Knowledge and Prevalence of Malaria among Rural Households in Ghana
Received: 15-Oct-2019 / Accepted Date: 02-Jan-2020 / Published Date: 10-Jan-2020 DOI: 10.4172/2161-0711.1000673
Abstract
Background: Malaria remains a global pressing issue despite several interventions to reduce its prevalence. This study aimed at determining the knowledge and prevalence of malaria among rural households in the Western-North region of Ghana.
Method: This was a cross-sectional study conducted in three rural communities in the Bibiani Anhwiaso-Bekwai Municipality of the Western-North region of Ghana. A total of 481 participants from 155 randomly selected households were screened for malaria using rapid diagnostic test (RDT). Socio-demographic data, information related to ITN and knowledge of malaria were collected using a semi-structured questionnaires. Multivariate logistic regression analysis was performed to determine independent association of variables with malaria.
Results: Malaria prevalence among participants was 39.1%. Prevalence was significantly higher among females (23.0%) and children under 5 years (12.6%) (p<0.05). Household ownership (83.9%) and the use of ITN (96.2%) were high in the Municipality. Although most participants had poor knowledge of malaria in terms of its transmission, the overall knowledge was good (54.2%). Having poor knowledge increased the risk of malaria infection (AOR=3.1, CI=0.89-10.7, p=0.07).
Conclusion: The prevalence of malaria was high among the study participants particularly among females and children under 5 years. Most of the participants had good knowledge of malaria in-terms of causative agent, signs and symptoms and preventive measures. However, knowledge on transmission pathways of malaria was poor. There is the need for increased community sensitization on malaria transmission and individual behaviours such as limiting time spent outside to minimize human-mosquito contact.
Keywords: Malaria; Prevalence; Knowledge; Ghana; Rural; Household
Abbreviations
ITN: Insecticide Treated Net; GDHS: Ghana Demographic and Health Survey; BABM: Bibiani-Anhwiaso Bekwai Municipality; RDT: Rapid Diagnostic Test; GHS: Ghana Health Service
Introduction
Malaria remains a public health concern and a pressing issue in the concept of human health improvement especially in Africa countries. Malaria is a parasitic infection caused by Plasmodium with the dominant species being P. falciparum which is responsible for about 90-99.7% of the Africa malaria burden [1]. Over the years, the disease has consistently been the leading cause of morbidity and mortality in resource poor Africa countries. For instance, of the respective 215 and 216 million global malaria cases in 2015 and 2016, the Africa region alone accounted for 90% [1].
Malaria is endemic in Ghana with all year transmission and it is associated with increased morbidity and mortality. Almost the entire Ghanaian population is at risk of malaria infection with children under the age of 5 years and pregnant women being the most vulnerable to the illness and death [2]. Although numerous impressive efforts including waiving of taxes in the importation of insecticide treated nets (ITNs), free provision of ITNs, indoor residual spraying, Roll back malaria programme, National Malaria Control programme, and the President’s Malaria programme have all been implemented to fight malaria in the country, achievements have been minimal. For instance, a recent report indicate a reduction in the number of admissions and deaths attributed to malaria from 409,947 to 379,986 and 2,137 to 1264 in 2015 and 2016 respectively [3].
On the other hand, of the global estimate of 216 million of malaria morbidity cases in the year 2016, Ghana accounted for 4% (8,640,000) [1]. In 2016, Ghana recorded 10.4 million suspected malaria cases at the outpatient department (OPD). This represented a 2.5% increase of cases reported during the same period in 2015. Averagely, nearly 28,606 suspected malaria cases are recorded daily in the country’s health facilities [3].
In Ghana, among the challenges for effective control of malaria include a more rational basis for stratified intervention delivery, better planning information and an ability to generate sufficient evidence to demonstrate impact of malaria on population. Besides the direct health impacts of malaria, there are also severe social and economic burdens on communities and the country as a whole. Earlier study in Ghana has estimated that every malaria episode recorded corresponds to an average of 5 workdays lost; 3 days to the patient and 2 days to the caretaker [4]. Prevalence of malaria occur with varying intensities depending on the cohort under investigation and other socialeconomic characteristics.
Knowledge level of malaria has been defined as the ability of a person to have correct understanding of malaria in terms of causative agent, mode of transmission, signs and symptoms, treatment and prevention [5]. Poor knowledge level of malaria infections and its prevention are considered a major risk factor in rural Africa. There is dearth of information on the prevalence and household knowledge of malaria at Bibiani-Anhwiaso Bekwai Municipality in the Western North region of Ghana. Information on prevalence and knowledge of the disease will guide public intervention, help in policy making and develop appropriate strategies to control malaria.
