Research Article Open Access
Lead-Induced Reduction In Body And Kidney Weight Of Wistar Albino Rats Ameliorated By Ginkgo Biloba Extract (EGb 761)
| Zaheer Amjad1, Muhammad Zia Iqbal2* and Amir Ali Shoro3 | |
| 1Department of Anatomy, Dow University of Health Sciences, Karachi, Pakistan | |
| 2Department of Anatomy, Suliman Alrajhi Colleges, Bukaryiah, Kingdom of Saudi Arabia | |
| 3Department of Anatomy, Liaquat National Medical College, Karachi, Pakistan | |
| *Corresponding Author : | Muhammad Zia Iqbal Department of Anatomy, Suliman Alrajhi Colleges Bukaryiah, Kingdom of Saudi Arabia Tel: 96653688772 Fax: 96663352601 Email: z.iqbal@sr.edu.sa |
| Received July 07, 2013; Accepted July 23, 2013; Published July 26, 2013 | |
| Citation: Amjad Z, Iqbal MZ, Shoro AA (2013) Lead-Induced Reduction in Body and Kidney Weight of Wistar Albino Rats Ameliorated by Ginkgo biloba Extract (EGb 761). Biochem Physiol 2:113. doi:10.4172/2168-9652.1000113 | |
| Copyright: © 2013 Amjad Z, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Biochemistry & Physiology: Open Access
Abstract
Objective To observe the effects of lead acetate on body and kidney weight of Wistar albino rats and the protective role of EGb 761 on weight changes. 1.2 Methodology One hundred and twenty young male Wistar albino rats were randomly selected and divided into three groups, A, B and C, comprising of 40 animals. Each group was further divided into four subgroups according to the duration of treatment, i.e., one, two, four and six weeks. Group A animals (control) were given 0.5 ml normal saline intraperitoneally (IP) daily. Group B animals received lead acetate 8mg/kg body weight IP daily, whereas Group C animals received 100mg/kg body weight of EGb 761 through gavage and 8mg/kg body weight lead acetate through IP. Initial and final body weights were taken and recorded. After sacrificing the animals, the kidneys were retrieved, dried and weighed using the Sartorius balance. 1.3 Results Group B animals showed overall decrease in the body weight, which was non-significant (p>0.05) after one week and became highly significant (p<0.001) after two, four and six weeks, when compared with the control group animals. Group C animals showed increase in the body weight which was less as compared to A group animals, being non-significant (p>0.05) after one and two weeks, while became significant (p<0.01) after four weeks and highly significant (p<0.001) after six weeks. There was a non-significant (p>0.05) decrease in kidney weight of group B rats after one week whereas the decrease was highly significant (p<0.001) after subsequent periods of treatment. When relative weight of the kidneys in group B animals was compared with A and C groups, it was significantly increased (p<0.001) after one, two and four weeks, whereas the change was non-significant (p>0.05) after six weeks of treatment. 1.4 Conclusion EGb 761 effectively ameliorated the lead-induced changes in body and kidneys weight of the albino rats.
| Keywords |
| Albino rats; Lead acetate; Ginkgo biloba; Kidneys |
| Introduction |
| Lead is a highly toxic heavy metal which is known to be present everywhere on the earth. Lead compounds are used as pigment in paints, ceramic glazes, dyes etc. Even natural soil is not free of lead [1]. Despite the admirable measures of phasing out the leaded petrol in Pakistan in July 2001 [2], it is still the most prominent identifiable cause of elevated blood lead levels in children living in the urban areas of Pakistan [3]. It is considered to be an abnormal trace element in humans and animals [4] and tends to accumulate in the body with the passage of time. There is no lower limit of blood lead level that can be considered safe for the body [5,6]. |
| Chronic exposure to lead leads to gradual loss of body weight [7]. This might be due to nausea, vomiting and anorexia, which usually accompany any metal toxicity [8]. One of the key factors in producing metal toxicity is the oxidative stress [9] In many studies it has been claimed that oxidative stress promotes catabolic states in the skeletal muscles, and chronic exposure to this type of stress leads to muscle wasting [10-12]. Hypothetically, any substance behaving as an antioxidant can ameliorate the toxic effects of the metal. |
| EGb 761 is a standardized extract of Ginkgo biloba leaves, one of the oldest and most resistant trees in the world [13]. Medicinal use of G. biloba has been documented since ancient times, but the standardized form was first introduced into allopathic practice in 1965 by Dr. Willmar Schwabe III, a renowned German physician and pharmacist [14]. Major constituent parts of EGb 761 are flavonoid glycosides (24%) and terpene lactones (6%) [15-17]. Some of the ingredients of flavonoids have been found to act as free radical scavengers, and may contribute to the anti-oxidant properties of the extract [18]. Most of the beneficial effects of EGb 761 have been attributed to its anti-oxidant properties [19]. In this research, we have studied changes in body weight and absolute and relative kidney weight of albino rats in which lead toxicity was produced by injecting lead acetate intraperitoneally (IP). The changes were compared with rats of the normal group and EGb 761 treated groups. The present study was based on the assumption that G. biloba extract ameliorates the toxic effects of lead. |
| Materials and Methods |
| The study was conducted in the Institute of Basic Medical Sciences (IBMS), Dow University of Health Sciences Karachi (Pakistan). This study was approved by Ethical and Research Committee, Dow University of Health Sciences Karachi (Pakistan). |
