Research Article Open Access
Laboratory Experiment of Advanced Oxidation Process Coupled Coagulation, Flocculation, and Biological Treatment
| Bhaskar G*, Naidu GRK and Madan Iyengar | ||
| Department of Environmental Sciences, Sri Venkateswara University College of Sciences, Sri Venkateswara University, India | ||
| Corresponding Author : | Bhaskar G Department of Environmental Sciences Sri Venkateswara University College of Sciences Sri Venkateswara University, India Tel: 91-877-2241526 E-mail: envibhaskar@gmail.com |
|
| Received August 15, 2014; Accepted November 25, 2014; Published November 28, 2014 | ||
| Citation: Bhaskar G, Naidu GRK, Iyengar M (2014) Laboratory Experiment of Advanced Oxidation Process Coupled Coagulation, Flocculation, and Biological Treatment. J Bioremed Biodeg 5:264. doi:10.4172/2155-6199.1000264 | ||
| Copyright: © 2014 Bhaskar G, et al. This is an open-a ccess article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | ||
Related article at Pubmed Pubmed  Scholar Google Scholar Google |
||
Visit for more related articles at Journal of Bioremediation & Biodegradation
Abstract
In recent environmental regulations have been stringent and requiring high quality of treated wastewater and also there is an increasing trend to require more efficient method wastewater from industries. We have developed a new treatment method called an advanced oxidation process coupled coagulation, flocculation, and biological treatment based on laboratory experiments to treat wastewater. The aim of research work was select optimal conditions of laboratory experiment. In this experiment NOXXALL is liquefying ozone, hydrated lime, poly aluminium chloride, polyelectrolyte, and intermittent cycle extended aeration system were used an oxidizing agent, coagulants, flocculent, and an aerobic treatment respectively. In this method reduction of suspended solids, total dissolved solids, chlorides, sulphate, biological oxygen demand, chemical oxygen demand, oil and grease, phenols, and arsenic were 97,54,79,78,98,96,85,66,53% respectively. Zebra fish used in bio assay test, and were survived 100% in final treated wastewater.
| Keywords |
| NAXXALL liquid; Hydrated lime; Poly aluminium chloride; Poly electrolyte; ICEAS; Hydroxyl radicals (–OH); Wastewater |
| Introduction |
| Currently water pollution is major problem faced by everyone all over the globe [1]. It is now pushing the government, industrialist, scientists and research scholar to develop new wastewater technologies with viable cost and more effective to treat different types of wastewater adequately before discharge into land and/or water bodies. The rapid industrialization has resulted in the rise of pollution [2]. Biotech industries are playing a vital role in economic growth of nations and also have high impact on environment with respective of water consumption and wastewater generation with high concentration of pollutants. The level of wastewater pollution varies from industry to industry depending on the type of process and size of industry. The pollution of water bodies by toxic materials from industrial effluent affects humans both directly and indirectly [3]. Wastewater comes from different processes takes place in same industry and poses different characteristics of wastewater depending upon process in day to day. Adaption of specific treatment for each process unit of wastewater is highly expensive. So common treatment system is required to treat total generation of wastewater from industries in order to reduce cost of treatment. |