Methods
Study area
This was a community based cross-sectional study among 481 participants in the Bibiani Anhwiaso Bekwai Municipality (BABM) in the Western-North region of Ghana. The Municipality is one of the 237 municipalities/districts in Ghana which falls within the Equatorial Rain Forest Zone. It is located between latitude 6° 3” N and longitude 2° 3” W. Majority of the people in the Municipality speak Sefwi. Farming constitutes the major occupation for livelihood. Others are miners, civil servants and traders.
Study population
The study included all members of the selected households. However, only household heads were respondents to the study questions. In the absence of the head of the household, a responsible adult above 18 years was chosen to respond to the questions on behalf of the family. A total of 481 participants were involved in the study which included 155 household heads and 326 household members.
Study design
This was a community based cross-sectional study conducted from May to June 2019 in three randomly selected villages, Fahiakobor, Tanoso and Akaasu in the BABM.
Sampling method
An approximated sample size of 155 households was arrived at taking into account prevalence of 26.5 % [6], desired precision of 7%, at 95% confidence interval [7]. The number of households sampled from each community was proportionate to the sample size. A multistage sampling method was used in the selection of representative sample of households. First, the Municipality was divided into three strata (Bibiani, Anhwiaso and Bekwai) because of its expansive nature. A list of all towns and villages were obtained from the Municipal assembly and a simple random sampling was employed in selecting three communities (Fahiakobor, Tanoso and Akaasu) from each strata. In each randomly selected community, the survey team selected every second house as they moved from the center of the community following a designated path using the “spin the bottle” approach [8]. Where a house served as a household, participants were invited to participate and where there were more than one household further simple random sampling was used to select a household in the house.
Data collection
A total of 155 households were involved in the study. Data collection took place from May-June 2019. Prior to the day of data collection, announcements were made publicly through the community information system. This facilitated both access and cooperation of the people. A pre-tested questionnaire was used to elicit knowledge of malaria from households. A household was defined as a group of people living together in one dwelling, eat from the same cooking pot and recognises one person as a head. The questionnaire had two parts, demographic information (age, sex, marital status, level of education, religion and occupation) and knowledge level of malaria which was ascertained based on participant’s level of understanding of malaria in terms of causative agent, mode of transmission, control and preventive measures as well as signs and symptoms. Household’ s management and preventive measures against malaria were also determined. Among household members, the demographic characteristic included were age and sex. In each selected household, all members were tested for the presence of malaria parasites using Rapid diagnostic test kits (RDT, CareStart™, ACESS BIO, USA).
Data analyses
Data were entered into SPSS version 23 (SPSS Inc., Chicago, Ill., USA). This was imported into STATA 15 (STATA Corps, Texas, USA) for analyses. Frequencies and proportions were used to for the descriptive analysis of data. Knowledge level of malaria was assessed based on participant’s responses to eight (8) questions which included understanding of malaria as a mosquito-borne disease, correctly mentioning at least one sign/symptom of malaria, answering correctly four of the transmission questions, knowing that malaria can kill and having knowledge of malaria prevention methods. Participants were assigned scores based on their responses, with 8 as the highest score. Categorization was done as follows, answering 75% (6/8) or more correctly (good), 50-74% (5/8, fair) and less than 50% (≤4, poor). Chisquare (χ2) test was used to determine the association between demographic, knowledge of malaria, ITN characteristics and malaria status of participants. All variables were used in a bivariate analysis and variables with significant associations (p < 0.05) with the dependent variable malaria status, were further considered for multivariate logistic regression to determine independent associations of each variable with the response variable, malaria status (Positive/ Negative). Significance level was determined at p < 0.05.
Results
Socio-demographic characteristics of the study participants
The study involved 481 participants from 155 households. Of the 481 participants, approximately a third (32.2%) were household heads with the rest (67.8%) being household members. Household heads were respondents to the study questionnaire. Their mean age was 42.6 ± 12.6 years. Most of them were males (56.8%) and married (86.5%). Majority of the respondents were Christians (91.6%). In terms of their educational level, approximately 21% had no formal education, 22.6% attained primary education, 38.1% had Junior high school (JHS) education, 16.1% had senior high school (SHS) education and few (2.6%) attained tertiary education. With respect to their occupation, most were farmers (69.7%) followed by traders (16.1%) and artisans (11.6%) who were mainly tailors, carpenters and seamstresses. Few (2.6%) were unemployed. Age and sex information of household members showed a mean age of 14.3 ± 12.9 years with more than half being females (57.5%). Age categorization showed one-third (33.2%) belonging to 5-10 years with 22.8% less than 5 years (Table 1).
