaf area by species. For each subplot, the value of each species’ contribution was adjusted to total 100 percent. The percent value of each species was then multiplied by the contribution of the subplot to the total frame. This information was combined with the total leaf area index within the 1-m2 frame measured using a LP- 80 Ceptometer (Decagon Devices, Pullman, Wash. USA) to obtain the total leaf area of the frame by species.
Data analysis
The estimated eight parameters (Table 1) were subjected to principal component analysis (PCA) to identify the general trend of the multi-dimensional data sets. Differences in photosynthetic parameters among plant species were tested using one-way analysis of variance (ANOVA). As ‘photosynthetic capacity’ is often used to refer to maximum photosynthetic rates under no resource limitations, we denote the PCA-summarized variable as ‘photosynthetic potential.’ This is correlated with the long-term averaged frequency data for the 26 species. Also, grand averages of photosynthetic rates of dominant species were correlated with the corresponding leaf area indices estimated from field surveys from the same location. Data of leaf area index were square root transformed in order to meet the assumptions of the correlation analysis.
Results and Discussion
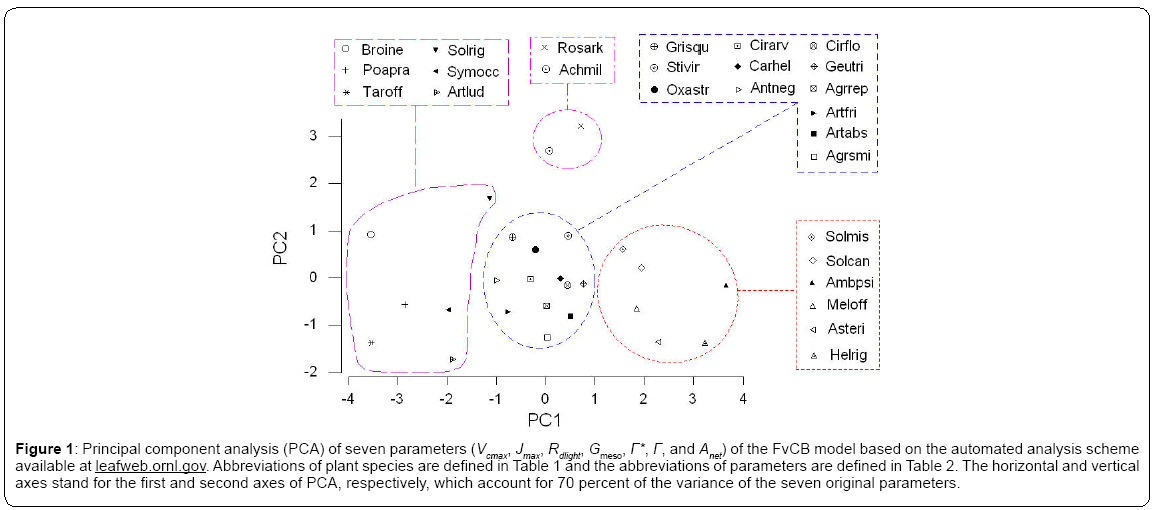
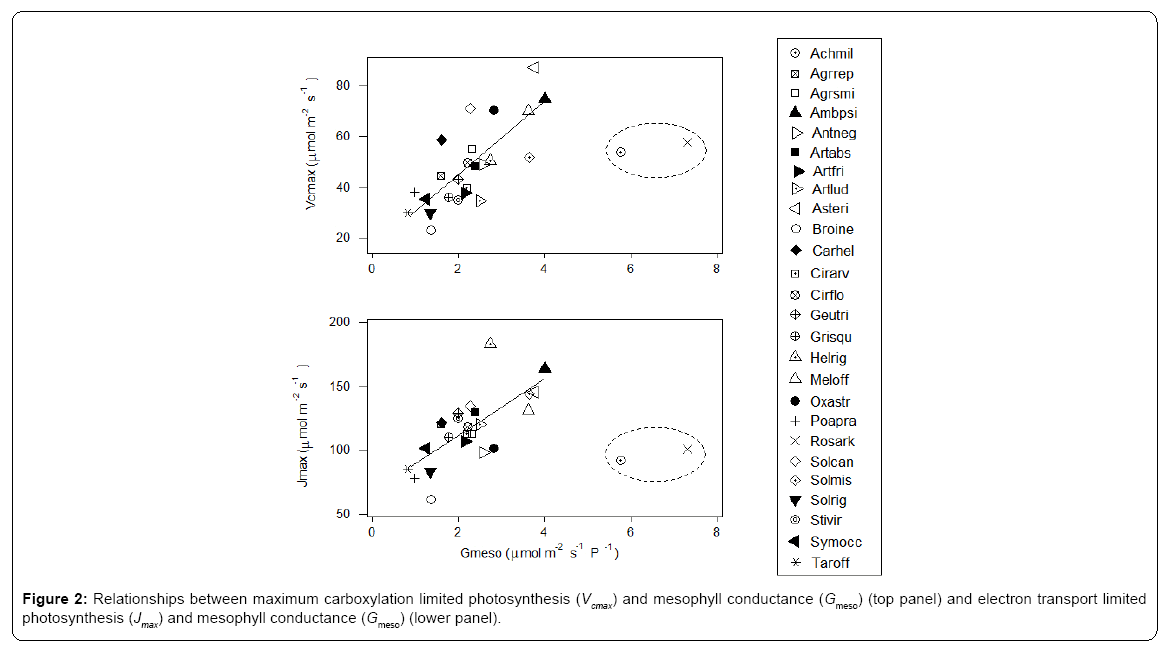
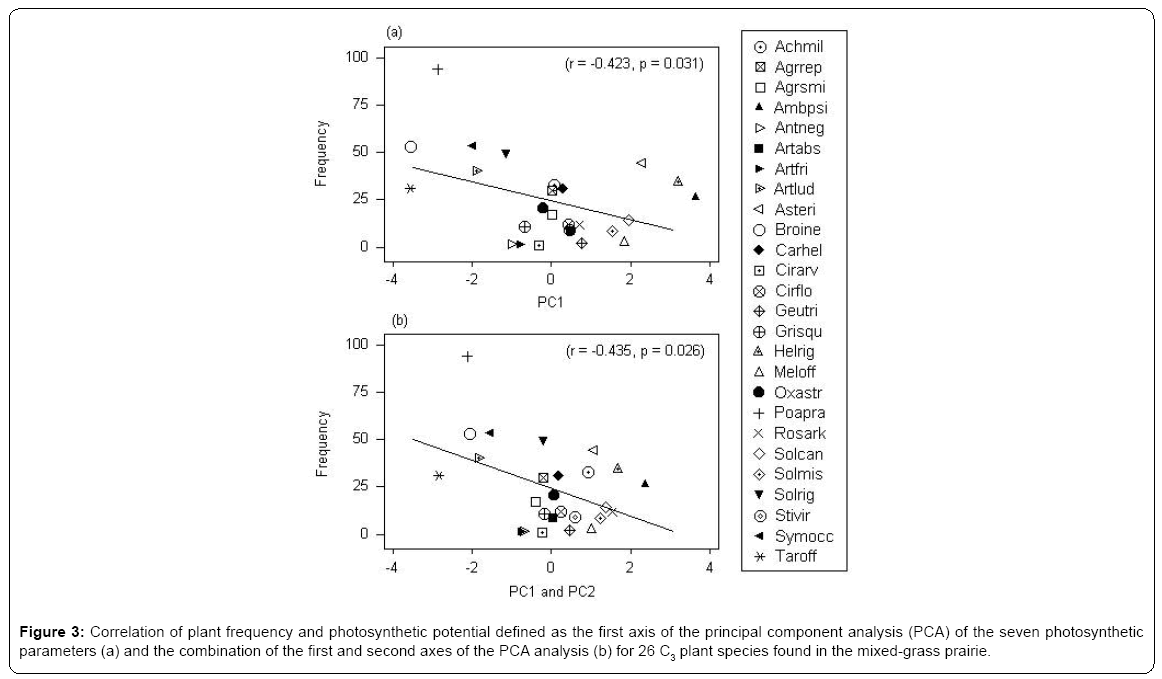
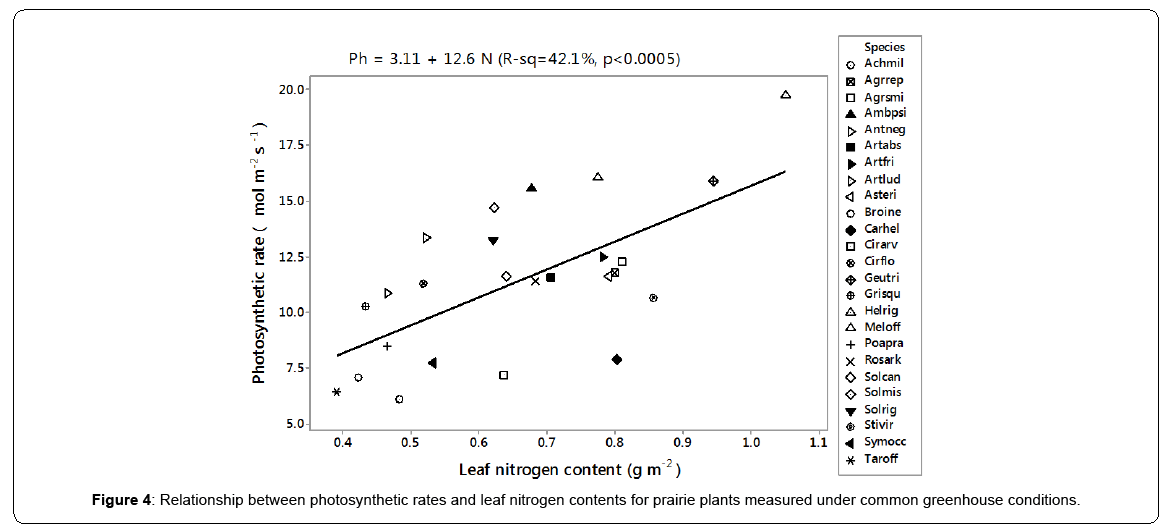
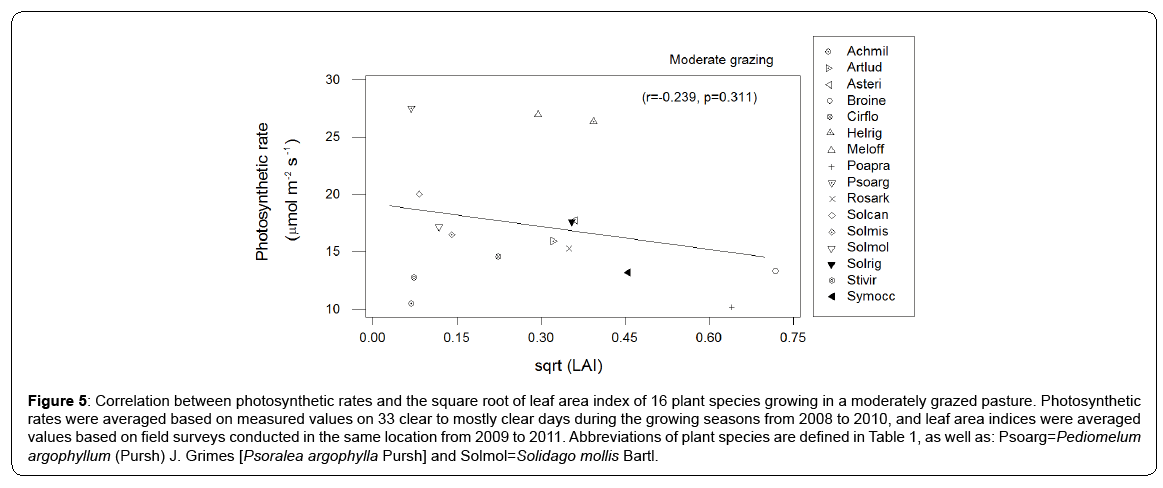
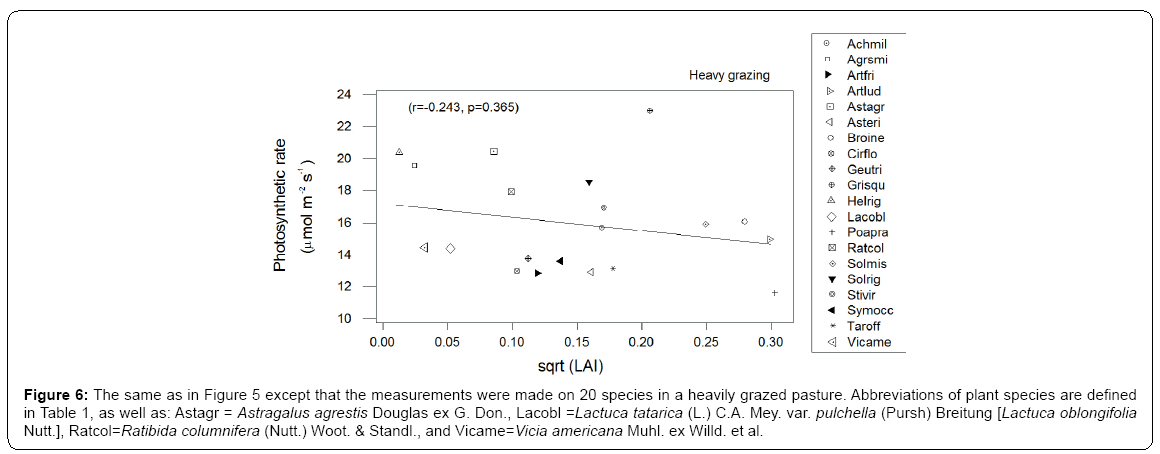
The first axis of PCA (Figure 1 - horizontal) is correlated with photosynthetic carboxylation (Vcmax) and electron transport (Jmax) and the second (vertical axis) with the mesophyll conductance (Gmeso) (Table 2). Six forbs (five from the Asteraceae family and one legume) had a greater intrinsic photosynthetic capacity than the remaining species, which occupied the middle or lower range of variation. Located at the lowest end of the horizontal axis were Poa pratensis, Bromus inermis and Taraxacum officinale F.H. Wigg. It appears that the maximum carboxylation rate (reflecting mainly the Vcmax in the first PCA axis) and the mesophyll conductance (the second PCA axis) may compensate to give similar maximum photosynthesis. In this respect, Achillea millefolium L. and Rosa arkansana Porter exhibited different patterns from other species (Figure 2). As shown in Figure 3, frequency was negatively correlated with photosynthetic potential of prairie plants, though the correlation was stronger when the information from the first two PCA axes were combined (Figure 3b, r=-0.435, p=0.026). Also, there was a positive correlation between photosynthetic rates and leaf nitrogen in these species (Figure 4). Furthermore, using our leaf photosynthesis data collected from 2008 through 2010 and leaf area index data for 25 species collected from 2009 through 2011, we observed also a negative correlation between leaf photosynthesis and plant success (here defined as leaf area within a unit land area) in both moderately and heavily grazed pastures, although the correlation was statistically weak (p=0.311 for moderate grazing and p=0.365 for heavy grazing), (Figures 5 and 6).
