Knockdown of NUDT21 Inhibits Cell Proliferation and Cell Migration, Promotes Apoptosis in Prostate Cancer
Received: 04-Apr-2022 / Manuscript No. CMB-22-59486 / Editor assigned: 06-Apr-2022 / PreQC No. CMB-22-59486 (PQ) / Reviewed: 13-Apr-2022 / QC No. CMB-22-59486 / Revised: 18-Apr-2022 / Manuscript No. CMB-22-59486 (R) / Accepted Date: 18-Apr-2022 / Published Date: 25-Apr-2022 DOI: 10.4172/1165-158X.1000230
Abstract
Nudix Hydrolase 21 (NUDT21) is a newly characterized oncogene involved in several types of cancer. However, the expression patterns and biological function of NUDT21 in prostate cancer (PCa) remain unclear. The present study aimed to investigate the roles of NUDT21 in the cell proliferation and metastasis of PCa. In the present study, the expression of NUDT21 was analyzed by qRT-PCR in PCa cell lines and human prostatic stromal cell line (WPMY). Compared with WPMY cells, NUDT21 expression was significantly increased in DU 145 and LNcap cells. Furthermore, we constructed the DU 145 and LNcap cell lines with stable low expression of NUDT21 to validate the function of NUDT21. Celigo cell count assay, flow cytometry, wound healing and Transwell assays were performed to analyze and compare cell viability and cell migration. These results showed that lentivirus-mediated NUDT21 knockdown significantly inhibited DU 145 and LNcap 786-O cell proliferation and migration, as well as induced cell cycle arrest and increased apoptosis in vitro. In overall, the present findings demonstrated that NUDT21 plays an oncogenic role in PCa and the potential of NUDT21 targeting in PCa treatment.
Keywords: PCa; NUDT21; Cell proliferation; Cell apoptosis; Cell migration
Keywords
PCa; NUDT21; Cell proliferation; Cell apoptosis; Cell migration
Introduction
Prostate cancer (PCa), an epithelial malignancy that occurs in the prostatic epithelium, is a most common malignant tumor with a high incidence in the male genitourinary system [1,2]. Due to the lack of effective therapies, the mortality rates of PCa are annually increasing. Additionally, PCa is one of chronic diseases with various severe complications, it has become one of the major threats worldwide to human life and health [3], focusing on therapeutic strategies for PCa is urgently necessary. Recently, various traditional methods for treating PCa including surgical excision, radiation therapy, hormonal therapy, and chemotherapy are the primary has been developed [4]. Unfortunately, a number of patients with advanced PCa often succumb to iatrogenic injury, drug toxicity, and therapy resistance, and the clinical treatment remains particularly therapeutically challenging [1,5]. Therefore, there is an unquestionable need for more effective therapeutic approaches and less toxic drugs. Although the immune system plays a huge role in preventing and treating of tumors, immunotherapy for PCa has not been efficacious for patients in the past [6]. Molecular diagnosis and targeted therapy have proved to be a promising approach for PCa treatment [7,8], however, these novel molecular targeting treatments have achieved little clinical progression. Moreover, the etiology and pathogenesis of PCa remain not fully understood. Hence, the proposal of new gene therapy programs is of great importance for the treatment and diagnosis of PCa.
Nudix Hydrolase 21 (NUDT21), a complex of RNA-binding proteins critical to regulate alternative transcript polyadenylation (APA) (9). NUDT21 has previously been reported to be involved in certain important biologic regulatory processes and contributes to the control of cell fate determination in a critical way [10]. It has been showed that NUDT21 increased the of risk preeclampsia by targeting 3'-UTR of EZH2 mRNA [11]. In recent years, several studies showed that NUDT21 act as tumor suppressors or promoters in carcinogenesis and cancer progression among different cancer types [9,10,12,13]. For instance, the expression level of NUDT21 was decreased in cervical cancer tissues and cells, and highly associated with the clinical prognosis of patients [14]. We also found that NUDT21 suppressed the pathologic progress of small cell lung cancer [15]. Moreover, up-regulation of NUDT21 inhibited bladder cancer growth, while NUDT21 knockdown promoted bladder cancer growth and metastasis [10]. Intriguingly, however, opposing studies indicates that NUDT21 was frequently overexpressed in a multitude of cancers [13,16]. Subsequent functional studies indicated that NUDT21 knockdown inhibits proliferation and promotes apoptosis of pancreatic ductal adenocarcinoma [13]. Additionally, silencing NUDT21 attenuated the mesenchymal Identity of glioblastoma cells via the NF-κB pathway [17].