| Nowadays advanced oxidation technologies (AOTs) are successfully adapting with coagulation and/ or biological treatment to treat various types of wastewater. These integrated treatments have received great attention in research and development of wastewater treatment technologies in the recent years. Advanced oxidation processes (AOPs) are used to oxidize complex organic constituents found in wastewater that are difficult to degrade biologically into simpler end products [4]. Which are facilitating the conversion of pollutants to less harmful and more biodegradable compounds [5]. AOPs may be coupled with other physical and chemical methods, and biological for mineralisation [6]. Several studies have been reported on the examination of coagulation, flocculation for the treatment of industrial wastewater [7]. |
| There are some literature papers on combination of advanced oxidation process with coagulation and/ or biological treatment. In the study of Alessandra Cesaro et al. [8], discussed most common AOPs used as pre-treatment of wastewater for its biological processing in order to highlight the enhancement of wastewater biological treatability supplied by different advanced oxidation methods. In the study of Vimala Ebenezerl et al. [9], the ozonation at flow rate of 0.015 m3/min and contact time of 1 h followed by reaction with Fenton's reagent for 1 h achieved 81% and 75% removal of colour and COD. This effluent from ozonation is carried over to sequential batch reactor which gave a removal efficiency of colour and COD at overall 90% and 88% respectively. In study of Van Huu Tap and Trinh Van Tuyen [10], landfilleachate was used for removal of COD, and color by using coagulation and ozonation processes to determine the optimal reaction conditions. The removal of COD and color in the pre-treatment stage (coagulation-PAC) was approximately 30 and 70%, respectively and ozonation process was applied to leachate at pH 8, after coagulation and achieved highest removal of COD and colour were 57 and 8%, respectively. In the study of Sangav et al. [11], laboratoryscale experiments were conducted in order to investigate the effect of ozone as pre-aerobic treatment and post-aerobic treatment for the treatment of the distillery wastewater. This integrated process (ozoneaerobic oxidation-ozone) achieved 79% COD reduction along with the complete discolouration of the effluent sample as compared to 34.9 % COD reduction for non ozonated sample. In the study of Hamzeh ali Jamali et al. [12], an integrated technique consisted of coagulation/ flocculation and ozonation process was examined to enhance COD and colour reduction and increasese the biodegradability of leachate for further treatment. In study of Noor Ainee Noor et al. [13], to determine the efficiency of polyaluminum chloride (PACL) in removing chemical oxygen demand (COD), ammoniacal (NH3-N) and colour from two different landfill sites in Malaysia. |
| There are varies technologies available to produce the reactive hydroxyl free radicals (–OH) in aqueous phase and oxidation power of varies oxidizing agents are presented in Tables 1 and 2 respectively [4]. |
| Objective of research work was characterization of wastewater mainly evaluated in terms of pH, suspended solids, total dissolved solids (TDS), biological oxygen demand (BOD), chemical oxygen demand (COD), oil & grease, chloride, phenols, arsenic, determine toxicity factor of treated wastewater, and select optimal conditions of experiment. |
| Materials and Methods |
| Source and characteristic of wastewater |
| Wastewater samples were collected from equalization tank of treatment plant, located at Anthem Biosciences, Bangalore, Karnataka state, India. And used for this research work. Characteristics of wastewater are essential in planning to evaluate laboratory experiment and presented in column 4 of Table 3. NOXXALL is nano engineered with a unique formulation and special process of manufacture into liquefying ozone. It was used for decontamination of wastewater, and it reverts back into oxygen and CO2. NOXXALL liquid oxidation power is 2.06 eV and reaction summary is as below. |
| NOXXALL → liquid + contaminants in wastewater free radicals + less harmful contaminants + H2O + CO2 |
