Large-size Intramuscular Nodular Fasciitis; A Challenging Histopathologic Diagnosis Confirmed by Molecular Detection of USP6 Gene Rearrangement: A Case Report and Literature Review
Received: 07-Apr-2022 / Manuscript No. DPO-22-59355 / Editor assigned: 11-Apr-2022 / PreQC No. DPO-22-59355(PQ) / Reviewed: 21-Apr-2022 / QC No. DPO-22-59355 / Revised: 29-Apr-2022 / Manuscript No. DPO-22-59355(R) / Published Date: 07-May-2022 DOI: 10.4172/ 2476-2024.7.S11.001
Abstract
The intramuscular subtype of Nodular Fasciitis (NF) is rare with lesions normally not more than 2 cm in size and characterized by pseudosarcomatous morphology. This paper presents the case of a 27-year-old man with a large-size intramuscular NF. The patient came for treatment complaining of an increasingly enlarged mass in the left upper arm for 4 months. Magnetic Resonance Imaging (MRI) confirmed the presence of a well-defined tumor measuring 5 cm within the outer edge of the middle humerus. Microscopically, the neoplasm was rich in fibroblasts and myofibroblasts in an interlaced pattern with high mitotic index and evident multinuclear giant cells. Erythrocyte extravasation was easily seen in the stroma. The tumor border was infiltrative. Immunohistochemically, the tumor cells were positive for Smooth Muscle Action (SMA) and negative for cytokeratin, desmin, CD34, S100, ALK, and β-catenin. Molecular detection demonstrated evidence of Ubiquitin-Specific Peptidase 6 (USP6) gene rearrangements in this tumor. Based on the findings, the tumor was diagnosed as intramuscular NF. At 56 months after the initial surgery, the patient had recovered with no evidence of recurrence or metastasis.
Keywords: Intramuscular nodular fasciitis; Nodular fasciitis; Ubiquitin-specific peptidase
Abbreviations
NF: Nodular Fasciitis; MYH9-USP6: Myosin Heavy Chain 9/Ubiquitin-Specific Peptidase 6; MRI: Magnetic Resonance Imaging; SMA: Smooth Muscle Actin; FISH: Fluorescence In Situ Hybridization; LGMFS: Low-Grade Myofibroblastic Sarcoma; LGFMS: Low-Grade Fibromyxoid Sarcoma
Introduction
Nodular Fasciitis (NF) is a self-limiting fibrous neoplasm harboring the fusion gene Myosin Heavy Chain 9/Ubiquitin-Specific Peptidase 6 (MYH9-USP6) as a recurrent somatic gene fusion event, as described in the current World Health Organization classification [1-5]. NF is mainly divided into three subtypes based on anatomical location: subcutaneous (most common), fascia, and intramuscular [6]. Of these, intramuscular NF is a rare subtype sharing similar clinical, histologic, and immunohistochemical features, as well as the gene expression profile, with subcutaneous NF [6]. Normally, the lesions of intramuscular NF are less than 2 cm in size, but occasionally can be >4 cm, which can be easily over-diagnosed as a soft tissue sarcoma because of the “sarcoma-like” morphology. Here, we present a case of intramuscular NF on the upper arm of a young man with a lesion 5 cm in size that was initially suspected as a sarcoma and diagnosed by detecting the ectopic rearrangement of USP6 gene.
Case Presentation
A 27-year-old man came for treatment complaining of a progressively enlarging mass on his left upper arm over 4 months with occasional slight discomfort. He reported no history of trauma, infection, or prior surgery of the lesion. Magnetic Resonance Imaging (MRI) confirmed the presence of a well-defined mass located deeply in the outer edge of the middle humerus muscle, but at the focal border, it had protruded into the surrounding muscle. The signal intensity was slightly enhanced on T1-weighted imaging (Figure 1A) and marked heterogeneous enhancement on T2-weighted imaging (Figure 1B). The MRI findings suggested the possibility of fibromatosis. The patient underwent surgery for mass excision. On gross examination, it was an irregular, solitary mass measuring 5.0 × 4.0 × 4.0 cm, in the muscle with a grayish-white cut surface (Figures 1A and 1B).