Despite a considerable amount of evidence showing the involvement of NUDT21 in several types of cancer progression, the role of NUDT21 in PCa has been less explored.
Herein, we investigated the expression of NUDT21 in the PCa cell lines by qRT-PCR. Moreover, loss-of-function experiments further explored the biological function of NUDT21 in the PCa cell lines in vitro. Our data indicated that NUDT21 knockdown papillary influenced PCa cell proliferation, apoptosis and migration. Therefore, this study demonstrated that NUDT21 plays a critical role in the progression of PCa and NUDT21 may have potential as a diagnostic and therapeutic target for the treatment of PCa.
Materials and Methods
Cell culture
Human prostatic stromal cell line (WPMY) and human PCa cell lines (DU 145, PC-3 and LNcap) obtained from American Type Culture Collection (ATCC, USA). WPMY, DU 145 and LNcap cell lines were grown in RPMI-1640 with 10% FBS, while PC-3 cells were cultured in F-12K medium supplemented with 10% FBS.
All cells were cultured in a 37℃ incubator containing 5% CO2 in air and saturated humidity.
Real-time Quantitative PCR Detecting System (qRT-PCR)
Relative expression levels of the NUDT21 transcript were analyzed by quantitative real-time PCR (qRT-PCR) assay. Briefly, all cells were collected and lysed with Trizal, and total RNA was isolated using TRIzol reagent (Invitrogen, USA) following the manufacturer’s instructions. RNA quality control was determined by a Nanodrop 2000/2000C spectrophotometer (Thermo Fisher Scientific, USA). Subsequently, RNA was reverse transcribed to synthesize cDNA and qPCR reactions were performed in triplicate with the SYBR Green qPCR Master Mix (Thermo Fisher Scientific, USA). NUDT21 and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) genes were amplified for normalization of cDNA loading. The primers used for amplification were as follows: forwards primer of NUDT21: 5’-AAGCCTTGTTTGCAGTCCC-3’; reversed primer of NUDT21: 5’-TCCATATCCTGGTGCATTGTC-3’; forwards primer of GAPDH: 5’-TGACTTCAACAGCGACACCCA-3’; reversed primer of GAPDH: 5’-CACCCTGTTGCTGTAGCCAAA-3’. Real-Time PCR was performed in a two-step method, and a melting curve was created. The relative expression levels of NUDT21 mRNA in the cell lines were analyzed using the 2−ΔΔCt method. The results were expressed as the mean values of relative quantification ± Standard Error of the Mean (SEM).
ShNUDT21 construction and lentivirus transfection
Lentiviral containing short hairpin RNA (shRNAs) targeting NUDT21 was supplied by BioSCI Res (shanghai, China) and transfected into DU 145 or LNcap cells according to the manufacturer's instructions. In brief, using NUDT21 gene as a template, three RNA interference target sequences interference were designed and the single-stranded DNA oligo were synthesized. Subsequently, doublestranded DNA oligo were formed by annealing the single-stranded DNA oligo and connected to the linearized BR-V-108 vector followed by transformed into competent E.coli cells for amplification. Positive clones were extracted to obtain high-purity plasmids. The tool vector plasmid carrying the interference target sequences and the viral packaging helper plasmid were co-infected into 293T cells. After culturing for 72h at 37℃, supernatants containing lentivirus with shCtrl or shNUDT21 were harvested, respectively. The lentivirus containing green fluorescent protein (GFP) was infected into PCa cell lines (DU 145 and LNcap). After 72h, the expression of green GFP was tested under the microscope to assess the transfection efficiency. The RNA interference target sequences of NUDT21 were as follows: shNUDT21-1: 5’-ATGAGGGAAGAATTTGATAAA-3’, shNUDT21-2: 5’-TATGACAATGCACCAGGATAT-3’, shNUDT21-3: 5’-TTGGAATGAGGAGGACTGTAG -3’.