| Experimental set up and procedures |
| This experimental setup was divided into 3 steps and conducted at ambient temperature. Advanced oxidation process coupled coagulation, and flocculation process, and Biological treatment coupled advanced oxidation process followed by sand and activated filters. Determine toxicity factor of treated wastewater. |
| Advanced oxidation process coupled coagulation and flocculation: This experiment was carried out at 15, 30, and 45 minutes with different pH and dosage of the NOXXALL liquid, coagulants (hydrated lime & poly aluminium chloride), and flocculent (Polyelectrolyte) by using Jar apparatus test to evaluate efficiency of this experiment. The control of pH is very important, maximum oxidation occurring between pH 6 to 9 [14]. The following optimal conditions were selected to obtain high quality of treated wastewater. |
| Wastewater samples were collected from equalization tank. Sample of pH was 8.0. Five beakers were filled with 500 ml of wastewater. To this 0.4 ml of NAXXALL liquid was dosed to each beaker and stirred it at 100 rpm for 2 minutes and allowed for 30 minutes of oxidation process. Hydrated lime was dosed to each beaker to raise pH between 8.5 to 9.5, and samples were agitated at a flash mixing speed of 100 rpm for 2 minutes. After that polyaluminum chloride was dosed to each beaker to bring down pH between 6.5-7.5, subsequently diluted polyelectrolyte was dosed to these samples and agitated at 60 rpm for 3 minutes. After that agitation was stopped and flocks were allowed to settle down for 15 minutes. Same experiment was carried out 15 times to get 15 litres of treated wastewater. Treated wastewater was stored in dosing tank and used for further removal of pollutants through aerobic treatment process. |
| Biological treatment coupled advanced oxidation process followed by sand and activated carbon filters: 25 litres of glass tank was used an intermittent cycle extended aeration tank and 4 air lines were connected from main airline, which comes from air supply pump. 15 litres of treated wastewater was introduced into pre-react zone using a diaphragm dosing pump, and it flowed through openings at the bottom of baffle wall into main react zone. 2850 ml of domestic sludge was seeded to it. Oxygen was supplied approximately 0.05 kg during 11 hours contact time of air and wastewater in aerobic treatment. Dissolve oxygen was 4-5 mg/L during aeration time. Air was stopped to settle down sludge, and 10 litres of clear treated wastewater was discharged into 20 litres of plastic cans by fixed pipe with manual open/close valve. To this 2.5 ml of NOXALL liquid was dosed and agitated it gently for 2 munities and allowed for 30 minutes of oxidation process. After that sample was passed through sand and granular activated carbon filters. The size of sand, and granular activated carbon was 0.5mm, and 12Χ40 mm respectively. Each filters 2 litres with dia of 10 centimetres. Final treated wastewater sample was analysed to evaluate efficiency of laboratory experiment. Results of treated wastewater are presented in colum 05 of Table 3. Stage wise reductions of biological oxygen demand and chemical oxygen demand in this experiment are presented in Table 4. |
| Determination toxicity factor of treated wastewater: Bioassay method was used to evaluate acute toxicity of treated wastewater by using toxicity factor to zebra fish (Brachydario rerio). 20 zebra fish (brachydanio rerio) and two test vessels were used in this test. 5 fish were used to two litres of each test vessel, and test duration was 48 hours. Treated wastewater toxicity factor (TF) was 01, and zebra fish were survived 100% in final treated wastewater. The test was conducted strictly as per procedure prescribed in Indian standard 6582 (Part 2):2001, bio-assay method for evaluating acute toxicity of industrial effluents and waste waters, part 2 using toxicity factor to zebra fish (brachydanio rerio). |