Figure 1: Principal component analysis (PCA) of seven parameters (Vcmax, Jmax, Rdlight, Gmeso, Γ*, Γ, and Anet) of the FvCB model based on the automated analysis scheme available at leafweb.ornl.gov. Abbreviations of plant species are defined in Table 1 and the abbreviations of parameters are defined in Table 2. The horizontal and vertical axes stand for the first and second axes of PCA, respectively, which account for 70 percent of the variance of the seven original parameters.
Figure 3: Correlation of plant frequency and photosynthetic potential defined as the first axis of the principal component analysis (PCA) of the seven photosynthetic parameters (a) and the combination of the first and second axes of the PCA analysis (b) for 26 C3 plant species found in the mixed-grass prairie.
Figure 5: Correlation between photosynthetic rates and the square root of leaf area index of 16 plant species growing in a moderately grazed pasture. Photosynthetic rates were averaged based on measured values on 33 clear to mostly clear days during the growing seasons from 2008 to 2010, and leaf area indices were averaged values based on field surveys conducted in the same location from 2009 to 2011. Abbreviations of plant species are defined in Table 1, as well as: Psoarg=Pediomelum argophyllum (Pursh) J. Grimes [Psoralea argophylla Pursh] and Solmol=Solidago mollis Bartl.
Figure 6: The same as in Figure 5 except that the measurements were made on 20 species in a heavily grazed pasture. Abbreviations of plant species are defined in Table 1, as well as: Astagr = Astragalus agrestis Douglas ex G. Don., Lacobl =Lactuca tatarica (L.) C.A. Mey. var. pulchella (Pursh) Breitung [Lactuca oblongifolia Nutt.], Ratcol=Ratibida columnifera (Nutt.) Woot. & Standl., and Vicame=Vicia americana Muhl. ex Willd. et al.
| Parameter1 | PC1 (48.4%) | PC2 (21%) | PC3 (16%) |
|---|---|---|---|
| Vcmax | 0.76*2 | -0.05 | 0.48* |
| Jmax | 0.88* | -0.36 | -0.07 |
| Rdlight | -0.22 | -0.89* | 0.09 |
| Gmeso | 0.51* | 0.53* | 0.56* |
| Γ* | -0.51* | -0.26 | 0.72* |
| Γ | -0.87* | -0.14 | 0.22 |
| Anet | 0.84* | -0.43* | -0.01 |
1 =Abb6eviations: Vcmax, maximum rate of carboxylation limited by the amount, activity and kinetics of rubisco; Jmax, maximum rate of carboxylation limited by electron transport; Rdlight, rate of respiration in the presence of light; Gmeso, mesophyll CO2 conductance; Γ*, chloroplast CO2photo-compensation point without considering Rdlight; Γ, CO2 compensation point considering Rdlight; Anet, net photosynthesis estimated at the transition from RuBP-limited to the TPU-limited photosynthesis. 2*=correlations are statistically significant (p=0.05).
Table 2: Correlation coefficients between the first three axes of the principal component analysis (PCA) and the seven photosynthetic parameters used in the PCA analysis. The analysis was based on the averaged data from photosynthesisintercellular CO2 concentration curves for 26 C3 rangeland plant species. Measurements were made at 28ºC, but the parameters were scaled to 25ºC. The percentages of variance of the original seven parameters explained by the PCA axes are included in the parentheses.
Averaged over the growing season from May to September, the leaf area indices of Poa pratensis and Bromus inermis combined accounted for 46 percent and 24 percent of the total LAI in plots of moderately and heavily grazed pastures, respectively (Tables 3 and 4). In terms of leaf nitrogen on ground area basis, the two exotic species combined represented 30 percent and 15 percent of the total leaf nitrogen of the plant communities under the two grazing treatments.