| Variable | Frequency | Percentage |
|---|---|---|
| Household heads (n=155) | ||
| Age (years) 42.6 ± 12.6* | ||
| 20-30 | 27 | 17.4 |
| 31-40 | 56 | 36.1 |
| 41-50 | 33 | 21.3 |
| >50 | 39 | 25.2 |
| Sex | ||
| Male | 88 | 56.8 |
| Female | 67 | 43.2 |
| Education | ||
| No formal education | 32 | 20.6 |
| Primary | 35 | 22.6 |
| JHS | 39 | 38.1 |
| SHS | 25 | 16.1 |
| Tertiary | 4 | 2.6 |
| Religion | ||
| Christian | 142 | 91.6 |
| Moslem | 9 | 5.8 |
| Traditionalist | 4 | 2.6 |
| Occupation | ||
| Farming | 108 | 69.7 |
| Trading | 25 | 16.1 |
| Artisanα | 18 | 11.6 |
| unemployed | 4 | 2.6 |
| Marital status | ||
| Married | 134 | 86.5 |
| Single | 12 | 7.7 |
| Divorced/widowed | 9 | 5.8 |
| Household members (n=326) | ||
| Age (years), 14.3 ± 12.9* | ||
| < 5 | 75 | 23 |
| 10-May | 108 | 33.1 |
| 20-Nov | 72 | 22.1 |
| 20+ | 71 | 21.8 |
| Sex | ||
| Male | 139 | 42.6 |
| Female | 187 | 57.4 |
*Mean ± standard deviation αArtisan-Carpenters, hairdressers, and seamstresses
Table 1: Socio-demographic information of participants
Of the 155 household heads interviewed, 139/155 (89.7%) agreed to take part in the malaria test. This reduced the total number to 465/481 participants. The overall prevalence of malaria was 182/465 (39.1%). Among household heads, malaria prevalence was significantly (p=0.01) higher in females (21.6%) than in males (20.1%) (Table 2). Malaria prevalence differed significantly with respect to education among household heads (p=0.02) with the lowest prevalence recorded among participants with senior high school (2.9%) and tertiary (0.7%) education. Prevalence of malaria did not differ significantly with other socio-demographic information of household head participants. With respect to household members, malaria prevalence was significantly higher among children less 5 years (12.6%), p=0.01 and females (24.5%), p=0.04.
| Characteristics | +ve | -ve | p-value |
|---|---|---|---|
| Household Heads (n=139) | |||
| Sex | |||
| Male | 28 (20.1) | 56 (40.3)* | 0.01 |
| Female | 30 (21.6) | 25 (18.0) | |
| Age | |||
| 20 – 30 | 14 (10.1) | 9 (6.5) | 0.2 |
| 31– 40 | 15 (10.8) | 30 (21.6) | |
| 41 – 50 | 19 (13.7) | 16 (11.5) | |
| > 50 | 10 (7.2) | 26 (18.7) | |
| Occupation | |||
| Farming | 36 (25.9) | 61 (43.9) | 0.34 |
| Trading | 12 (8.6) | 11 (7.9) | |
| Artisan | 9 (6.5) | 2 (1.4) | |
| Unemployed | 1 (0.7) | 7 (5.0) | |
| Education | |||
| No formal education | 11 (7.9) | 18 (12.9) | 0.02 |
| Primary | 19 (13.7) | 13 (9.4) | |
| JHS | 23 (16.5) | 26 (18.7) | |
| SHS | 4 (2.9) | 21 (15.1) | |
| Tertiary | 1 (0.7) | 3 (2.2) | |
| Household Members (325) | |||
| Sex | |||
| Male | 44 (13.5) | 95 (29.1) | 0.04 |
| Female | 80 (24.5) | 107 (32.8) | |
| Age | |||
| < 5 | 41 (12.6) | 34 (10.4) | 0.01 |
| 5 – 10 | 40 (12.3) | 68 (20.9) | |
| 11 – 20 | 23 (7.1) | 49 (15.0) | |
| > 20 | 20 (6.1) | 51 (15.6) | |
| Sex (All participants) | |||
| Male (n = 223) | 75 (16.1) | 148(31.8) | 0.02 |
| Female (n = 242) | 107 (23.0) | 135 (29.0) | |
* n (%), +ve (positive) -ve (negative)
Table 2: Relationship between socio-demographic characteristics and malaria status.