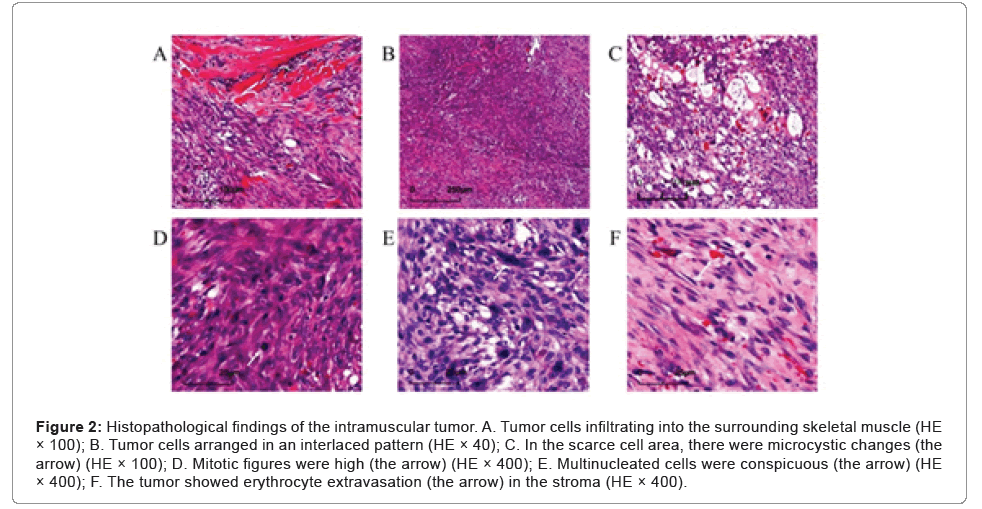
Microscopic examination showed that the tumor was relatively well demarcated, but in the focal, it was infiltrating the adherent muscle (Figure 2A). It was highly cellular and composed of fibroblasts and myofibroblasts arranged in bundles with an interlaced pattern (Figure 2B). Part of the lesion was discohesive and myxoid with a microcystic appearance (Figure 2C). Some tumor cells had mild atypia with active mitotic figures (mitotic rate up to 20 per 10 high-power fields, (Figure 2D) and mingled conspicuous multinuclear or atypical giant cells (Figure 2E). Erythrocyte extravasation was easily seen on a background of collagenous stroma (Figure 2F), with some hemosiderin deposition and scattered lymphoplasmacytic cells infiltration (Figures 2A-2F).
Figure 2: Histopathological findings of the intramuscular tumor. A. Tumor cells infiltrating into the surrounding skeletal muscle (HE × 100); B. Tumor cells arranged in an interlaced pattern (HE × 40); C. In the scarce cell area, there were microcystic changes (the arrow) (HE × 100); D. Mitotic figures were high (the arrow) (HE × 400); E. Multinucleated cells were conspicuous (the arrow) (HE × 400); F. The tumor showed erythrocyte extravasation (the arrow) in the stroma (HE × 400).
Imunohistochemical detection, the primary antibodies used were SMA (clone UMAB237, 1:300 dilution), CD34 (clone EP88, 1:150 dilution), S100 (clone 15E2E2+4C4.9, 1:400 dilution), ALK (clone 1A4, 1:100 dilution), CK (clone AE1/1E3, 1:150 dilution), Desmin (clone OTI4A8, 1:150 dilution), CD68 (clone KP1, 1:200 dilution), β-catenin (clone UMAB15, 1:50 dilution), Ki-67 (clone UMAB107, 1:100 dilution). All antibodies were purchased from Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd, PR China. The secondary antibody of Horseradish Peroxidase (HRP) labeled pika universal antibody (GK500705) and DAB chromogenic solution were purchased from Dako Company. Immunohistochemistry adopted En-Vision two- step method. After deparaffinization and hydration of tumor sections, high temperature and high-pressure antigen repair (10% citrate antigen repair buffer, pH 6.0) was performed, and 100 μl primary antibodies was added to each section. Incubated overnight at 4°C, wash, incubated with 100 μl secondary antibody for 30 minutes at room temperature, wash, DAB color, and observed the results with a Leica microscope (DM2500). The tumor cells were only positive for Smooth Muscle Actin (SMA) (Figure 3A), negative for cytokeratin, desmin, CD34, S100, ALK, and β-catenin. The multinuclear giant cells were positive for CD68 (Figure 3B). The Ki67-positive rate was 10% (Figure 3C). According to histopathological and immunohistochemical studies, fibromatosis was first excluded for the tumor’s growth pattern and β-catenin negative. However, it was still difficult to distinguish the tumor from a (myo) fibroblastic sarcoma.