Western blotting (WB)
PCa cells (DU 145 and LNcap) were cytolysis to total protein by using ice-cold cell lysate. The protein concentration was determined with a BCA Protein Assay Kit (HyClone-Pierce) according to the instruction. Equal amounts of harvested protein samples were solubilized in SDSPAGE with 10% separating gel and then transferred to the PCDF membranes. Membranes were blocked with 5% dried non-fat milk followed by incubation for 1 h at room temperature. Subsequently, the membranes were incubated with the primary antibodies against NUDT21 (Rabbit, 1:1000 dilution, ProteinTech Group, 10322-1-AP) or GAPDH (Mouse, 1:30000 dilution, ProteinTech Group, 60004-1- Ig) for 2h at room temperature. After washing, the membranes were incubated with the secondary antibodies HRP Goat anti-rabbit IgG (1:3000 dilution, Beyotime, A0208) or HRP Goat anti-mouse IgG (1:3000 dilution, Beyotime, A0216) for 1h at room temperature and then washed 3 times for 10 minutes in TBST. Immunoreactive proteins were visualized using the enhanced chemiluminescence ECL+PlusTM Western blotting detection system (Amersham Pharmacia Biotech, USA) according to the manufacturer's instructions. Finally, the protein bands were processed by image processing software.
Celigo cell count assay
DU 145 and LNcap cell lines in the logarithmic growth phase were re-suspended with complete medium and seeded into 96-well plates at a density of 2000 cells/well in a 5% CO2 atmosphere of at 37℃. From the second day after plating, cells were count and record by using Celigo S Imaging Cell Cytometer (Nexcelom Bioscience) at the same time point every day for 5 days and calculated by image analysis software. The medium was changed every 3 days. Statistical analysis was performed on the data and cell growth curve was drawn according to the absorbance value.
Apoptosis assay
Following transfection, DU 145 and LNcap cells were assessed using an Annexin V-APC apoptosis detection kit (BD Biosciences) according to the manufacturer's protocol. Briefly, the cells were cultured into a 6-well plate at a seeding density of 1,000 cells/well for 5 days after lentivirus transfection. The medium was changed every 3 days. Next, cells were digested with trypsin and washed with PBS buffer, and complete medium was re-suspended into cell suspension at a final density of ≥5×105 cells/ml. Cells were collected and washed twice with D-Hanks (pH 7.2 ~ 7.4). After 5 min centrifugation at 1300 rpm, the cell precipitation was washed successively with the precooling D-Hanks (pH = 7.2~7.4) and 1×binding buffer (eBioscience, Thermo Fisher Scientific). Cells were collected by centrifugation again followed by the addition of 200 μL 1× binding buffer. Subsequently, the cells were stained with 10 μL Annexin V-APC and incubated in the dark for 15 min at room temperature. Then the cell apoptosis was detected on the flow cytometer (Millipore).
Cell cycle assay
PCa cell cycle was evaluated by performing flow cytometry. DU 145 and LNcap cell lines were transduced with NUDT21-shRNA lentivirus or shCtrl lentivirus. After incubation for 72h, cell suspensions were generated and plated in 6-well plate for further culture. Subsequently, cells were collected and fixed with 4℃ cold 70% alcohol for at least 1h. Following centrifugation and re-rinse of PBS, cells were incubated in dark at 4℃ for 30min with 1.5ml propidium iodide (PI) (40×, 2 mg/ mL: 100×RNase, 10 mg/mL: 1×PBS =25:10:1000). The cell suspension was filtered through a 300-mesh nylon screen and added to the flow cytometry tube, and flow cytometry was performed. Finally, cell cycle distribution (G1, S and G2) was detected by Flow Jo software (version vX 0.7) (BD Franklin Lakes, USA).
Wound healing assay
The 96-well wound healing assay (VP scientific) was used to analyze migration of DU 145 and LNcap cells treated with or without NUDT21 knockdown cells. After lentiviral infection, cells were incubated for 72h and then the assay was performed according to the manufacturer's protocol. Briefly, cells in the logarithmic growth phase after transfection were digested in each experimental group, and then re-suspended in complete medium and counted. Subsequently, the cells were seeded into 96-well plates at a density of 5 × 104 cells per well and the cultured in a 37 ℃ incubator with 5% CO2 for 24h. Wounds were generated by using a 96 Wounding Replicator (VP scientific) across the cell layers while the confluence reached greater than 90%, and detached cells were removed by rinsing the cells with serum-free medium three times. Subsequently, the medium with 0.3% FBS was added into the 96-well plate and cells were cultured in a 5% CO2 incubator at 37℃. The 96-well plate was scanned at 0, 24, 48, and 72 h to obtain scratch pictures by using a fluorescence microscope (Olympus Corporation; magnification, ×200). The migration rate was calculated as the ratio of the width of the wound at a given time point relative to the width of wound at the 0h time point
Transwell assay
Briefly, 100μL serum-free medium was added to the membrane in the Transwell upper chamber and incubated at 37℃ for 2h. After transfection, DU 145 and LNcap cells in the logarithmic growth phase were digested and then re-suspended in low serum medium and counted. Subsequently, the medium in the upper chamber of 24-well plates were removed followed by the addition of cells with serum-free medium were added whereas 600μL medium containing 30% FBS) was added to the lower chamber, and then the upper chamber was transferred to the lower chamber of 24-well plates. Following incubation for 24h at 37℃ with 5% CO2, non-metastatic cells were gently removed with a cotton swab and metastatic cells were stained with Giemsa at room temperature for 5min. After rinsing with double distilled H2O three times, samples were allowed to air dry. Finally, pictures were taken with a fluorescence microscope (Olympus Corporation; magnification, ×100) and the number of transferred cells was counted.