Majority of the respondents understood malaria as a mosquitoborne disease (92.3%) that can cause death (94.2%) (Table 3). All the participants answered correctly that malaria is acquired through the bite of infected female anopheles mosquito. However, most participants incorrectly answered that malaria can be caused by eating contaminated food (71.0%), drinking contaminated water (79.4%) and transmitted through sexual intercourse with an infected person (53.5%). The common signs/symptoms mentioned by participants were fever (41.9%), followed by body weakness (19.4%), head ache (14.2%), high body temperature (11.0%) and vomiting (7.1%). Others (6.5%) include bitterness in the mouth, diarrhoea, cold, anaemia and loss of appetite. All the participants knew that malaria can be prevented and mentioned various preventive methods including use of medication (6.5%), ensuring proper sanitation (47.1%) and sleeping under mosquito net (46.1%).
| Variables | Frequency | Percentage |
|---|---|---|
| Understanding of malaria | ||
| Disease caused my mosquito | 143 | 92.3 |
| Disease caused by poor sanitation | 12 | 7.7 |
| Malaria is transmitted through the bite of an infected female anopheles mosquito | ||
| Yes | 155 | 100 |
| Malaria is transmitted through physical contact with infected people | ||
| Yes | 53 | 34.2 |
| No | 102 | 65.8 |
| Malaria is caused by eating contaminated food | ||
| Yes | 110 | 71 |
| No | 45 | 29 |
| Malaria is caused by drinking contaminated water | ||
| Yes | 123 | 79.4 |
| No | 32 | 20.6 |
| Malaria is transmitted through sexual contact with infected people | ||
| Yes | 83 | 53.5 |
| No | 72 | 46.5 |
| Malaria can kill | ||
| Yes | 146 | 94.2 |
| No | 9 | 5.8 |
| Signs/symptoms of malaria | ||
| Body weakness | 30 | 19.4 |
| Fever | 65 | 41.9 |
| Headache | 22 | 14.2 |
| High body temperature | 17 | 11 |
| Vomiting | 11 | 7.1 |
| Other* | 10 | 6.5 |
| Malaria can be prevented | ||
| Yes | 100 | 100 |
| No | 0 | 0 |
| Prevention methods | ||
| Use of medication | 10 | 6.5 |
| Ensuring proper sanitation | 73 | 47.1 |
| Sleeping under mosquito net | 72 | 46.4 |
Other* - cold, bitter mouth, diarrhea, anaemia, loss of appetite
Table 3: Knowledge of malaria among participants.
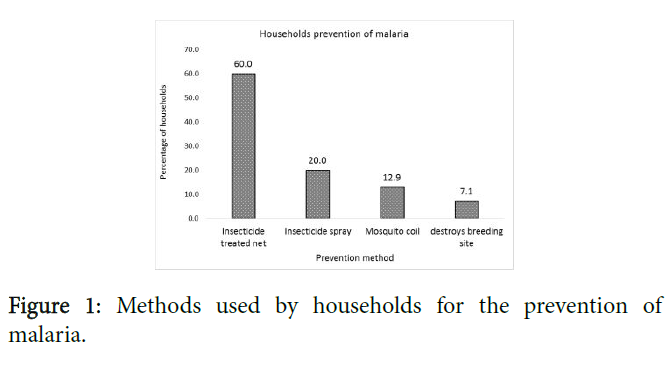
The methods used by households to prevent themselves from being attacked by mosquitoes are shown in Figure 1.
Most participants (60.0%) mentioned the use of Insecticide treated net (ITN), followed by mosquito spray (20.0%), mosquito coil (12.9%) and destroying breeding sites of mosquitoes (7.1%).
Majority of the households possessed at least one ITN (83.9%) (Table 4). Almost all the participants (98.5%) indicated free provision of the ITN by the government. Majority mentioned their frequent use of the ITN (96.2%) and slept under ITN (90.8%) a night prior to the survey.
| Characteristic | Frequency | Percentage |
|---|---|---|
| Have ITN | ||
| Yes | 130 | 83.9 |
| No | 5 | 16.1 |
| Source of ITN | ||
| Bought it | 2 | 1.5 |
| Government | 128 | 98.5 |
| Use of ITN | ||
| Yes | 125 | 96.2 |
| No | 5 | 3.8 |
| Slept under ITN yesterday | ||
| Yes | 118 | 90.8 |
| No | 12 | 9.2 |
Table 4: Information on insecticide treated nets (ITN).