Molecular detection was performed on 4 μm paraffin-embedded tumor section by Fluorescence In Situ Hybridization (FISH) using a USP6 dual-color break-apart probe. The DNA probe USP6 (17p13) Gene Break Double Fluorescence Probe Kit (catalogue number F.01229-01) was purchased from Guangzhou LBP Medicine Science and Technology Co., Ltd., Guangzhou, PR China, and the green signal was labeled at the telomere end, the red signal was marked at the centromere end. Taken 10 uL of the probe working solution and dropped it on the tissue section, placed the section on a hybridization instrument, co-denaturation at 85°C for 5 minutes, hybridization at 42°C for 12 hours. Washed and dried after hybridization, added 10 uL 4’,6-Diamino-2-Phenylindole (DAPI) counterstain, and observed the result under Leica fluorescence microscope (DM2500). The FISH results revealed evidence of USP6 gene rearrangement given the division of green and red signals (Figure 3D). Molecular findings confirmed the diagnosis of intramuscular NF. At 56 months after surgery, the patient recovered well with no evidence of recurrence or metastasis by the imaging examination (Figures 3A-3D).
Figure 3: Immunohistochemical staining. A. Tumor cells were strongly immuno-reactive for SMA. (× 100); B. The multinuclear giant cells were positive for CD68 (× 100); C. Ki-67 proliferation index was 10% (× 100); D. FISH illustrated rearrangement of the USP6 gene locus 17p13 using a break-apart probe set, with separation of red and green signals (the arrow).
Results and Discussion
Intramuscular NF is a rare benign fibrous tumor, accounting for only 5.9% of 272 NF cases in a previous retrospective study [6]. Unfortunately, there is limited datas about intramuscular NF, particularly regarding the clinicopathological features. As far as we know, until now, only seven cases of intramuscular NF have been reported, including our case, in the English literature via a search of the PubMed database [7-12]. All seven cases are reviewed and summarized in Table 1. Although there was no predominant gender difference (male to female ratio, 3:4) among these seven cases, there was a wide age range from 11 to 46 years (mean age, 32.5 years). Tumors were mainly found in deep locations of the limbs and trunk, including the thigh, rectus abdominis muscle, gluteal region, right axillary tail, erector spinae muscle, neck, and upper arm. The preoperative duration was relatively short, no more than 2 months in four cases, while our case was 4 months, and the other two were over 1 year in duration. All seven patients complained of pain, from mild to severe. Other clinical symptoms included nerve compression, swelling, and numbness. The patient in this report just felt a little discomfort, but the mass in the upper arm gradually increased. The follow-up periods ranged from 6 months to 10 years. Five patients recovered with no evidence of recurrence or metastasis. However, one patient developed multiple recurrences and ultimately metastasis at their 10-year follow- up, which was caused by inadequate surgery for the complexity of the deep anatomical location and poorly infiltrated border. Fortunately, our patient had been followed up 56 months with no signs of recurrence by the adequate surgery. Grossly, the maximum dimensions ranged from 1.8 to 10 cm (median, 4.9 cm) with grayish-white and solid cut surfaces. The lesions in three cases were >4 cm in size with less defined borders including our case. Microscopically, most cases had hyper and hypocellular areas with a focally myxoid or microcystic appearance. The predominant growth pattern in the hypo cellular areas was S- or C-shaped fascicles, or storiform-like. The tumor cells all were fibroblasts or myofibroblasts with plump and regular spindle- shaped nuclei lacking hyperchromatism and pleomorphism. Mitotic figures were active, but no atypical forms were present. Most cases had a typical background of collagen and erythrocyte extravasation with or without lymphocyte infiltration and hemosiderin deposition. However, in distinct contrast, in the present case, (myo) fibroblasts were mildly atypic with many multinuclear giant cells mixed, it was extremely mimic soft tissue sarcoma. Immunohistochemically, the immunophenotype of the tumor cells was (myo) fibroblastic expressing SMA (Table 1).