| Species | ǂLAI-2009 | LAI-2010 | LAI-2011 | LAI-mean | N (g/m2 ground area)§ |
|---|---|---|---|---|---|
| Bromusinermis | 0.3233 | 0.5416 | 0.6826 | 0.5158 | 0.2182 |
| Poapratensis | 0.3081 | 0.4069 | 0.5165 | 0.4105 | 0.1908 |
| Helianthus pauciflorus | 0.1174 | 0.1496 | 0.1976 | 0.1548 | 0.1626 |
| Symphoricarposoccidentalis | 0.1746 | 0.196 | 0.2518 | 0.2075 | 0.1106 |
| Aster ericoides | 0.115 | 0.1257 | 0.1494 | 0.13 | 0.1029 |
| Rosa arkansana | 0.0882 | 0.1168 | 0.1618 | 0.1223 | 0.0834 |
| Oligoneuronrigidum | 0.0941 | 0.1178 | 0.1654 | 0.1258 | 0.0782 |
| Melilotusofficinalis | 0.0316 | 0.0933 | 0.1355 | 0.0868 | 0.0672 |
| Artemisia ludoviciana | 0.0907 | 0.0941 | 0.1242 | 0.103 | 0.0537 |
| Cirsiumflodmanii | 0.0301 | 0.042 | 0.0774 | 0.0499 | 0.0258 |
| Solidagomissouriensis | 0.0237 | 0.0053 | 0.0299 | 0.0196 | 0.0122 |
| Solidagomollis* | 0 | 0.0152 | 0.0259 | 0.0137 | 0.0090 |
| Tragopogondubius* | 0.0047 | 0.0187 | 0.0101 | 0.0111 | 0.0073 |
| Lotus purshianus* | 0 | 0 | 0.0305 | 0.0102 | 0.0067 |
| Stipaviridula | 0.0021 | 0 | 0.0137 | 0.0053 | 0.0045 |
| Solidagocanadensis | 0.0179 | 0 | 0.0023 | 0.0067 | 0.0043 |
| Anemone cylindrica* | 0.0005 | 0.0044 | 0.0127 | 0.0059 | 0.0039 |
| Psoraleaargophylla* | 0.0019 | 0 | 0.0119 | 0.0046 | 0.0030 |
| Asclepiasovalifolia* | 0 | 0 | 0.0117 | 0.0039 | 0.0026 |
| Cirsiumarvense* | 0.0045 | 0.003 | 0.0044 | 0.004 | 0.0025 |
| Achilleamillefolium | 0.0032 | 0.0038 | 0.0068 | 0.0046 | 0.0022 |
| Carexspp. | 0.0042 | 0.0023 | 0.001 | 0.0025 | 0.0020 |
| Phleum pretense* | 0 | 0 | 0.0066 | 0.0022 | 0.0014 |
| Taraxacumofficinale | 0.0025 | 0 | 0 | 0.0008 | 0.0003 |
| Total | 1.4383 | 1.9365 | 2.6297 | 2.0015 | 1.3156 |
ǂLAI was measured five times from May to September in 2009 through 2011. Value for each species was estimated by the relative leaf area occurring within 1×1 m frames as well as the absolute total seasonal LAI measured using a ceptometer (see main text for detail).
§Leaf nitrogen per unit ground area for each species was estimated by multiplying LAI of each species by area-based leaf nitrogen concentration (based on Figure 4).
*Species for which leaf nitrogen was not measured and an average value of 0.66 g/m2 (based on 25 species) was assumed.
Table 3: Leaf area indices (LAI) and leaf nitrogen per unit ground area for 24 plant species found in a moderately grazed pasture in the mixed-grass prairie near Streeter N.D., USA.
| Species | ǂLAI-2009 | LAI-2010 | LAI-2011 | LAI-mean | N (g/m2ground area)§ |
|---|---|---|---|---|---|
| Artemisia ludoviciana | 0.0373 | 0.0788 | 0.1524 | 0.0895 | 0.0467 |
| Poapratensis | 0.0321 | 0.0766 | 0.1665 | 0.0917 | 0.0426 |
| Solidagomissouriensis | 0.0452 | 0.0665 | 0.0749 | 0.0622 | 0.0387 |
| Bromusinermis | 0.0301 | 0.0707 | 0.1337 | 0.0782 | 0.0331 |
| Stipaviridula | 0.0128 | 0.0245 | 0.0502 | 0.0292 | 0.0250 |
| Aster ericoides | 0.0115 | 0.0253 | 0.0407 | 0.0259 | 0.0205 |
| Grindelia squarrosa | 0.0353 | 0.047 | 0.0456 | 0.0426 | 0.0184 |
| Oligoneuronrigidum | 0.0111 | 0.0322 | 0.0328 | 0.0253 | 0.0157 |
| Carexspp. | 0.0199 | 0.019 | 0.0155 | 0.0181 | 0.0145 |
| Achilleamillefolium | 0.008 | 0.0194 | 0.0584 | 0.0286 | 0.0138 |
| Taraxacumofficinale | 0.0188 | 0.0367 | 0.0394 | 0.0317 | 0.0124 |
| Geumtriflorum | 0.0038 | 0.0129 | 0.0209 | 0.0125 | 0.0118 |
| Boutelouagracilis* | 0.0081 | 0.0155 | 0.0286 | 0.0174 | 0.0114 |
| Artemisia frigida | 0.0134 | 0.0134 | 0.0158 | 0.0142 | 0.0111 |
| Symphoricarposoccidentalis | 0.0128 | 0.018 | 0.0255 | 0.0188 | 0.0100 |
| Cerastiumarvense | 0.0054 | 0.0186 | 0.0176 | 0.0139 | 0.0089 |
| Antennarianeglecta | 0.0088 | 0.0143 | 0.0265 | 0.0165 | 0.0077 |
| Oxalis stricta* | 0 | 0.0035 | 0.0309 | 0.0115 | 0.0076 |
| Dichantheliumwilcoxianum* | 0.0043 | 0.004 | 0.0244 | 0.0109 | 0.0072 |
| Medicagolupulina* | 0.0017 | 0.0104 | 0.0187 | 0.0103 | 0.0068 |
| Ratibidacolumnifera* | 0.0018 | 0.0075 | 0.0202 | 0.0098 | 0.0064 |
| Stipacurtiseta* | 0 | 0.0151 | 0.0112 | 0.0088 | 0.0058 |
| Cirsiumflodmanii | 0 | 0.0069 | 0.0252 | 0.0107 | 0.0055 |
| Astragalusflexuosus* | 0.0026 | 0 | 0.0198 | 0.0074 | 0.0049 |
| Astragalusagrestis* | 0.0026 | 0.0121 | 0.0071 | 0.0073 | 0.0048 |
| Anemone cylindrica* | 0.0028 | 0.0042 | 0.0143 | 0.0071 | 0.0047 |
| Viola pedatifida* | 0.001 | 0.0043 | 0.0137 | 0.0063 | 0.0041 |
| Lactucatatarica* | 0.0006 | 0.0014 | 0.006 | 0.0027 | 0.0018 |
| Sphaeralceacoccinea* | 0.0011 | 0.0031 | 0.0028 | 0.0023 | 0.0015 |
| Viciaamericana* | 0 | 0.0003 | 0.0027 | 0.001 | 0.0007 |
| Potentillapensylvanica* | 0.0003 | 0 | 0.0024 | 0.0009 | 0.0006 |
| Pascopyrumsmithii | 0 | 0 | 0.0017 | 0.0006 | 0.0005 |
| Ambrosia psilostachya | 0.0008 | 0.0003 | 0 | 0.0004 | 0.0003 |
| Euphorbia serpyllifolia* | 0 | 0.0013 | 0 | 0.0004 | 0.0003 |
| Helianthus pauciflorus | 0 | 0.0005 | 0 | 0.0002 | 0.0002 |
| Agrostishyemalis* | 0 | 0.0009 | 0 | 0.0003 | 0.0002 |
| Onosmodium, molle* | 0 | 0.0008 | 0 | 0.0003 | 0.0002 |
| Polygonum convolvulus* | 0 | 0.0001 | 0 | 0 | 0.0000 |
| Total | 0.3340 | 0.6661 | 1.1461 | 0.7155 | 0.4703 |