| One hundred and twenty young male Wistar albino rats weighing between 150-180 grams were procured from the animal house of IBMS and divided into three groups. Each group was comprised of 40 animals. Each of the three groups A, B and C was further subdivided into four according to the duration of treatment, i.e. one, two, four and six weeks, and the numbers 1, 2, 3 and 4 were assigned to these, respectively. All the animals were tagged by punching their ears and were kept in well maintained scientific metallic cages under standard conditions with 12 hour day and night cycle. They were provided with food and water ad libitum and were kept under observation for one week prior to starting the experiment so as to get them acclimatized to the laboratory conditions. Throughout the experiment, the general behavior and activity of the animals was observed and any change was noted. The facilities in which the rats were maintained and the studies described were conducted in accordance with the Guide for Care and Use of Laboratory Animals provided by the Dow University of Health Sciences Karachi (Pakistan). |
| The Group A animals were given IP normal saline injection 1 ml per day until the time of their sacrifice whereas Group B animals were given a solution of lead acetate (Merck, Germany) dissolved in distilled water IP in a dose of 8mg/kg body weight daily [20], Group C animals were given daily 100 mg/kg body weight of EGb 761 orally [21], 2 hours prior to injecting lead acetate in saline solution. Body weights of the animals were noted with the help of a digital balance at the start of the experiment and at the time of sacrifice. At the end of the respective periods of treatment, the animals were anaesthetized with ether and the kidneys were retrieved after giving a midline incision in the abdomen. The kidneys were dried in filter paper and weighed on Sartorius balance. The mean weight of the kidneys was recorded. |
| The relative weight of the kidneys was calculated by the following formula: |
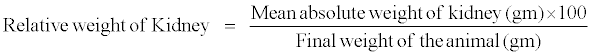 |
| Statistical Analysis |
| SPSS version 16 was used for statistical analysis of these data. Mean �?± standard deviation of the body and kidney weight was calculated for each group. The groups were compared using ANOVA (Analysis of Variance) and post hoc tests were applied where necessary. P-value>n 0.05 was considered statistically significant. |
| Observations and Results |
| All the animals in the control group remained alive and healthy throughout the period of treatment. There was gradual increase in their body weight and they responded promptly to the external stimuli throughout the treatment period. The lead treated animals on the other hand exhibited sluggish and lethargic behavior and showed slow response to the external stimuli. Moreover, their weight was also reduced gradually. The lead+EGb 761 treated group showed less drastic changes in body and kidney weight as compared to the lead-treated group. |
| Body weight of the animals was observed both at the start of the experiment and at the time of sacrifice, that is, at the end of one, two, four and six weeks. Difference in the initial body weight of all the three groups was statistically non-significant with a p-value<0.05. |
| There was a gradual and steady increase in the final body weight of the group A animals, as shown in Table 1. However in lead-treated group B animals, there was gradual weight loss, which when compared with Group A animals, was statistically non-significant after one week (p>0.05), but became highly significant after two, four and six weeks (p<0.001). |
| In EGb 761 treated Group C animals, there was a gradual increase in body weight which when compared with Group B animals, was statistically non-significant after one week (p>0.05), but became highly significant after two, four and six weeks (p<0.001). When compared with group A animals, the weight gain in group C animals was nonsignificantly lower after one and two weeks (p<0.05), whereas it was significantly lower after four weeks (p<0.01) and highly significant drop in weight gain was observed after six weeks (p<0.001). |
| Both kidneys of the animals were weighed at the time of sacrifice and an average was taken. As depicted in Table 2, the weight of the kidneys gradually increased in group A animals, whereas it gradually decreased in group B animals. The statistical difference was non-significant after one week (p > 0.05), whereas it was statistically highly significant after two, four and six weeks. The weight of the kidneys in group C animals also increased, although to a lesser extent as compared to the group A animals. When compared with group B animals, the change was nonsignificant after one week (p>0.05), whereas it was highly significant after two, four and six weeks (p<0.001). When compared with group A animals, the change in weight was statistically non-significant after one and two weeks (p>0.05), whereas it was significant after four weeks (p<0.01) and highly significant after six weeks (p<0.001). |
| Table 3 shows changes in the relative weight of the kidneys in different animal groups. The mean relative weight in group B animals when compared with group A was significantly higher after one, two and four weeks (p<0.001). However, the difference was non-significant after six weeks of treatment (p>0.05). |
| When the mean relative weight of kidneys in group C animals was compared with group A animals, the rise was statistically significant after one and two weeks (p<0.01), whereas it was non-significant after four and six weeks (p > 0.05), i.e. relative weight significantly dropped to control group. |