| Case(Ref) | Age(years)/Gender | Preoperative symptom | Preoperative duration (months) | Tumor location | Tumor size(cm) | Tumor border | Molecular genetics | Follow-up (months) |
|---|---|---|---|---|---|---|---|---|
| 1 (7) | 11/F | Occasional discomfort | 2 | Rectus abdominis muscle | 2.6 | Well-circumscribed | ND | NEOD after 6 |
| 2 (9) | 19/M | Dull ache and swelling | 24 | Left gluteal region | 10 | Locally infiltrative margin | ND | NEOD after 6 |
| 3 (10) | 31/F | Persistent pain | 2 | Right axillary tail | 1.8 | Well-circumscribed | ND | NR |
| 4 (8) | 42/F | Nerve compression, severe pain and numbness | 12 | Proximal right thigh | 2.5 to 9.7 | Extensively infiltrative margin | PPPR6-USP6 gene fusion | Four times recurrences and eventually local multiple metastasis during 120 |
| 5 (11) | 46/M | Back pain | 1 | Right erector spinae muscle | 2.8 | Well-circumscribed | ND | Spontaneous regression after 1 |
| 6 (12) | 45/F | Neck swelling | 2 | over right side of neck | 3 | Partially capsulated and surrounded by skeletal muscle fibers | ND | NEOD after 6 |
| 7 (present case) | 27/M | Little discomfort | 4 | Left upper arm | 5 | Locally infiltrative margin | USP6 gene rearrangement | NEOD after 6 |
Note: F: Female; M: Male; ND: Not Done; NEOD: No Evidence of Disease; NR: Not Reported
Table 1: Clinicopathological and genetic features of the current and previously reported cases of intramuscular NF.
USP6 (17p13), a deubiquitinating protease involved in cell trafficking, protein degradation, signaling, and inflammation, is a valuable adjunct for the diagnosis of intramuscular NF. Initially, a chromosome 17p13 rearrangement was observed as an oncogenic-activated event in aneurysmal bone cysts [13-18]. In 2011, Erickson-Johnson et al observed that the MYH9 promoter region fused with the entire coding region of USP6, which resulted in overexpression of USP6 in NF [1]. Based on this observation, they were the first to put forward a new term of transient neoplasia induced by MYH9-USP6 gene fusion in NF. In 2014, a study by Oliveira et al confirmed that detection of USP6 genomic rearrangements was valuable for the diagnosis of NF, since they had observed high sensitivity and specificity (respectively 93% and 100%) using FISH for USP6 in their clinical practice [3]. Subsequently, several other studies also found that USP6 rearrangements in the NF has the same characteristics [4,5]. However, in 2017, a study by Patel et al identified seven other novel pathogenic fusions of USP6 in NF by RT-PCR, including RRBP1, CALU, CTNNB1, MIR22HG, SPARC, THBS2, and COL6A2 [19]. Thus, in our clinical practice, we prefer to use the USP6 break-apart probe to detect USP6 gene translocation in NF. However, USP6 gene rearrangement is not a unique genetic alteration for aneurysmal bone cysts and NF, as it can exist in multiple lesions, including myositis ossificans, giant cell lesions of the small bones, fibromas of the tendon sheath, and fibro-osseous pseudotumors of the digits [13,16,20-25]. These lesions may belong to the same disease spectrum, but the clinical features, histopathological characteristics, and gene fusions differ.