| Analytical methods |
| To quantify efficiency of laboratory experiment, wastewater samples were analysed before, and after experiment as per APHA et al. [15]. Standard methods for the examination of water and wastewater, 19th edition, Washington, USA. |
| Results and Discussion |
| Each test was performed three times, and average results of advanced oxidation process coupled coagulation, flocculation, and biological treatment followed by sand and activated carbon filters are presented in Table 3, and stage wise reduction of biological oxygen demand and chemical oxygen demand are presented in Table 4, and discussed here. |
| Colour and odour |
| Colour industrial wastewaters may require colour removal before discharge into water body and/or on land. It is important parameter from the standpoint of aesthetics. Wastewater was muddy colour and treated water was colour less. Offensive odour can cause poor appetite impaired respiration, nausea and vomiting, and mental perturbation. In extreme situation offensive odour can lead to the deterioration of personal and community. We have observed that wastewater was unpleasant odour and treated wastewater was odour free. |
| pH |
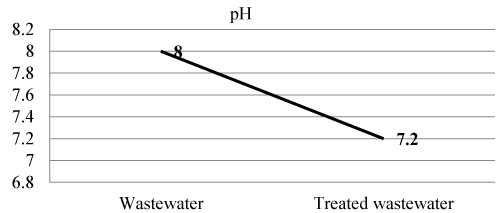
| Measurement of pH in one of the most important and frequently used test in water and wastewater treatment. The effect of pH on the chemical and biological properties of liquid makes its determination very important. The extremes of pH of wastewater are generally not acceptable as extremes of pH cause problems to survival of aquatic life. It also interferes with the optimum operation of wastewater treatment facilities. Water with high or low pH is not suitable for irrigation. At low pH most of the metals become soluble therefore could be hazardous in the environment. At high pH most of the metals become insoluble and accumulate in the sludge and sediments [16]. pH of Wastewater and treated wastewater shown in Figure 1 and Table 3. |
| Suspended solids |
| Suspended solids consists slit, clay, fine particles inorganic and organic matter, soluble organic compounds and microorganisms. It will play an important role in selection of chemical coagulation and biological treatment process. Suspended solids presence in water sample cause depletion of oxygen level. |
| Suspended solids in wastewater, and treated wastewater shown in Figure 2 and Table 3. The efficiency of laboratory experiment was 97% in removal of suspended solids. |
| Total dissolved solids |
| Excess amount of dissolved substances are undesirable in water and wastewater. Dissolved minerals, gases, and organic constituents may produce aesthetically displeasing colour, tastes, and odour. Total dissolved solids are mainly inorganic matters. There are a large variety of salts such as chlorides, carbonates, bicarbonates, nitrates, phosphates and sulphates of calcium, magnesium, sodium, potassium, iron etc which import certain taste to water. If any one of these becomes in excess, the water is unfit for drinking and on long range use in irrigation causes salinity of soil. On evaporation of such waters, the salts are left behind on soil surface in form of thin crust. The high level of total dissolved solids will interfere biological treatment process, and soil contamination. It is important that total dissolved solids should be brought down to required level before passes into biological treatment, and treated wastewater discharged on land and/or water bodies. |
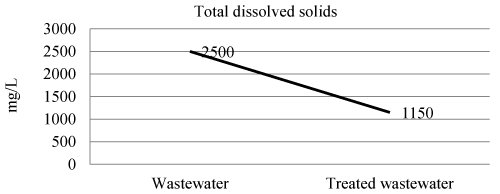
| Total dissolved solids of wastewater and treated wastewater shown in Figure 3 and Table 3. The efficiency of laboratory experiment was 54% in removal of total dissolved solids. |
| Chlorides |