| Comparing the mean relative weight of the kidneys in group B with group C animals, it was significantly higher in group B animals after one, two and four weeks (p<0.001), whereas the difference was nonsignificant after six weeks (p>0.05). |
| Discussion |
| It was suggested that animals having a continuous exposure to heavy metals usually lose weight [22,23]. In this study we observed that lead administration resulted in striking reduction in body weight of the albino rats. Similar types of findings were observed in a study conducted by Rafique et al. in which lead was given IP to observe its toxic effects on testes of albino rats [8]. In our study, the weight loss was not much significant in B1 group, which is in agreement with a study by Khalil-Manesh et al. who observed rather an initial increase in the body weight, although less than the control group [24]. They attributed this gain to the initial increase in the absolute organ weight because of accumulation of lead in the form of nuclear inclusion bodies in the proximal tubular cells. However, overall loss of body weight with continuous exposure to lead might be explained on the basis of anorexia which is induced by heavy metal ingestion [25]. In a study conducted on Mediterranean fish species, it has been observed that a negative correlation exists between heavy metal levels and the size of the fish species [26]. Another possible explanation for the loss of body weight may be the decreased muscle mass and cachexia due to the oxidative stress induced by lead. Because there is evidence that heavy metal toxicity is associated with oxidative stress [19,27] which according to many researchers is associated with muscle wasting and cachexia leading to low body weight [10,11,28]. |
| In our study, although there was not a significant change in the kidney weight after one week, the relative weight of the kidney was significantly higher in this group as well as in groups B2 and B3. In group B4, the relative weight was comparable to the control group, although the absolute weight was markedly reduced. This means that the weight of the kidneys did not reduce as rapidly as the body weight after one, two and four weeks. Apart from nuclear inclusion bodies, another possible explanation for this relative increase in the kidney weight may be the initial DNA replication and proximal tubular proliferation induced by lead acetate, as proposed in a study carried out by Choie and Richter [29]. According to Vogetseder et al. [30] the rapid proliferation of proximal tubules may be in response to injury by the metal. The overall reduction in the absolute kidney weight observed in our study is in agreement with the study of Afonne et al. who observed decrease in weight of the kidneys of mice after administration of mercury [31]. |
| It has been suggested that one of the mechanisms of metal induced toxicities is the oxidative stress that results from depletion of the cellular anti-oxidants and enzymes [9], and several studies have approved the anti-oxidant role of EGb 761 [18,19]. The less dramatic changes in the body weight and weight of the kidneys in group C rats, which were given both lead acetate as well as EGb 761, may be attributed to the anti-oxidant property of EGb-761. Improvement in weight gain has also been observed in a study by Chao et al. in which they treated rats having duodenal ulcer by giving EGb 761 intravenously for one to two weeks, and attributed this weight gain to the anti-oxidant and cytoprotective effects of EGb 761 [32]. In a recent study conducted on old rats it was found that administration of EGb 761 was associated with increased performance and weight gain in the soleus muscle of the rats [33]. However this increase in muscular weight was associated with a decrease in body weight of the old rats. Thus, EGb 761 might have an optimizing effect on body weight, which is also observed in our study. |
| Conclusion |
| Lead hazards have not been fully wiped off from developing countries like ours, and people are still at risk of developing organ damage. Anorexia, weight loss and organ damage produced by lead toxicity can be prevented to large extent by giving some anti-oxidant medicine which is time-tested, cost-effective and easily available. EGb 761 is one of such medicines which not only ameliorates the toxic effects of metals, but is also beneficial in obesity related disorders. However, prior to generalizing the use of EGb 761, it is highly recommended to conduct clinical trials to appreciate its benefits in humans. |
References
|
Tables and Figures at a glance
| Table 1 | Table 2 | Table 3 |
Relevant Topics
- Analytical Biochemistry
- Applied Biochemistry
- Carbohydrate Biochemistry
- Cellular Biochemistry
- Clinical_Biochemistry
- Comparative Biochemistry
- Environmental Biochemistry
- Forensic Biochemistry
- Lipid Biochemistry
- Medical_Biochemistry
- Metabolomics
- Nutritional Biochemistry
- Pesticide Biochemistry
- Process Biochemistry
- Protein_Biochemistry
- Single-Cell Biochemistry
- Soil_Biochemistry
Recommended Journals
- Biosensor Journals
- Cellular Biology Journal
- Journal of Biochemistry and Microbial Toxicology
- Journal of Biochemistry and Cell Biology
- Journal of Biological and Medical Sciences
- Journal of Cell Biology & Immunology
- Journal of Cellular and Molecular Pharmacology
- Journal of Chemical Biology & Therapeutics
- Journal of Phytochemicistry And Biochemistry
Article Tools
Article Usage
- Total views: 14617
- [From(publication date):
July-2013 - Sep 18, 2024] - Breakdown by view type
- HTML page views : 10056
- PDF downloads : 4561