Owing to its pseudosarcoma-like morphology, deep location, and invasive border, large size intramuscular NF is easily misdiagnosed as other soft tissue sarcomas or neoplasms. A study by Erber et al in 2018 found that only 33% of 71 lesions were correctly diagnosed as NF, including intramuscular NF, and 33% were initially over-diagnosed as malignant tumors or low malignant potential tumors [5]. The main differential diagnoses include Low-Grade Myofibroblastic Sarcoma (LGMFS), which is also composed of spindle cells arranged in bundles and storiform pattern. But by contrast, LGMFS have moderate cellular atypia and diffusely infiltrative margin, local recurrence is frequent, even at multiple sites, and treatment should combine complete surgical excision with radiotherapy [26]. Intramuscular NF thus must be differentiated from LGMFS. However, both lesions show that the (myo) fibroblastic immunophenotyped, FISH detection of USP6 gene rearrangement is essential for differential diagnosis of LGMFS and intramuscular NF. Low-Grade Fibromyxoid Sarcoma (LGFMS) is also similar to intramuscular NF, both with a background of myxoid and collagenous stroma. However, LGFMSs commonly show a mixed composition of alternately distributed collagen like and mucoid areas, and nearly 40% of LGFMSs are scattered with giant collagen rosettes [27]. LGFMS is specifically expressed MUC4 but not SMA, and FUS fusion gene exists in most cases [28]. Fibromatosis is another (myo) fibroblast neoplasm which is characterized by the proliferation of elongated, uniformly shaped spindle cells in the collagenous stroma, with obvious infiltrating borders, and shows nuclear β-catenin positive for the β-catenin (CTNNB1) gene mutation [29].
Most patients with intramuscular NF recovered well after adequate surgery, and local recurrence was quite rare following incomplete resection [8,30]. However, among the seven reported cases of intramuscular NF, one presented as a low-grade malignant tumor that developed multiple recurrences and eventually metastasized during the 10-year follow-up period. Molecular analysis showed that there was an amplified new fusion gene PPPR6-USP6 [8]. Different USP6 fusion genes may have various biological behaviors. Further research is required to confirm the relationship between the behaviors of intramuscular NFs and different pathogenic fusion partners of USP6.
Conclusion
In conclusion, the clinicopathological characteristics of intramuscular NF were based on previous reports and the present case. A histopathological diagnosis of a large-size intramuscular NF is challenging due to the pseudosarcomatous morphology, larger size, deep location, and infiltrative margin. Fsortunately, USP6 gene rearrangement is a valuable adjunct for the differential diagnosis of intramuscular NF and other tumors. Further investigations are required to detect different pathogenic fusion partners of USP6, which would be meaningful to expand the biological potentials of intramuscular NF.
Acknowledgement
The authors would like to thank International Science Editing for their language editing Service.
Funding
Not applicable.
Availability of Data and Materials
The data used to support the findings of this study are available from the corresponding author upon request.
Authors’ contributions
CRW designed the study and wrote manuscript. WW and RJX collected clinical in-formation and associated literature of NF. JJX contributed to the revision of manuscript. All authors read and approved the final manuscript.
Ethics Approval and Consent to Participate
The study was approved and consented by the Ethics Committee of the Affiliated Hangzhou First People’s Hospital, Zhejiang University School of Medicine.
Patient consent for publication
The written consent to publish was obtained from the patient.
Competing Interests
The authors declare that they have no competing interests.
References
- Tan P H, Ellis I, Allison K, Brogi E, Fox, SB, et al. (2020) The 2019 World Health Organization classification of tumours of the breast. Histopathology 77:181-185.
[Crossref] [Google Scholar] [Pubmed]
- Tan BY, Acs G, Apple SK, Badve S, Bleiweiss IJ, et al. (2016) Phyllodes tumours of the breast: A consensus review. Histopathology 68:5-21.
[Crossref] [Google Scholar] [Pubmed]
- Krings G, Bean GR, Chen YY (2017) Fibroepithelial lesions; The WHO spectrum. Semin Diagn Pathol 34:438-452.