| Chlorides are major inorganic anions in water and wastewater. Discharges of chloride content beyond maximum permissible limit (250 mg/L) can result in contamination of both surface water and groundwater and corrode the metals. The salty taste produces by chloride depends on the chemical composition of water. Chloride content evaluation in water used for irrigation gain more important because salty water after evapo-transpiration tend to settle in the soil and creates difference in the osmotic pressure of water thus blocking the uptake by the roots. This affects the yield of crops and therefore chloride content is restricted to waters used for irrigation salt-sensitive crops. It is interesting to note that in areas where water supply is very scarce, chloride level is very high. Determination of chlorides very important parameter in wastewater and ensure chlorides bring down to required level before discharge into water bodies and/ or on land. |
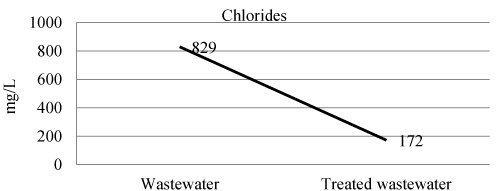
| Chlorides of wastewater, and treated wastewater shown in Figure 4 and Table 3. The efficiency of laboratory experiment was 79% in removal of chlorides. |
| Sulphate |
| High amounts of sulphate salts may give an offensive taste in drinking water at concentration ranges from 250 to 1000 mg/L. Sulphates salts may increase corrosive properties. When treated wastewater discharged into aquatic system in excess amount, they cause cathartic effect in living organisms. |
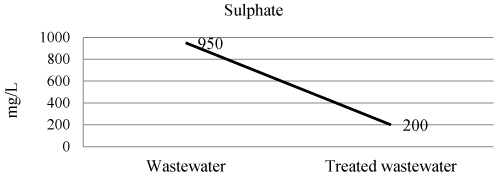
| Sulphate of wastewater and treated wastewater shown in Figure 5 and Table 3. The efficiency of laboratory experiment was 78% in removal of sulphate. |
| Biological oxygen demand |
| Biological oxygen demand is of, the amount of oxygen that require for bacteria to degrade the organic components present in water/ wastewater. This test is used in selection of biological treatment method and to evaluate efficiency of treatment in terms of biological oxygen demand. |
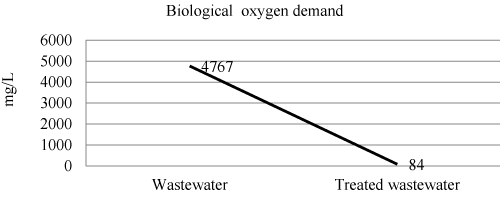
| Biological oxygen demand of wastewater and treated wastewater shown in Figure 6 and Table 3. The efficiency of laboratory experiment was 98% in removal of biological oxygen demand. |
| Chemical oxygen demand |
| Chemical oxygen demand is the total measurement of all chemicals (organic and in-organic) in the water/wastewater. This test can be used in evaluate the amount of oxidizable and non-oxidizable wastes in water and wastewaters. |
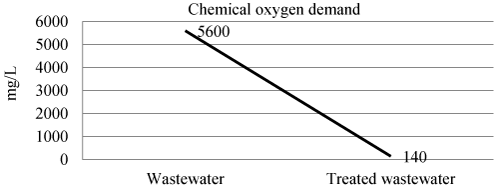
| Chemical oxygen demand in wastewater, and treated wastewater shown in Figure 7 and Table 3. The efficiency of laboratory experiment was 97% in removal of chemical oxygen demand. |
| Oil and grease |
| Oil and grease present in excessive amounts, they may interfere with aerobic and anaerobic biological processes and lead to decreased efficiency of wastewater treatment. Determination of oil and grease in domestic and industrial wastewater helps to choose the suitable treatment method for its removal and helps in evaluation of efficiency of the treatment employed. |
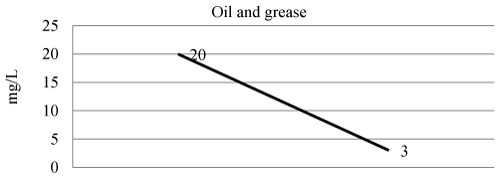
| Oil and grease in wastewater and treated wastewater shown in Figure 8 and Table 3. The efficiency of laboratory experiment was 85% in removal of oil and grease. |
| Phenols as phenolic compounds (C6H5OH) |
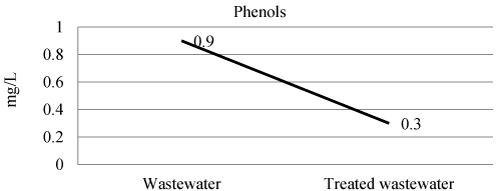
| Phenols, defined as hydroxyl derivatives of benzene and its condensed nuclei, may occur in industrial wastewater. It may be toxic to phyto and zoo-plankton beyond certain level. A total of 1.5 g may be fatal. Ozone is used effectively to reduce the concentration of phenols in the wastewater [17]. Commonly used methods for the treatment of phenol bearing wastewater include solvent extraction, physical absorption, chemical oxidation and aerobic biological processes. Phenols in wastewater and treated wastewater shown in Figure 9 and Table 3. The efficiency of laboratory experiment was 66% in removal of phenols. |
| Arsenic |