ǂLAI was measured five times from May to September in 2009 through 2011. Value for each species was estimated by the relative leaf area occurring within 1×1 m frames as well as the absolute total seasonal LAI measured using a ceptometer (see main text for detail).
§Leaf nitrogen per unit ground area for each species was estimated by multiplying LAI of each species by area-based leaf nitrogen concentration (based on Figure 4).
*Species for which leaf nitrogen was not measured and an average value of 0.66 g/m2 (based on 25 species) was assumed.
Table 4: Leaf area indices (LAI) and leaf nitrogen per unit ground area for 38 plant species found in a heavily grazed pasture in the mixed-grass prairie near Streeter N.D., USA.
Past studies indicate that photosynthetic capacities of prairie forbs when compared to grasses are similar [20], higher [21] or lower [14,22]. Our results suggest that photosynthetic capacity of prairie forbs is widely variable, which is not surprising considering the number of plant families and life forms represented. The negative correlation between photosynthesis and plant frequency as observed in our study contrasts the findings of McAllister et al. [14] done in a highly productive tallgrass prairie. We speculate that the negative photosynthesis-plant success relationship as found in our study might indicate an outcome of severe interspecific competition for soil resources in the mixed-grass prairie.
The data from this study has several implications for understanding plant community and ecosystem functions in the mixed-grass prairie. First, the negative correlation between leaf photosynthesis and plant success suggests that the instantaneous photosynthetic characteristics measured at leaf level can only represent shortly-lived trends of leaf carbon gain, which cannot portray the overall photosynthetic capacity of the plant species during the entire growing season. Our measurements were conducted in the warm months, and did not capture photosynthetic activity in early spring and late fall when Poa pratensis and Bromus inermis maintain photosynthesis and growth while many other species in the prairie are dormant. Taking advantage of ample spring soil water for rapid growth is especially important for the competitive success of these two exotic species. Second, maintaining a low photosynthetic rate for the most abundant species also reduces excessive depletion of soil resources, and thus favors a more diverse plant community. This can also be seen from the peak-season value of the ground area-based nitrogen for each species co-occurring in the moderate and heavy grazing pastures (Tables 3 and 4). We can see that on a unit ground area basis on this prairie, these two exotic species are strong competitors for nitrogen, especially in the moderately grazed pasture. This occurred despite their low nitrogen content and low photosynthetic rate per unit leaf area.
Soil in this mixed-grass prairie typically is low in nitrogen (N) and phosphorus (P) and fertilization of N and P can increase forage production [23]. At the same time, high N application tends to favor exotic grasses such as Poa pratensis and reduce plant diversity, depending on stocking history [24]. Studies in the tallgrass prairie [25,26] also demonstrate that nitrogen addition may encourage native warm-season grasses to be displaced by exotic cool-season grasses such as Poa pratensis and Elymus repens (L.) Gould. It has been estimated that atmospheric nitrogen deposition due to burning of fossil fuels, crop fertilization and intensive livestock operations may increase plant N supply by 10-25 percent in the tallgrass prairie [27]. The relative contribution of nitrogen deposition may be higher in the mixed-grass prairie, perhaps as high as 50 percent, considering an estimated annual N mineralization rate of 1.2 g m-2 [28] and an annual total atmospheric N deposition of 0.6 g m-2 based on data of NADP for the year 2000. Bromus inermis and Poa pratensis, which are considered invasive [3] and investing larger proportion of its production to belowground biomass compared with co-occurring natives [29], have been shown to increase in both biomass production and cover on the silty range sites of the mixed-grass prairie over past 26 years, especially under rest and light grazing situations [15]. Wedin and Tilman [27] used a positive feedback model to describe the interaction between tallgrass prairie natives and soil nitrogen cycling. Perhaps the same framework can be used to speculate why in the mixed-grass prairie Poa pratensis and Bromus inermis have extensively displaced native grasses and forbs may partly be due to the low photosynthesis/low nitrogen, and low litter quality nature of these exotic species. Nevertheless, as the positive feedback system is inherently unstable, the future of the mixed-grass prairie will to a substantial extent be influenced by how the plant species, especially those representing a large fraction of LAI and per land area based nitrogen, such as Poa pratensis and Bromus inermis, allocate nitrogen among different organs and adjust their physiology strategies in response to various disturbances, including atmospheric N deposition at regional scale spanning tallgrass and mixed-grass prairies.