Having ITN, the use of ITN, and sleeping under ITN a night prior to the survey was not significantly associated with malaria (Table 5). Having overall good and fair knowledge of malaria was significantly associated with lower cases of malaria (p<0.05).
| Variable | +ve | -ve | p-value |
|---|---|---|---|
| Malaria is transmitted through contact with infected people | |||
| Yes | 26 (18.7)* | 21 (15.1) | 0.02 |
| No | 32 (23.0) | 60 (43.2) | |
| Malaria is caused by eating contaminated food | |||
| Yes | 50 (36.0) | 50 (36.0) | 0.01 |
| No | 8 (5.8) | 31 (22.3) | |
| Malaria is caused by drinking contaminated water | |||
| Yes | 51 (36.7) | 61 (43.9) | 0.06 |
| No | 7 (5.0) | 20 (14.4) | |
| Malaria is transmitted through sexual contact with infected people | |||
| Yes | 24 (17.3) | 45 (32.4) | 0.09 |
| No | 34 (24.5) | 36 (25.9) | |
| Overall knowledge | |||
| Good | 20 (14.4) | 56 (40.3) | <0.01 |
| Fair | 14 (19.0) | 11(7.9) | |
| Poor | 24 (32.8) | 14 (10.1) | |
| Have ITN | |||
| Yes | 45 (32.4) | 70 (50.4) | 0.74 |
| No | 13 (9.4) | 11 (7.9 | |
| Use ITN | |||
| Yes | 43 (37.4) | 68 (59.1) | 0.65 |
| No | 2 (1.7) | 2 (1.7) | |
| Slept under mosquito net yesterday | |||
| Yes | 42 (36.5) | 61 (53.0) | 0.29 |
| No | 3 (2.6) | 9 (7.8) | |
* n (%), +ve (positive) -ve (negative)
Table 5: Relationship between respondent’s knowledge, ITN characteristics and malaria status.
Younger participants, less than 5 years (aOR=4.4, CI: 1.1-18.8) and 6-20 years (aOR=4.1, CI: 1.0-16.4) were more likely to be have malaria infection compared to older age groups. Households with household heads with no formal education (aOR=6.5, CI: 1.2-36.3) and poor knowledge of malaria (aOR=3.1, CI: 0.89-10.70) were more likely to have malaria infection (Table 6).
| Variable | aOR (CI) | p-value |
|---|---|---|
| Age (years) | ||
| <5 | 4.4 (1.1-18.8) | 0.04 |
| 20-Jun | 4.1 (1.0-16.4) | 0.04 |
| 21-30 | 1.1 (0.4-3.8) | 0.84 |
| 31-40 | 2.3 (0.54-10.0) | 0.26 |
| ≥ 41 | 1 | - |
| Sex | ||
| Male | 1 | - |
| Female | 1.3 (0.50-3.60) | 0.55 |
| Education | ||
| No formal education | 6.5 (1.2-36.3) | 0.03 |
| Primary/JHS | 2.4 (0.35-16.5) | 0.37 |
| SHS/tertiary | 1 | - |
| Knowledge of malaria | ||
| Good | 1 | - |
| Fair | 1.6 (0.46-5.90) | 0.45 |
| Poor | 3.1 (0.89-10.70) | 0.07 |
aOR- Adjusted odds ratio, CI-Confidence interval; Predictors - age, sex, education, and knowledge of malaria. Outcome variable- malaria (Positive/Negative) by RDT. Data (age and sex) include all participants.
Table 6: Factors associated with malaria.
Discussion
Over the years, impressive efforts have led to the reduction of malaria morbidity and mortality in Ghana. Despite this, malaria is still highly prevalent among many communities including Bibiani- Anhwiaso Bekwai Municipality where malaria continues to top the chart for the top ten reported cases in terms of OPD attendance and death [9]. According to the Ghana demographic and health survey, malaria prevalence range from 11.2% to 40.0 % [2]. In this study, the prevalence of malaria was found to be 39.1%. This result indicate a high burden of malaria among rural households in the Municipality. This is similar to the study by Deku et al. on their retrospective study on malaria in the Western North region where this study was conducted [6]. Similar to our findings is the high prevalence of 58% reported by Owusu Agyei et al. in the Brong-Ahafo region of Ghana [10]. The high prevalence documented is also consistent with studies in other Africa countries [11-15]. The high prevalence could be due to the period of data collection (May-June) which is a major raining season in the Municipality. In Ghana, the major rainy season commences in March or April and runs until mid-July followed by a short dry period in July–August and the minor rainy season in September–October as well as a long dry season (harmattan) usually lasting from November through March. During the raining season, the number of breeding grounds for Anopheles mosquitoes increases enhancing oviposition by the vector leading to an increase in malaria. Again, the municipality is one of the mining areas in Ghana and this may increase malaria burden since mining activities have been linked to high prevalence of malaria [16, 17]. Mining often create ditches where erosion takes place often leading to flooding to create suitable environment for breeding of mosquitoes increasing the incidence of malaria and other vector-borne diseases. Thus, mining activities play an important role in the maintenance of malaria transmission and imposes an important barrier to malaria elimination [16].