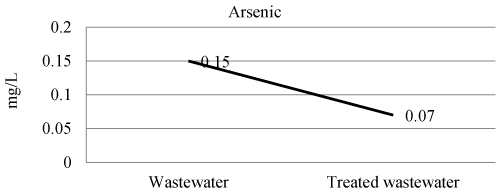
| Chronic effects may result from the accumulation of arsenic compounds in the body at low intake levels. The toxicity of arsenic depends on its chemical form. The United Nations Food and Agriculture Organisation recommended maximum level for irrigation waters in 100 μg/L. The U.S EPA primary drinking water standard is 0.05 mg/L. Arsenic in wastewater and treated wastewater shown in Figure 10 and Table 3. The efficiency of laboratory experiment was 53% in removal of arsenic. |
| Bio-assay test |
| Bio-assay test was used to evaluate acute toxicity of treated wastewater. Acute toxic effects of treated wastewater determined in terms of death of fish in test water. A fish is declared death if it does not move when touched. Counted dead of fish in each vessel at 2 h, 6h, 24 h, and 48 hours. Toxicity factor of treated wastewater was 01. Zebra fish were survived 100 % in treated wastewater during 48 hours of test period. On this observation treated wastewater not much harm to aquatic environment. |
| Conclusion and Recommendation |
| NOXXALL was used in this advanced oxidation process to generate reactive hydroxyl radicals (OH-) in aqueous phase. The experimental conditions are as follows NOXXALL was applied at pH 8. pH was maintained 8.5 to 9, and 6.5 to 7.5 in coagulation, and flocculation process respectively. Contact time of NOXXALL liquid with wastewater and treated wastewater was 30 minutes, 0.4, and 0.25 ml of NOXXALL liquid dosed to 500 ml of wastewater, and 1 litre of treated wastewater of ICEAS respectively. Oxygen was supplied approximately 0.05 kg during 11 hours contact time of air and wastewater in aerobic treatment. 0.5 mm size of sand and 12Χ40 mm size of 1200 iodine value (mg/gm) of granular activated carbon used in sand and activated filters respectively. Each filter dia was 10 cm with two litres capacity. Determined acute toxicity factor of treated wastewater was 01, and zebra fish were survived 100% during 48 hours of test period in tested treated water. Results are indicated that it has been proven more effective method to treat wastewater. |
| we are suggesting interested students, research scholar, scientists and industrialists on water and wastewater treatment technologies to evaluate NOXXALL liquid alone and/ or combination with other physical, chemical, and biological treatment method in laboratory scale and full scale treatment system. |
| Acknowledgements |
| It is my pleasure to express my gratitude and sincere thanks to Dr.T. Dhamodharam, Head, Department of Environmetal Sciences, Sri Venkateswar University, Tirupati, Andhara Pradesh, India. Management and emplyees of Anthem Biscinecs Pvt Ltd, Bangalore, karnataka state, India. Mr. Srinivas, Director -OZO SCIENCES, Bangalore, Karnataka State, Bangalore, India for thier able guidance, encouragement and co operation, throught my research work. |
References
|
Tables and Figures at a glance
| Table 1 | Table 2 | Table 3 | Table 4 |
Figures at a glance
 |
 |
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 | Figure 4 | Figure 5 |
 |
 |
 |
 |
 |
| Figure 6 | Figure 7 | Figure 8 | Figure 9 | Figure 10 |
Relevant Topics
- Anaerobic Biodegradation
- Biodegradable Balloons
- Biodegradable Confetti
- Biodegradable Diapers
- Biodegradable Plastics
- Biodegradable Sunscreen
- Biodegradation
- Bioremediation Bacteria
- Bioremediation Oil Spills
- Bioremediation Plants
- Bioremediation Products
- Ex Situ Bioremediation
- Heavy Metal Bioremediation
- In Situ Bioremediation
- Mycoremediation
- Non Biodegradable
- Phytoremediation
- Sewage Water Treatment
- Soil Bioremediation
- Types of Upwelling
- Waste Degredation
- Xenobiotics
Recommended Journals
Article Tools
Article Usage
- Total views: 15606
- [From(publication date):
December-2014 - Apr 04, 2025] - Breakdown by view type
- HTML page views : 10893
- PDF downloads : 4713
