Commentary Open Access
Lactate Dehydrogenase-5 (Ldh-5) Immunohistochemical Expression as Predictor of Efficacy of First-Line Therapy in Patients with Advanced Colorectal Cancer Treated with Chemotherapy and Bevacizumab
Garde-Noguera J1*, Gil-Raga M2, Evgenyeva E3, Bernet L4, Llombart-Cussac A1 and Camps-Herrer C5
1Medical Oncology Department, Hospital Arnau de Vilanova, Valencia, Spain
2Medical Oncology Department, Hospital de Sagunto, Valencia, Spain
3Anatomic Pathology Department, Hospital Marina Salud, Denia, Spain
4Anatomic Pathology Department, Hospital Arnau de Vilanova, Valencia, Spain
5Medical Oncology Department, Hospital General Universitario de Valencia, Spain
- *Corresponding Author:
- Javier Garde-Noguera
Medical Oncology Department
Hospital Arnau de Vilanova
Valencia, Spain
Tel: 34699307265
E-mail: javiergarde@icloud.com
Received date: August 19, 2016; Accepted date: September 13, 2016; Published date: September 15, 2016
Citation: Garde-Noguera J, Gil-Raga M, Evgenyeva E, Bernet L, Llombart-Cussac A, et al. (2016) Lactate Dehydrogenase-5 (Ldh-5) Immunohistochemical Expression as Predictor of Efficacy of First-Line Therapy in Patients with Advanced Colorectal Cancer Treated with Chemotherapy and Bevacizumab. J Clin Exp Pathol 6: 293. doi:10.4172/2161-0681.1000293
Copyright: © 2016 Garde-Noguera J, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Clinical & Experimental Pathology
Abstract
Purpose: The aim of this study is to analyse the prognostic role of LDH5 expression measured by immunohistochemistry in patients with advanced colorectal cancer treated with standard chemotherapy with or without bevacizumab. Methods: Retrospective multicentre study, carried out at four hospitals in the Valencian Community (Spain). We investigated the immunohistochemical expression of LDH-5 in a series of 112 patients with advanced colorectal adenocarcinomas treated with oxaliplatin-based chemotherapy with or without bevacizumab. Results: Histological samples for the LDH5 analysis were available for 87 of the 112 patients selected. Sixteen (18.3%) had undetectable or mild expression of LDH5 (Group 1) and 71 (87.7%) showed moderate or high expression of LDH5 (Group 2). Response rate in Group 1 was 56.2% compared to 60.8% in Group 2 (p=0.47). Progression-free survival (PFS) 11 vs. 12 months (p=0.28), and overall survival (OS) 20 vs. 24 months, (p=0.17), were numerically but not significantly higher in patients from Group 2 vs. Group 1. Patients from Group 2 who received bevacizumab presented a significantly higher PFS (13 vs. 12, p=0.039) and a numerically higher OS (27 vs. 20 months, p=0.27) than those treated exclusively with chemotherapy. Conclusions: Our results suggest that the absence or low expression of LDH5 is not associated with a better prognostic profile in patients with advanced colorectal cancer treated with chemotherapy and bevacizumab. Patients with high expression of LDH5, benefit from the combination of chemotherapy with bevacizumab.