Statistical analysis
The data involved in this study were all expressed as mean ± standard deviation (SD). SPSS 2.0 was used for statistical analysis and GraphPad Prism was used for graph drawing. T-test and one-way ANOVA were used to analyze the statistical analysis of two groups and more than two groups, respectively. In all analyses, two-tailed P<0.05 was considered to indicate a statistically significant difference.
Results
Expression and knockdown of NUDT21 in PCa cells
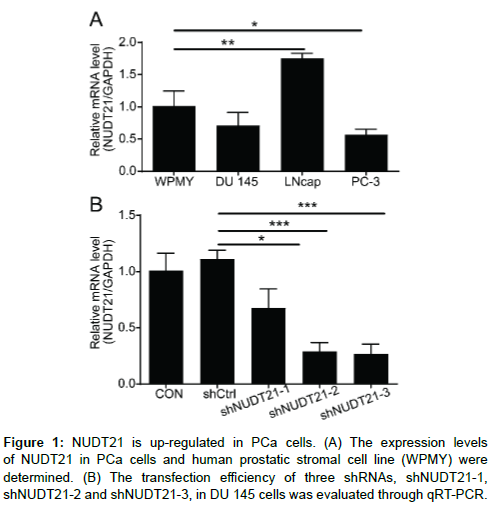
Recently, studies have shown that NUDT21 is elevated in many types of human cancers and involved in many biological functions. Simultaneously, we observed that the mRNA expression level of NUDT21 was also showed a significant up-regulation in LNcap cell line compared to human prostatic stromal cell line (WPMY) (Figure 1A). Thus, we speculated that the NUDT21 is involved in the progression of PCa. To further assess the biological roles of NUDT21 in the PCa cells, NUDT21 knockdown cell models were constructed. Briefly, stable NUDT21 knockdown in the PCa cells was established using a lentiviral delivery system. A total of three different sequences of shRNA (shNUDT21-1, shNUDT21-2 and shNUDT21-3) were used to determine the most effective RNA interference sequence in the DU 145 cells. Compared with the shCtrl group, the NUDT21 mRNA expression of shNUDT21-1, shNUDT21-2 and shNUDT21-3 were decreased by 39.2%, 74.0% and 76.0%, respectively (P < 0.05; P < 0.001; P < 0.001) (Figure 1B). The shNUDT21-3 with higher knockdown efficiency was used for further experiments.
Knockdown efficiency of NUDT21 in the DU 145 and LNcap
We next stably transfected DU 145 and LNcap cells with shNUDT21-3, while cells transfected with shCtrl were used as negative controls. As shown in Figure 2A, most DU 145 and LNcap cells transfected with shCtrl or shNCAPG2 were green fluorescent protein (GFP) positive, which confirmed the high transduction efficiency of vectors. Subsequently, the efficiency of NUDT21 knockdown was assessed at both the protein and mRNA levels. Compared to the shCtrl of each cell lines, knockdown efficiency of NCAPG2 was 92.45% in DU 145 cells and 92.59% in LNcap cells (Figure 2B). Moreover, Western blot analysis showed that the protein levels of NUDT21 were significantly decreased upon NUDT21 knockdown in both cell lines compared to the shCtrl group (Figure 2C). Altogether, these results suggested the NUDT21-knockdown models were successfully established and also confirmed that the up-regulation of NUDT21 protein in DU 145 and LNcap cell lines.
Figure 2: Knockdown efficiency of NUDT21 in the DU 145 and LNcap. (A) The transfection efficiencies of shNUDT21-3 in DU 145 and LNcap cells were evaluated through observing the fluorescence of GFP. Magnification times: 100 ×. (B-C) The NUDT21 expression in DU 145 and LNcap cells after transfection was analyzed by qRT-PCR (B) and western blot (C).