In addition to nitrogen deposition, other global change factors, such as increases in atmospheric CO2 concentration and temperature, may have differential impacts to exotic vs. native species. A synthesis of available data suggests that invasive, noxious weeds have a larger than expected growth increase to both recent and projected atmospheric CO2 increase as compared to native plant species [30]. Actually, it has been shown that, in Poa pratensis, increased atmospheric CO2 concentration not only mitigated high temperature stress on net carbon gain, but also significantly increased root growth [31]. It will be interesting to test experimentally if this CO2-related growth enhancement would increase the invasive potential of this and related species. Although our data showed a relative low photosynthetic potential in several invasive plants (Figure 1 and Table 2), the low mesophyll conductance measured in several invasive plants (Supplementary Table 1) suggests potentials for photosynthesis to increase under high atmospheric CO2. To what extent this will be integrated into whole plant growth and competitive success is yet to be seen using manipulative experiments contrasting the invasive against the native plants, for which comprehensive data is merger yet critically important for understanding the integrity of rangeland ecosystems under future climate and land use changes [30]. As the invasion and naturalization of exotic plant species is influenced by not only biophysical but also social economic factors, including global trade [32], it will be a challenging task to constantly contain the vigor of the exotic species using a combination of tools, such as early grazing and prescribed fire targeted at the most vulnerable growth stages while maintaining the integrity of native species [4-6,33,34]. This highlights the importance of integrated, transdisciplinary studies for the rehabilitation of degraded rangelands [35].
Acknowledgements
This paper was part of North Dakota Agricultural Experiment Station projects ND6146, ND6147 and ND6149. We appreciate editors and reviewers for valuable suggestions that helped to improve the paper.
References
- Weidenhamer JD, Callaway RM (2010) Direct and indirect effects of invasive plants on soil chemistry and ecosystem function. J Chem Ecol 36:59-69.
- Murphy RK, Grant TA (2005) Land management history and floristics in the mixed-grass prairie, North Dakota, USA. Natural Areas J25: 351-358.
- Grant TA, Flanders-Wanner B, Shaffer TL, Murphy RK, Knutsen GA (2009) An emerging crisis across northern prairie refuges: Prevalence of invasive plants and a plan for adaptive management. Ecol Restoration 27: 58-65.
- DeKeyser S, Clambey G, Krabbenhoft K, Ostendorf J (2009) Are changes in species composition in central North Dakota rangelands due to non-use management? Rangelands 31: 16-19.
- DeKeyser S, Meehan M, Sedivec K, Lura C (2010) Potential management alternatives for invaded rangelands in the Northern Great Plains. Rangelands 32: 26-31.
- Helzer C (2012) Dealing with a pervasive invasive – Kentucky bluegrass in prairies.
- Dong X, Patton B, Nyren P, Limb R, Cihacek L, et al. (2011) Leaf-water relations of a native and an introduced grass species in the mixed-grass prairie under cattle grazing.ApplEcol Environ Res 9: 311-331.
- Zelitch I (1982) The close relationship between net photosynthesis and crop yield. Bioscience 32: 796–802.
- Wullschleger SD (1993) Biochemical limitations to carbon assimilation in C3 plants–a retrospective analysis of the A/Ci curves from 109 species. J ExpBot 44: 907–920.
- McDowell SCL (2002) Photosynthetic characteristics of invasive and noninvasive species of Rubus (Rosaceae). Am J Bot 89:1431-1438.
- He Z, Bentley LP, Holaday, AS (2011) Greater seasonal carbon gain across a broader temperature range contribute to the invasive potential of Phalarisarundinacea (Poaceae; Reed canary grass) over the native sedge Carexstricta (Cyperaceae). Am J Bot 98: 20–30.
- Nagel JM, Griffin KL (2004) Can gas-exchange characteristics help explain the invasive success of Lythrumsalicaria? Biol Invasions 6: 101–111.
- Ugoletti P, Stout JC, Jones MB (2011) Ecophysiological traits of invasive and non-invasive introduced Impatiens species. Biology Environ: Proc Royal Irish Acad 111B: 1-14.