Keywords
Advanced colorectal cancer; LDH5 expression; Angiogenesis
Introduction
Colorectal cancer (CRC) is the third the most common malignancy, and is responsible for approximately 10% of cancer deaths in both sexes [1]. Over more than 40 years, the only treatment for patients with advanced CRC was chemotherapy. At first, with 5-fluorouracil (5FU), achieved a response rate of 10-20% as a single agent [2]. The addition of oxaliplatin to chemotherapy regimens became a step forward for these patients, as it added efficacy in terms of increased both response rates (RR) and overall survival (OS) [3]. Next big advance in the field was the arrival to the therapeutic arsenal of bevacizumab (Avastin), a humanised monoclonal antibody capable to inhibit vascular endothelial growth factor, a key mediator of angiogenesis [4-6]. Several randomised clinical trials have demonstrated the benefit of the addition of bevacizumab to the various standard chemotherapy regimens in patients with advanced CRC: 5-fluorouracil/leucovorin (5- FU/LV) [7], 5-FU/LV plus irinotecan (IFL) [8], 5-FU/LV plus oxaliplatin (FOLFOX) [9] and 5-FU/LV plus capecitabine (XELOX) [10]. With increases in response rates (RR), OS and progression free survival (PFS). The combination XELOX/bevacizumab offers a PFS of 9.4 months and an OS of 21.3 months [10] and is currently one of the most widely used first-line treatment regimens in patients with advanced CRC. The efficacy obtained by the addiction of an antiangiogenic drug to chemotherapy seems to be due to the inhibition of the formation of new vessels, and the normalisation of abnormal tumour vasculature [11,12]. Since Bevacizumab was approved and incorporated into first line treatment protocols, an intense effort in the search of biomarkers has been done. Identification of molecular markers capable of predicting patients’ prognosis and the response to cytostatic and anti-angiogenic treatments is a challenging task, since these potential markers would allow selecting the patients who would benefit the most from each of the drugs available. Unfortunately, despite the different attempts carried out, there has been no success to relate several molecular markers with tumour response to the treatment with bevacizumab; therefore, currently there is no way to predict in advance which patients will benefit from this treatment [13]. It is still unknown the place where anti-VWGF therapies produce its main effect, they might have a direct effect on the tumour cell, but it could also base its activity on the endothelial cells of the blood vasculature and/or the lymphatic vessels supporting the tumour, where it has been detected a overexpression of VEGFR-2 (KDR) and VEGFR-3 (13) receptors. VEGFR-2 is involved in the initiation of molecular pathways that induce proliferation and survival of endothelial cells [14]. Overexpression of the activated form of VEGFR-2 or pKDR measured by immunohistochemistry as well as of lactate dehydrogenase 5 (LDH5), protein involved in the regulation of the transformation of pyruvate to lactate in anaerobic conditions for acquisition of energy, and therefore an indirect marker cellular hypoxia, have been associated with worse prognosis in patients with colon cancer, and are postulated as markers of angiogenesis process [15]. Lactate dehydrogenase 5 (LDH5) is one of the five LDH isoenzymes and, apparently, the most important for promoting anaerobic glycols. LDH5 is transcriptionally regulated by the hypoxia inducible factors (HIF) 1-alpha and 2-alpha. LDH5 up-regulation has been related with HIF1a and HIF2a accumulation, what leads to a VEGF activation, increased vascular density and also with extramural invasion, followed by nodal and distant metastases [16]. These observations suggest that a high LDH5 content in tumour cells is directly related to an up-regulated HIF pathway and is linked with an aggressive phenotype in colorectal cancer in fact, LDH-5 has been associated with a worse prognosis in patients with CRC who have undergone surgery [17]. The objective of our study was to retrospectively evaluate the prognostic role of LDH5 expression in patients with advanced CRC with non-resectable metastasis who have received treatment with oxaliplatin and fluoropyrimidine-based chemotherapy, with and without bevacizumab. In addition, we analysed the clinical variables associated with the expression of LDH5 as well as its influence on the effectiveness of the anti-angiogenic treatment.
Materials and Methods
This is a retrospective multicentre study of patients diagnosed with stage IV colorectal cancer treated in four hospitals of Valencian Community over three years with oxaliplatin and fluoropyrimidine combination chemotherapy, with or without bevacizumab.
Patients’ characteristics
We performed a systematic review of patients diagnosed with stage IV colorectal cancer with histological confirmation of colorectal adenocarcinoma and treated between January 2011 and December 2014 with first-line standard chemotherapy regimen based on Oxaliplatin and Fluoropyrimidines (FOLFOX or XELOX), either in combination or not with bevacizumab. The evaluation method consisted in a review of medical records and baseline tumour radiologic measurements before the start of the treatment. Baseline disease extension and tumour response evaluation was carried out by CT scans. Blood laboratory data were collected from each test prior to each cycle of chemotherapy to assess blood counts and kidney and liver function. Tumour response was evaluated according to RECIST criteria. OS was calculated as the period from the start of treatment until the date of death.