In this study, a significant gender disparity of malaria cases was observed with females significantly recording higher percentage than males. There is discordance in literature on gender susceptibility to malaria infection. This is in agreement with earlier studies [11, 15, 18]. The higher prevalence in females may be due to the characteristics female dresses that expose some parts of their body to malaria vectors during outdoor activities. Household heads with lower level of education (no formal education, primary and JHS) significantly had higher prevalence of malaria. This is in agreement with findings of Sultana et al. where malaria cases were higher among children with less-educated (10.98%) and illiterate (7.82%) household heads [19]. Among household members in this study, malaria prevalence was significantly higher, 12.6% (p=0.01) among the under-fives compared to the other age groups. This is different from the studies by Sultana et al., who reported highest prevalence among children aged 11–14 years [19]. The highest prevalence in this age group may be due to decreased immunity to fight malaria parasites infection.
In Ghana, the use of ITN is one of the central interventions for preventing malaria infection. The 2014-2020 National Malaria Control Programme Strategic Plan aims to achieve universal coverage with LLINs, defined as one net for every two people. To achieve this objective, the plan supports time-limited, national, free distribution campaigns of LLINs [2]. In this study, majority of the participants (83.9%) owned at least one ITN which was mainly provided by the government. This is similar to the study by Paulander et al. where 85.9% of the household interviewed owned a bed net [20] and also consistent with a recent report by the Ghana malaria indicator survey (GMIS) and the Ghana Demographic and Health Survey [2,21]. The GDHS reported 70% ownership of ITN of any type while the GMIS indicated that 73% of households in Ghana owned at least one LLIN largely (85%) obtained free from mass distribution campaign. Almost all participants (96.2%) who indicated possession of ITN reported usage of the net. However, 6.2% did not sleep under the ITN a night prior to the survey. This is in agreement with the study by Jenkins et al. where approximately 92.11% of the respondents reported using a mosquito net for sleeping [15] but contrary to the findings of Rakhshani et al. and Hla-Shein et al. where most subjects never used a mosquito net [22,23]. The high possession and usage of the ITN was not surprising because there had been a mass distribution of ITN by the government two months prior to the survey.
Some of the participants who had ITN but did not sleep under it indicated how uncomfortable they feel under the net because of heat. Others indicated the presence of fun in the room so there was no need to sleep under the net. Malaria prevalence did not differ significantly from households that owned and slept under ITN and those that did not. This is contrary to the findings of Jenkins et al. [15]. Despite the high percentage of household’s ownership and usage of ITN, malaria prevalence in this study was high. Possible reason could be due to the behavior of the participants like spending much time outside and receiving bite of mosquito before going to sleep under the ITN and not wearing long protective clothing to prevent human mosquito contact.
Poor knowledge level about malaria infections and its prevention are considered a major risk factor in rural Africa. In this study, majority of the participants understood malaria as a deadly disease (94.2%) caused by the bite of mosquitoes (92.3%). This is consistent with earlier studies [23-26]. All the participants were able to mention at least one signs/symptoms of malaria with fever (41.9%) been the most common sign/symptom mentioned followed by body weakness (19.4%), head ache (14.2%) and high body temperature (11.0%). This is in agreement with the an Ethiopian study where most of the respondents (92.7%) were able to mention at least one sign/symptom of malaria like fever (55.0%), and head ache (28.5%) [20]. A Thailand study has also reported similar findings [27]. All the participants knew that malaria can be prevented and mentioned various preventive methods including the use of medication, ensuring proper sanitation and sleeping under mosquito net. This in agreement with a study in Columbia where 93.5% and 94.3% of people in moderate risk (MR) and high risk areas respectively indicated the use of insecticide-treated nets (ITNs) to prevent malaria [28]. With respect to questions on malaria transmission, majority of the respondents admitted that malaria is transmitted through sexual contact with infected people (53.5%), eating contaminated food (71.0%) and drinking contaminated water (79.4%). This indicates poor knowledge of the participants in terms of malaria transmission in the municipality. This is consistent with a study in Northern Uganda where participants mentioned cold food, playing in the rain, cold weather and eating mangos as cause of malaria [24] but contrary to a study in Bangledesh [29]. Findings from earlier study in Ghana indicate that some participants thought overworking oneself caused malaria [26].