NUDT21 knockdown impaired cell proliferation and induced cell cycle arrest and apoptosis
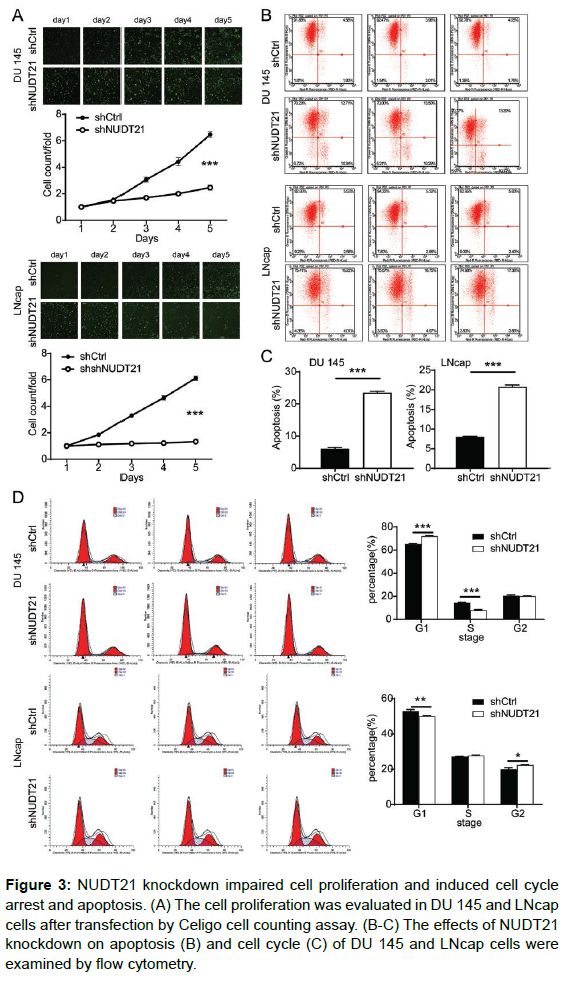
To elucidate the biological functions of NUDT21 in PCa cells in vitro, we further explore whether the NUDT21 was critical for cell proliferation, cell cycle arrest and apoptosis upon NUDT21 knockdown. The effect of NUDT21 knockdown on PCa cell proliferation was determined using Celigo cell counting assay. As shown in Figure 3A, compared with the shCtrl group, cell growth was significantly suppressed in DU 145 cells (2.6-fold change; P < 0.001) and LNcap cells (4.6-fold change; P < 0.001) with stable knockdown of NUDT21 by shRNA transfection at different time points. Thus, NUDT21 knockdown suppresses the proliferation of human PCa cells in vitro. Subsequently, the influence of NUDT21 knockdown on the apoptosis and cell cycle were analyzed by flow cytometry. Compared with shCtrl group, Knockdown of NUDT21 significantly increased the proportion of apoptotic cells by 3.8 fold change in DU 145 cells (P < 0.001) and LNcap cells (P < 0.001) (Figure 3B). The results revealed that the reduced the expression of NUDT21 significantly increased the induction of apoptosis in PCa cells. Furthermore, our results also proved that silencing NUDT21 induced a dramatic alteration in the cell cycle distribution of both DU 145 and LNcap cells. As shown in Figure 3C, the cell ratio in the G1 phase was increased (P < 0.001) and the cell ratio in the G2 phase was decreased significantly in the shNUDT21 group in comparison with that in the shCtrl group of DU 145 cells (P < 0.001), suggesting the increase of DU 145 cells were arrested in the G1 phase. Strikingly, silencing NUDT21 significantly decreased the G1 phase cell fraction (P < 0.001) and induced a G2 cell cycle arrest (P < 0.05) in LNcap cells compared with the shCtrl group cells. These findings indicate that NUDT21 silencing induced cell cycle arrest and affected cell cycle distribution in PCa cells.
Figure 3: NUDT21 knockdown impaired cell proliferation and induced cell cycle arrest and apoptosis. (A) The cell proliferation was evaluated in DU 145 and LNcap cells after transfection by Celigo cell counting assay. (B-C) The effects of NUDT21 knockdown on apoptosis (B) and cell cycle (C) of DU 145 and LNcap cells were examined by flow cytometry.