- McAllister CA, Knapp AK, Maragni LA (1998) Is leaf-level photosynthesis related to plant success in a highly productive grassland? Oecologia 117: 40–46.
- Patton B, Dong X, Nyren P, Nyren A (2007) Effect of grazing intensity, precipitation and temperature on forage production. Rangeland Ecol Manage 60: 656-665.
- Lura CL, Barker WT, Nyren PE (1988) Range plant communities of the Central Grasslands Research Station in south central North Dakota. Prairie Nat 20: 177-192.
- Bonham CD (1989) Measurements for Terrestrial Vegetation. John Wiley and Sons Inc., New York.
- Gu L, Pallardy SG, Tu K, Law BE, Wullschleger SD (2010) Reliable estimation of biochemical parameters from C3 leaf photosynthesis-intercellular carbon dioxide response curves. Plant Cell Environ 33: 1852–1874.
- Sun Y, Gu L, Dickinson RE, Pallardy SG, Baker J, et al. (2014) Asymmetrical effects of mesophyll conductance on fundamental photosynthetic parameters and their relationships estimated from leaf gas exchange measurements. Plant Cell Environ 37: 987-994.
- Reich PB, Buschena C, Tjoelker MG, Wrage K, Knops J, et al. (2003) Variation in growth rate and ecophysiology among 34 grassland and savanna species under contrasting N supply: a test of functional group differences. New Phytol 157: 617–631.
- Lee TD, Tjoelker MG, Ellsworth DS, Reich PB (2001) Leaf gas exchange responses of 13 prairie grassland species to elevated CO2 and increased nitrogen supply. New Phytol 150: 405–418.
- Tjoelker MG, Craine JM, Wedin D, Reich PB, Tilman D (2005) Linking leaf and root trait syndromes among 39 grassland and savannah species. New Phytol 167: 493–508.
- Lorenz RJ, Rogler GA (1973) Growth rate of mixed prairie in response to nitrogen and phosphorus fertilization. J Range Manage 26: 365-368.
- Volk JM (2006) Impacts of slow release phosphorus and urea on intensively and moderately grazed mixed grass prairie and Impacts of Rotational Grazing, Intensive Season-long Grazing, and Idle Land Use on Soil Health in the Missouri Couteau. North Dakota State University, USA
- Tilman D (1987) Secondary succession and the pattern of plant dominance along experimental nitrogen gradient. EcolMonogr 57: 189-214.
- Tilman D (1990) Constraints and tradeoffs: toward a predictive theory of competition and succession. Oikos 58: 3-15.
- Wedin DA, Tilman D (1992) Nitrogen cycling, plant competition, and the stability of tallgrass prairie. Conference: Recapturing the Vanishing Heritage. University of Northern Iowa Press, Iowa.
- Biondini ME, Patton BD, Nyren PE (1998) Grazing intensity and ecosystem processes in a northern mixed-grass prairie, USA. EcolAppl 8: 469-479.
- Dong X, Patton J, Wang G, Nyren P, Peterson P (2014) Effect of drought on biomass allocation in two invasive and two native grass species dominating the mixed-grass prairie. Grass Forage Sci 69: 160-166.
- Ziska LR, George K (2004) Rising carbon dioxide and invasive, noxious plants: Potential threats and consequences. World Resource Rev 16: 427-447.
- Song Y, Yu J, Huang B (2014) Elevated CO2-mitigation of high temperature stress associated with maintenance of positive carbon balance and carbohydrate accumulation in Kentucky bluegrass. PLoS ONE, USA.
- Guo Q, Rejmánek M, Wen J (2012) Geographical, socioeconomic, and ecological determinants of exotic plant naturalization in the United States: insights and updates from improved data. NeoBiota 12: 41-55.
- Gifford M, Otfinowski R (2013) Landscape disturbances impact affect the distribution and abundance of exotic grasses in northern fescue prairies. Invasive Plant Sci Manage 6: 577-584.
- Dillemuth FP (2012) Invasion of smooth brome into North American tall-grass prairies: Impact on native plant/herbivore species and mechanisms responsible for successful invasion. Louisiana State University, USA.
- Sheley RL, James JJ, Rinella MJ, Blumenthal D, DiTomaso JM (2011) Invasive plant management on anticipated conservation benefits: A scientific assessment. Natural Resources Conservation Service, USA.
Relevant Topics
- Aquatic Ecosystems
- Biodiversity
- Conservation Biology
- Coral Reef Ecology
- Distribution Aggregation
- Ecology and Migration of Animal
- Ecosystem Service
- Ecosystem-Level Measuring
- Endangered Species
- Environmental Tourism
- Forest Biome
- Lake Circulation
- Leaf Morphology
- Marine Conservation
- Marine Ecosystems
- Phytoplankton Abundance
- Population Dyanamics
- Semiarid Ecosystem Soil Properties
- Spatial Distribution
- Species Composition
- Species Rarity
- Sustainability Dynamics
- Sustainable Forest Management
- Tropical Aquaculture
- Tropical Ecosystems
Recommended Journals
Article Tools
Article Usage
- Total views: 64456
- [From(publication date):
December-2014 - Jul 11, 2025] - Breakdown by view type
- HTML page views : 59811
- PDF downloads : 4645