In general, categorization of knowledge of malaria (good, fair or poor) was done based on eight questions with the highest score of 8. Most participants had good knowledge of malaria with average score of 5.5 ± 1.21 (min=2, max 8). This is contrary to the low knowledge score of 5.5 (maximum 15.0) documented in an earlier study in Iran [22]. The difference in scores could be due to the number of questions used and the assessment criteria. Their knowledge questions were 15 compared with the 8 questions used in this study. The good knowledge documented is in agreement with other studies [5,25,30,31]. Other authors have reported lower knowledge of malaria [29,32]. Having poor knowledge increased the risk of malaria infection (aOR = 3.1, CI = 0.89-10.7, p=0.07).
Conclusion
This study reports a high risk of malaria in the Bibiani-Anwhiaso Bekwai Municipality with a prevalence of 39.1%. Prevalence was significantly higher in females (23.0%) and children under 5 years (12.6%) (p<0.05). Majority of the households in this study possessed at least one ITN which was mainly provided by the government. Participants demonstrated a relative good knowledge of malaria. Malaria control efforts should take into consideration local prevalence, and its influencing factors. With much focus on the achievement of the sustainable development goals (SDGs), it is also imperative to pay much attention and consider local community behaviors and knowledge of malaria to enhance national, regional and global goal to reduce and eliminate malaria burden.
Acknowledgement
Much appreciation is extended to the Municipal Health Director, Dr. Francis Takyi and Mr. Isaac Tettefio for their assistance and the community leaders for their support. We are grateful to the participants who volunteered to take part in this study. To Erica Intsin and Emmanuella Igwebuike Nneamaka, God bless you for the constant reminder to finish this work. We acknowledge the financial contribution given to the first author, IB, by the EACEA department of the European Union (EU) through the TreccAfrica scholarship programme to complete his MSc. work.
Ethics Approval and Consent to Participate
Ethical approval for the study was obtained prior to data collection from the Health Research Ethics committee, University of Nigeria Teaching hospital, Enugu (NHREC/05/01/2008BFW00002458- 1RB00002323). Permission to conduct the study was obtained from the Municipal Health Directorate and the leaders in each community. In addition, individual oral and written informed consent were obtained from all the study participants.
Authors’ Contributions
IB, FCO, and PO designed the research. IB and WI collected and analysed the data, and wrote the first draft of the manuscript. FCO and PO supervised the study concept, participated in interpreting the data, reviewed and edited the manuscript. All authors approved the final manuscript.
References
- Ghana Health Service (20119) 8th issue of the Ghana malaria control programme periodic bulletin.
- Asante FA, Asenso-Okyere K (2003) Economic burden of malaria in Ghana. World Health Organization, Geneva 1-81.
- Dhawan G, Joseph N, Pekow PS, Rogers CA, Poudel KC, et al. (2014) Malaria-related knowledge and prevention practices in four neighbourhoods in and around Mumbai, India: A cross-sectional study. Malar J 13: 303.
- Deku JG, et al. (2018) Malaria burden and trend among clients seeking healthcare in the western region: A 4-year retrospective study at the sefwi-wiawso municipal hospital, Ghana. Open Microbiol J 12: 404-411.
- Daniel WW, Cross CL (2018) Biostatistics: A foundation for analysis in the health sciences (11th edn) Wiley.
- Kimbi HK, Nkesa SB, Ndamukong-Nyanga JL, Sumbele IU, Atashili J, et al. (2014) Knowledge and perceptions towards malaria prevention among vulnerable groups in the buea health district, Cameroon. BMC Public Health 14: 883.
- Asibet BQ (2015) Determinants of healthcare utilization in rural communities in bekwai municipality, Ghana.
- Owusu-Agyei, S, Asante KP, Adjuik M, Adjei G, Awini E, et al. (2009) Epidemiology of malaria in the forest-savanna transitional zone of Ghana. Malaria J 8: 220.
- Onyishi GC, Aguzie ION, Nwani CD, Obiezue RNN, Okoye IC (2018) Malaria-vector dynamics in a tropical urban metropolis, Nigeria. Pakist J Zoology 50: 1035-1041.
- Wogu M, Nduka F (2018) Evaluating malaria prevalence using clinical diagnosis compared with microscopy and rapid diagnostic tests in a tertiary healthcare facility in rivers state, Nigeria. J tropical med 18: 16-19.
- Dawaki S, Al-Mekhlafi HM, Ithoi I, Ibrahim J, Atroosh WM, et al. (2016) Is Nigeria winning the battle against malaria? Prevalence, risk factors and KAP assessment among Hausa communities in Kano State. Malaria J 15: 351.