Immunohistochemistry
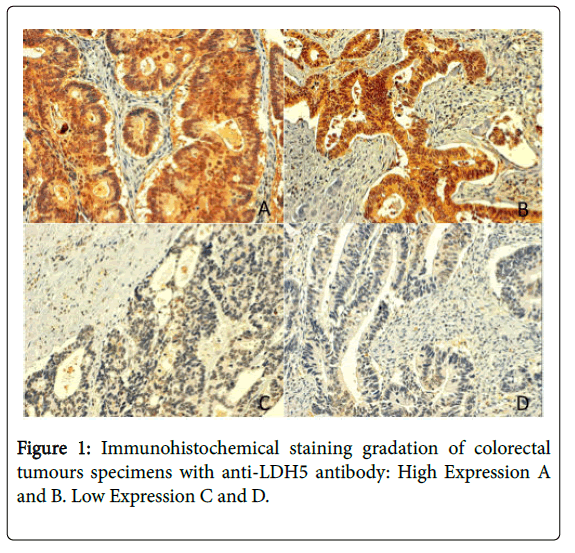
After an initial review of all the samples of haematoxylin and eosinstained tumour biopsies, a paraffin block representative of each of the study subjects was selected. The blocks selected had to include viable tissue and were used to create a tissue microarray (TMA), with two tumour cylinders for each patient. Once the TMAs had been put together, consecutive sections of 4 lm were created. One of the sections from each sample was stained with haematoxylin and eosin, and the subsequent sections were immunofixed with anti-LDH5 antibodies. The immunofixation was performed using the immunoperoxidase technique followed by predigestion and trypsinization. The antibody used was sheep polyclonal antibody 9002 (Abcam, Cambridge, UK) raised against human LDH5, purified from human placenta was used for immunohistochemistry [17]. The percentage of cancer cells with strong cytoplasmic and nuclear LDH5 expression was assessed separately, following inspection of the entire embedded tissue. In each optical field, the percentage was recorded and the final score for each case was the mean value obtained. Values lower than 10% were scored as 0%. The expression of LDH5 was quantified using a grading system that classified the samples into 2 groups: high/mild vs. low/negative expressing tumours based on previous studies (Figure 1) [14].
Statistical methods
All the statistical analyses and the results were processed using the statistical software SPSS 19.0. A descriptive statistical analysis was carried out, including central tendency and dispersion parameters for the quantitative variables, and absolute and relative frequencies for categorical variables. The Chi-squared test was used to compare two or more independent subject groups for categorical variables. The survival curve was estimated using the Kaplan–Meier method and compared using the log-rank test.
Results
Patient characteristics
In our database, we found 112 patients diagnosed with advanced CRC treated with oxaliplatin and fluoropyrimidines combination chemotherapy (XELOX or FOLFOX) with or without bevacizumab. Eighty-seven of them had sufficient material for histological analysis of LDH5 immunohistochemistry. Sixteen patients (18.3%) had low expression (group 1); while seventy-one patients (81.7%) showed strong expression of LDH 5 (group 2): 20 nuclear expression in >10% of cells with no cytoplasmic expression, 16 strong cytoplasmic expression without nuclear expression, and 35 over-expression both nuclear and cytoplasmic. Patients’ characteristics are summarised (Table 1). The median age of patients in Group 1 was 71 years, and 68 years in Group 2. No significant differences were observed between the groups in terms of sex, performance status (PS), and presence of KRAS mutations, number of metastatic sites or the presence of rectal bleeding at the time of diagnosis. The percentage of patients who received treatment with Bevacizumab was lower in Group 1 (37.5 vs 69%, p=0.02), and this group also showed a non-significant tendency towards an inferior rate of serum LDH elevation [18,19].
| Global population N=112 |
GRUPO 1. Low LDH5 expression N=16 |
GRUPO 2 High LDH5 expression N=71 |
p Value | |
|---|---|---|---|---|
| Median Age | 68 years | 71 years | 68 years | |
| Sex Male/Female | 66/46 (58.4 vs. 40.7%) | 9/7 (56 vs. 44%) | 44/27 (61.9 vs. 38.1%) | 0.4 |
| PS 0-1 vs. 2 | 87 vs. 22 (77 vs. 23%) | 11 vs. 5 (68.7 vs. 31.3%) | 58 vs. 13 (81.6 vs. 18.4%) | |
| High blood pressure at time of diagnosis | 43 (38%) | 4 (70%) | 33 (54%) | 0.1 |
| Weight loss >10% | 21 (22.8%) | 4 (30.7%) | 12 (21.4%) | 0.34 |
| Rectal bleeding | 35 (37.6) | 6 (46.1%) | 21 (36.8%) | 0.37 |
| Occlusive Symptoms | 10 (10.8%) | 1 (7.6%) | 6 (10.7%) | 0.6 |
| >1 metastatic site | 52 (46.4%) | 7 (43%) | 31 (43.6%) | 0.86 |
| High serum CEA | 75 (79.7%) | 10 (66.6%) | 48 (78.6%) | 0.25 |
| Anaemia | 47 (54.6%) | 5 (35.7%) | 27 (47.3%) | 0.31 |
| High serum LDH | 34 (52.3%) | 3 (33%) | 22 (46.8%) | 0.35 |
| Chemotherapy with Bevacizumab | 75 (66.9%) | 6 (37.5%) | 49 (69%) | 0.02* |
| KRAS mutations | 42 (51%) | 4 (40%) | 28 (52%) | 0.3 |
Table 1: Patient’s characteristics.