[Crossref] [Google Scholar] [Pubmed]
- Vasanthakumar A, Godley LA (2015) 5-hydroxymethylcytosine in cancer: significance in diagnosis and therapy. Cancer Genet 208:167-77.
[Crossref] [Google Scholar] [Pubmed]
- Mariani CJ, Madzo J, Moen EL, Yesilkanal A, Godley LA (2013) Alterations of 5-hydroxymethylcytosine in human cancers. Cancers (Basel) 5:786-814.
[Crossref] [Google Scholar] [Pubmed]
- Chapel DB, Husain AN, Krausz T (2019) Immunohistochemical evaluation of nuclear 5-hydroxymethylcytosine (5-hmC) accurately distinguishes malignant pleural mesothelioma from benign mesothelial proliferations. Mod Pathol 32:376-386.
[Crossref] [Google Scholar] [Pubmed]
- Lian CG, Xu Y, Ceol C, Wu F, Larson A, et al (2012) Loss of 5-hydroxymethylcytosine is an epigenetic hallmark of melanoma. Cell 150:1135-46.
[Crossref] [Google Scholar] [Pubmed]
- Tsai KW, Li GC, Chen CH, Yeh MH, Huang JS, et al. (2015) Reduction of global 5-hydroxymethylcytosine is a poor prognostic factor in breast cancer patients, especially for an ER/PR-negative subtype. Breast Cancer Res Treat 153:219-34.
[Crossref] [Google Scholar] [Pubmed]
- Huang KT, Dobrovic A, Yan M, Karim RZ, Lee CS, et al. (2010) DNA methylation profiling of phyllodes and fibroadenoma tumours of the breast. Breast Cancer Res Treat 124:555-65.
[Crossref] [Google Scholar] [Pubmed]
- Kim JH, Choi YD, Lee JS, Lee JH, Nam JH (2009) Borderline and malignant phyllodes tumors display similar promoter methylation profiles. Virchows Arch 455: 469-75.
[Crossref] [Google Scholar] [Pubmed]
- Schnitt SJ, Collins, LC (2013) Biopsy interpretation of the breast. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins.
- Laé M, Vincent-Salomon A, Savignoni A, Huon I, Fréneaux P, et al. (2007) Phyllodes tumors of the breast segregate in two groups according to genetic criteria. Mod Pathol 20:435-44.
[Crossref] [Google Scholar] [Pubmed]
- Lv S, Niu Y, Wei L, Liu Q, Wang X, et al. (2008) Chromosomal aberrations and genetic relations in benign, borderline and malignant phyllodes tumors of the breast: a comparative genomic hybridization study. Breast Cancer Res Treat 112:411-8.
[Crossref] [Google Scholar] [Pubmed]
- Ng CC, Tan J, Ong CK, Lim WK, Rajasegaran V et al. (2015) MED12 is frequently mutated in breast phyllodes tumours: a study of 112 cases. J Clin Pathol 68:685-91.
[Crossref] [Google Scholar] [Pubmed]
- Yoshida M, Ogawa R, Yoshida H, Maeshima A, Kanai Y, et al. (2015) TERT promoter mutations are frequent and show association with MED12 mutations in phyllodes tumors of the breast. Br J Cancer 113:1244-8.
[Crossref] [Google Scholar] [Pubmed]
Citation: Wang C, Wang W, Xu R, Xiang J (2022) Large-size Intramuscular Nodular Fasciitis; A Challenging Histopathologic Diagnosis Confirmed by Molecular Detection of USP6 Gene Rearrangement: A Case Report and Literature Review. Diagnos Pathol Open S11 :001. DOI: 10.4172/ 2476-2024.7.S11.001
Copyright: © 2022 Wang C, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Open Access Journals
Article Tools
Article Usage
- Total views: 5748
- [From(publication date): 0-2022 - Nov 18, 2025]
- Breakdown by view type
- HTML page views: 5196
- PDF downloads: 552