- Babamale OA, Ugbomoiko US, Heukelbach J (2018) High prevalence of plasmodium falciparum and soil-transmitted helminth co-infections in a periurban community in Kwara State, Nigeria. J Infect Public Health 11: 48-53.
- Jenkins R, Omollo R, Ongecha M, Sifuna P, Othieno C, et al. (2015) Prevalence of malaria parasites in adults and its determinants in malaria endemic area of Kisumu county, Kenya. Malaria J 14: 263.
- Castellanos A, Chaparro-Narváez P, Morales-Plaza CD, Alzate A, Padilla J, et al. (2016) Malaria in gold-mining areas in Colombia. Memorias do Instituto Oswaldo Cruz 111: 59-66.
- Kumi-Boateng B, Yakubu I (2010) The use of gis to study the spatial variation of diseases: A case of western region, Ghana. Ghana Mining J 11: 1-8.
- Kunihya IZ, Samaila AB, Nassai I, Sarki A, Haruna MY (2016) Prevalence of malaria infection among children attending specialist hospital Yola, Adamawa State, Nigeria. J Med Biol Sci Res 2: 136-142.
- Sultana M, Sheikh N, Mahumud RA, Jahir T, Islam Z, et al. (2017) Prevalence and associated determinants of malaria parasites among Kenyan children. Trop Med Health 45: 25.
- Paulander J, Olsson H, Lemma H, Getachew A, Sebastin M (2009) Knowledge, attitudes and practice about malaria in rural Tigray, Ethiopia. Global Health Action 2: 1839.
- Rakhshani F, Ansari Moghadam AR, Alemi R, Moradi A (2003) Knowledge, perceptions and prevention of malaria among women in Sistan va Baluchestan, Islamic Republic of Iran. East Mediterr Health J 9: 248-256.
- Shein H, Sein TT, Soe S, Aung T, Win N, et al. (1998) The level of knowledge, attitude and practice in relation to malaria in Oo-do village, Myanmar. Southeast Asian J Trop Med Public Health 29: 546-549.
- Obol J, Lagoro DK, O, Garimoi C (2011) Knowledge and misconceptions about malaria among pregnant women in a post-conflict internally displaced persons' camps in Gulu district, Northern Uganda. Malar Res Treat 11: 107987.
- Mazigo HD, Obasy E, Mauka W, Manyiri P, Zzinga M, et al. (2010) Knowledge, attitudes, and practices about malaria and its control in rural northwest Tanzania. Malar Res Treat 10: 794261.
- De La Cruz N, Crookston B, Dearden K, Gray B, Ivins N, et al. (2006) Who sleeps under bednets in Ghana? A doer/non-doer analysis of malaria prevention behaviours. Malaria J 5: 61.
- Van Benthem BH, Khantikul N, Panart K, Somboon P, Oskam L (2006) Knowledge and use of preventive measures against malaria in endemic and non-endemic villages in northern Thailand. Southeast Asian J Trop Med Public Health 37: 243-249.
- Forero DA, Chaparro PE, Vallejo AF, Benavides Y, Gutiérrez JB, et al. (2014) Knowledge, attitudes and practices of malaria in Colombia. Malar J 13: 165.
- Ahmed SM, Haque R, Haque U, Hossain A (2009) Knowledge on the transmission, prevention and treatment of malaria among two endemic populations of Bangladesh and their health-seeking behaviour. Malar J 8: 173.
- Singh R, Musa J, Singh S, Ebere UV, et al. (2014) Knowledge, attitude and practices on malaria among the rural communities in aliero, northern Nigeria. J Family Med Prim Care 3: 39-44.
- Shimaponda-Mataa NM, Mwase ET, Gabreslasie M, Mukaratirwa S (2016) Knowledge, attitudes and practices in the control and prevention of malaria in four endemic provinces of Zambia. Southern African J Infectious Diseases 32: 29-39.
- Legesse Y, Tehehn A, Belachew T, Tushune K (2007) Knowledge, attitude and practice about malaria transmission and its preventive measures among households in urban areas of Assosa Zone, Western Ethiopia. Ethiop J Health Dev 21: 157-165.
Citation: Boadu I, Nsemani W, Ubachukwu P, Okafor FC (2020) Knowledge and Prevalence of Malaria among Rural Households in Ghana. J Community Med Health Educ 10: 673. DOI: 10.4172/2161-0711.1000673
Copyright: © 2020 Boadu I, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 3153
- [From(publication date): 0-2020 - Nov 24, 2025]
- Breakdown by view type
- HTML page views: 2264
- PDF downloads: 889