Treatment and response rate
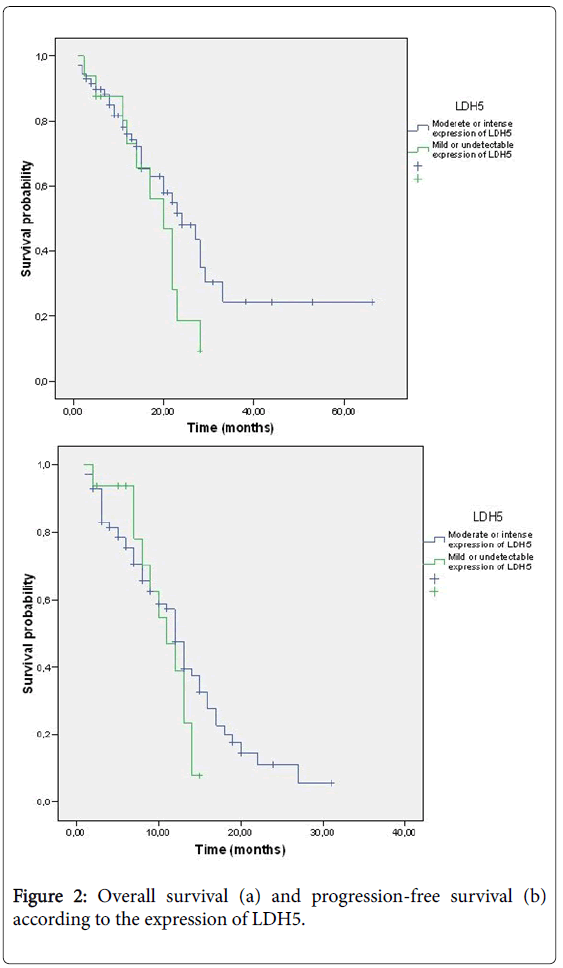
Of the 16 patients in Group 1, 6 (37.5%) received chemotherapy in combination with bevacizumab, while the other 10 received only chemotherapy. Of the 71 patients in Group 2, 49 (69%) received chemotherapy with bevacizumab, and the other 22 (31%) received chemotherapy (Figure 2). A total of 80 patients were evaluable in terms of response; all the patients in Group 1 and 64 from Group 2. The seven non-evaluable patients died before the re-evaluation (5 after the second cycle of chemotherapy and 2 after the first cycle) (Table 2).
| Response | GROUP 1 Low LDH5 |
GROUP 2 High LDH5 |
p Value |
|---|---|---|---|
| Complete Response Partial Response |
0 9 (56.2%) |
1 (1.5%) 38 (59.3%) |
0.47 |
| Stable Disease | 5 (31.2%) | 14 (21.8%) | |
| Progression Disease | 2 (12.5%) | 7 (10.9 %) |
Table 2: Response to treatment.
The treatment RR in the patients with low LDH5 expression was 56.2% (9 PR), compared to 60.8% in the group of patients with high LDH5 expression (1 complete response, 37 partial responses, 19 stable disease and 13 progressive disease); differences in the RR between the two groups were not statistically significant (p=0.47). There were no significant differences in the efficacy of chemotherapy and bevacizumab in Group 2 according to the location of LDH5 expression: cytoplasmic vs. nuclear vs. both (87.5% vs. 55% vs. 51.6%, p=0.09).
Overall survival and progression-free survival
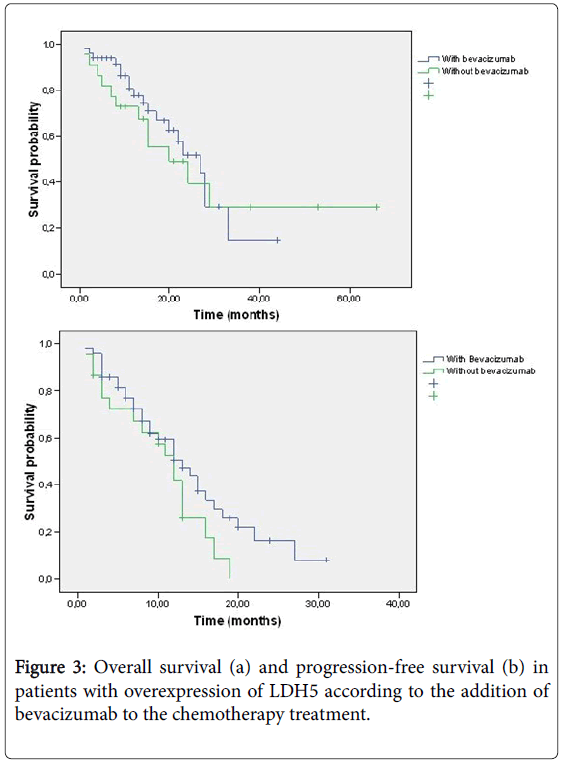
The median survival in Group 1 was 20 months, compared to 24 months in Group 2 (p=0.17). There was neither difference in PFS, 11 months vs . 12 months in Group 1 and 2 respectively (p=0.28). The median OS in those patients in Group 2 who received treatment with chemotherapy in combination with bevacizumab was 27 months, compared to 20 months in the patients treated with chemotherapy alone (p=0.27). PFS was significantly higher in Group 2 for patients treated with bevacizumab (13 vs. 12 months, p=0.039) (Figure 3).
Discussion
This retrospective study was designed to analyse the prognostic role of the overexpression of lactate dehydrogenase 5 (LDH5) in patients diagnosed with advanced colorectal adenocarcinoma treated with chemotherapy with oxaliplatin and fluoropyrimidines with and without bevacizumab. Our study did not find any relationship between the overexpression of LDH5 with an increased Response Rate, neither with significant differences in PFS or OS based on its presence.
The frequency of LDH5 overexpression in advanced colorectal cancer tumour cells detected in our series of patients was 87.7%. The elevated rate of expression of this angiogenesis marker is in line with other research published to date. Kourkourakis et al. reported a series of 128 patients with resected colon cancer (stages IIa-III) in which the rate of LDH5 overexpression was 77.3% [17], and same authors communicated in another series with 75 patients diagnosed with advanced colorectal cancer, a LDH5 overexpression rate of 68% [16]. Our findings confirm that this protein is highly expressed in colorectal tumours.
LDH5 is one of the five LDH isoenzymes and, apparently, the most important for promoting anaerobic glycolysis. LDH5 is transcriptionally regulated by the hypoxia inducible factors (HIF) 1a and 2a, and its overexpression has been related with aggressive advantages that colorectal tumours may gain from a high LDH5 content, for instance the increase capability for lymph node involvement and distant metastases spread [16]. In fact, its presence has been associated with worse outcome in patients with stage I-III operable colorectal cancer, who presented a poorer survival when compared with those with negative expression of LDH5 (HR 15.1, p=0.0003) [17]. Similar results have been communicated in patients diagnosed of advanced Non-Small Cell Lung Cancer (NSCLC) [20], were a significantly poorer survival was noted in the group of patients with high LDH5 immunohistochemical reactivity, both when measured at cytoplasm and at nucleus. In addition, Scartozzi et al. observed similar findings in patients with high LDH serum levels [21], who presented a significant shorter PFS (4.2 vs. 8 months, p=0.003) and OS (19.6 vs. 34.9 months, p=0.0014), when compared with patients with low LDH levels in serum. Despite all this data, in our series the high expression of LDH5 was not associated with classical clinical factors associated with worse prognostic features, such as loss of weight, bad performance status or more than one metastatic site. It should be pointed out that those patients with low LDH5 expression presented similar clinical characteristics to those with high expression, for instance we observed no differences neither in levels of serum CEA or serum LDH, or rectal bleeding or occlusive symptoms. This contrast with what our group observed in the prognostic role of other angiogenesis marker, VEGFR-2/pKDR in a previous report [22], were patients with an overexpression of this protein showed a worse clinical profile. VEGFR-2/KDR is the main receptor of the vascular endothelial growth factor (VEGF) and is frequently overexpressed in CRC. The binding of VEGF to VEGFR-2/KDR leads to phosphorylation, and the activation of an intracellular signal pathway, which ultimately potentiates processes such as cell proliferation, migration, apoptosis inhibition, and maturation of endothelial cell vascular structures [23,24]. An association between LDH-5 and pKDR expression in cancer cells and in tumour-associated vasculature was recorded in 128 colorectal carcinomas examined. In fact, 75% and 60% of tumours with high LDH-5 expression had high pKDR expression in cancer cells and in vessels, respectively. These percentages were clearly lower (30% and 23%, respectively), in tumours with low LDH-5 expression [17].
As said, we have not found any differences en response rate or progression-free and overall survival in our analysis. It is important to mention that there was significant difference in the rate of patients treated with bevacizumab between patients with low and high LDH5 expression (37,5 vs. 69%, p=0.02), which means, that patients with the hypothetical worse molecular feature had been treated with a more active treatment schedule. This important imbalance could explain the non-statistically significant but numerically better outcome of patients with LDH5 overexpression. Furthermore, the retrospective nature of this study carried out based on small sample of patients, with different stage of the disease from previous series, makes it very difficult to do any comparison.
The analysis of patients with LDH5 overexpression according to treatment with anti-angiogenics confirms that patients treated with bevacizumab presented a longer PFS, and showed a favourable trend in terms of OS compared to those treated exclusively with chemotherapy. The limited number of patients with low LDH5 expression identified in our study prevented us to analyse the benefit that bevacizumab could add to chemotherapy in patients with low expression of this angiogenesis marker, and also prevented us to assess the hypothetical prognostic role of that marker in this context. Previous reports found similar results regarding to the better outcome of patients with overexpression of pro-angiogenic proteins when treated with Bevacizumab. Both patients with high levels of pKDR measured by immunohistochemistry, and patients with high serum level of LDH presented a benefit in RR, PFS and OS, when compared with patients with high expression of these markers and treated with chemotherapy alone [21,22]. All these experiences show that the addition of bevacizumab to chemotherapy permits to improve clinical outcome in a subgroup of patients who usually present with an adverse natural history. In this context, bevacizumab appears to be capable to reverse the poor prognosis conditioned by tumour biology. The finding of an improved response rate and median PFS for patients with high LDH5 expression levels receiving bevacizumab over patients with the same LDH5 overexpression not receiving bevacizumab could represent corroboration to this observation. At the moment, we still do not have response predicting markers for anti-angiogenics such as bevacizumab in advanced CRC, and their identification continues to be a challenge for which in-depth knowledge of the biological processes involved in angiogenesis is essential.
In conclusion, the results of our study suggest that LDH5 overexpression is observed in the majority of patients with advanced CRC. The low expression of LDH5 does not seem to be related to a patient profile with better prognostic clinical characteristics, and in our series it was not associated with favourable Response Rate, OS or PFS. Patients with high expression of LDH5 showed a significant improvement of PFS and numerically higher OS when treated with bevacizumab.
Compliance with Ethical Standards
Due to the characteristics of the study, the ethics committee approved the conduction of the study without obtaining the informed consent from the participating patients since a previous consent for a prior analysis of biomarkers in tumour samples had been obtained and most of the patients had died at the moment of the current analysis.
Research involving human participants and/or animals Ethical standards: the study was approved by an Ethics Committee of Clinical Research and was conducted according to the 1964 Declaration of Helsinki for studies in humans and its later amendments. This article does not contain any studies with animals performed by any of the authors.
Acknowledgment
The study was funded by a grant for projects of emerging groups in care centres for the promotion of health research in Valencia (Spain) in 2011.
References
- Garfinkel L, Mushinski M (1999) U.S. cancer incidence, mortality and survival: 1973-1996. Stat Bull MetropInsur Co 80: 23-32.
- Bleiberg H (1996) Role of chemotherapy for advanced colorectal cancer: new opportunities. SeminOncol 23: 42-50.
- de Gramont A, Vignoud J, Tournigand C, Louvet C, André T, et al. (1997) Oxaliplatin with high-dose leucovorin and 5-fluorouracil 48-hour continuous infusion in pretreated metastatic colorectal cancer. Eur J Cancer 33: 214-219.
- Ferrara N (2001) Role of vascular endothelial growth factor in regulation of physiological angiogenesis. Am J Physiol Cell Physiol 280: C1358-1366.
- Ferrara N, Gerber HP, LeCouter J (2003) The biology of VEGF and its receptors. Nat Med 9: 669-676.
- Jain RK (2001) Normalizing tumor vasculature with anti-angiogenic therapy: a new paradigm for combination therapy. Nat Med 7: 987-989.
- Kabbinavar FF, Hambleton J, Mass RD, Hurwitz HI, Bergsland E, et al. (2005) Combined analysis of efficacy: the addition of bevacizumab to fluorouracil/leucovorin improves survival for patients with metastatic colorectal cancer. J ClinOncol23: 3706-3712.
- Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, et al. (2004) Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 350: 2335-2342.
- Giantonio BJ, Catalano PJ, Meropol NJ, O’Dwyer PJ, Mitchell EP, et al. (2007) Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200. J ClinOncol25: 153-1544.
- Saltz L, Clarke S, Diaz-Rubio E, Scheithauer W, Figer A, et al. (2008) Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J ClinOncol26: 2013-2019.
- Kerbel R, Folkman J (2002) Clinical translation of angiogenesis inhibitors. Nat Rev Cancer 2: 727-739.
- Jain RK (2005) Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science 307: 58-62.
- Smith NR, Baker D, James NH, Ratcliffe K, Jenkins M, et al. (2010) Vascular endothelial growth receptors VEGFR-2 and VEGFR-3 are localized primarily to the vasculature in human primary solid cancers. Clin Cancer Res 16: 3548-3561.
- Karkkainen MJ, Petrova TV (2000) Vascular endothelial growth factor receptors in the regulation of angiogenesis and lymphangiogenesis. Oncogene 19: 5598-5605.
- Gerber HP, Ferrara N (2005) Pharmacology and pharmacodynamics of bevacizumab as monotherapy or in combination with cytotoxic therapy in preclinical studies. Cancer Res 65: 671-680.
- Koukourakis MI, Giatromanolaki A, Simopoulos C, Polycronidis A, Sivridis E (2005) Lactate dehydrogenase 5 (LDH5) relates to up-regulated hypoxia inducible factor pathway and metastasis in colorectal cancer. ClinExpMetast22: 25-30.
- Koukourakis MI, Giatromanolaki A, Sivridis E, Gatter KC, Harris AL (2006) Tumour Angiogenesis Research Group. Lactate dehydrogenase 5 expression in operable colorrectal cancer: strong association with survival and activated vascular endothelial growth factor pathway. A report of the tumour angiogenesis research Group. J ClinOncol24: 4301-4308.
- Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, et al. (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45: 228-247.
- Zaman K, Ryu H, Hall D, O´Donovan K, Lin KI, et al. (1999) Protection from oxidative stressinduced apoptosis in cortical neuronal cultures by iron chelators is associated with enhanced DNA binding of hypoxia-inducible factor-1 and ATF-1/CREB and increased expression of glycolytic enzymes, p21(waf1/cip1), and erythropoietin. J Neurosci19: 9821-9830.
- Koukourakis MI, Giatromanolaki A, Sivridis E, Bougioukas G, Didilis, et al. (2003) Lactate dehydrogenase-5 (LDH-5) overexpression in non-small-cell lung cancer tissues is linked to tumour hypoxia, angiogenic factor production and poor prognosis. Br J Cancer 89: 877-885.
- Scartozzi M, Giampieri R, Maccaroni E, Del Prete M, Faloppi L, et al. (2012) Pre-treatment lactate dehydrogenase levels as predictor of efficacy of first-line bevacizumab-based therapy in metastatic colorectal cancer patients. Br J Cancer 106: 799-804.
- Garde-Noguera J, Gil-Raga M, Evgenyeva E, Garcia JA, Llombart-Cussac A, et al. (2016) High pKDR immunohistochemical expression is an unfavourable prognostic biomarker in patients with advanced colorectal cancer treated with chemotherapy plus bevacizumab. Clin Trans Oncol 18: 405.
- Takahashi T, Shibuya M (1997) The 230 kDa mature form of KDR/Flk-1 (VEGF receptor-2) activates the PLC-gamma pathway and partially induces mitotic signals in NIH3T3 fibroblasts. Oncogene 14:2079-2089.
- Quinn TP, Peters KG, De Vries C, Ferrara N, Williams LT(1993) Fetal liver kinase 1 is a receptor for vascular endothelial growth factor and is selectively expressed in vascular endothelium. ProcNatlAcadSci USA90: 7533-7577.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 12147
- [From(publication date):
October-2016 - Apr 02, 2025] - Breakdown by view type
- HTML page views : 11257
- PDF downloads : 890