NUDT21 knockdown inhibited PCa cell migration in vitro
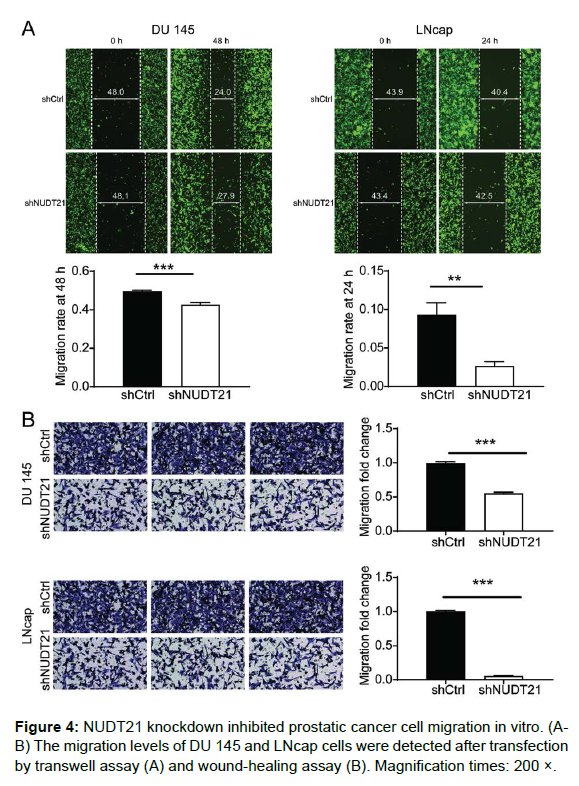
We further evaluated the effect of NUDT21 deficiency on the migration capacity of PCa cells by using a wound healing assay and a Transwell assay. For quantification of migration ratio, images were captured and measured the width of wound at the same site on 0h, 24h and 48h. As shown in Figure 4A, the distance between the scratches was significantly lower in the shCtrl group (48h for DU 145 cells; 24h for LNcap cells), while the distance between the scratches was not significantly lower in the NUDT21 knockdown group, indicating the migration ability of DU 145 and LNcap cells was significantly inhibited by NUDT21 depletion. Similarly, the results of Transwell assay showed that the cell migration rate was decreased by 45% in DU 145 cells with NCAPG2 knockdown (P < 0.001) and 94% in LNcap cells with NUDT21 knockdown (P < 0.001) (Figure 4B). Altogether, these findings suggest that NUDT21 plays a crucial role in migration of PCa cells.
Discussion
PCa is a common slow growing malignant tumor with complex pathogenesis in the male reproductive system [18]. Although PCa can be treated effectively in different ways, its treatment remains a major clinical challenge due to multidrug resistance and almost advancedstage patients suffer from the side effects and recurrences [19,20]. Recent studies have investigated that the gene-targeted therapy for cancers was a promising treatment for cancers [21,22]. Various theoretically attractive targets for PCa treatment have emerged; however, few effective targets are used in clinical studies. In the present study, our results provided insights into the molecular mechanism of PCa progression, suggesting NUDT21 contributing to the development and progression of PCa.
NUDT21, a member of the Nudix family of hydrolases, was considered as a novel post-transcriptional regulator of cell fate, which involves in many physiological processes and participate in many diseases, especially in cancer [23]. It has previously been established that NUDT21 gene regulates the protein level of MeCP2, and ultimately leads to neuropsychiatric diseases [24]. Moreover, accumulating evidence has revealed that have demonstrated that NUDT21 can act as a tumor promoter or suppressor in certain types of cancer, thereby affecting tumor development and progression [9,10,12,13]. Previous study shown that NUDT21 is reduced all four subtypes of high grade glioma [25] bladder cancer [10] and breast cancer [23] Brumbaugh. found that NUDT21 suppression facilitates generation of induced pluripotent stem cells, and delays progenitor cell differentiation [26]. NUDT21 inhibits HCC proliferation, metastasis and tumorigenesis [27]. Conversely, it has been reported that NUDT21 also acts as a prooncogenic role in several cancers. Zhu et al NUDT21 promoted cell proliferation, colony formation, cell migration and invasion in gastric cancer cells [9]
. It has also been reported that NUDT21 suppression significantly inhibited cell proliferation and promoted apoptosis of pancreatic ductal adenocarcinoma [13]. More importantly, NUDT21 was related to drug resistance in childhood acute leukemia [28]. Nevertheless, the role of NUDT21 in PCa remains understudied. Therefore, the the characterization of NUDT21 in the pathological progression of PCa deserves further study. To the best of our knowledge, the present study was the first to provide the evidence of the pro-oncogenic role of NUDT21 in PCa.
In this study, we first contrasted the NUDT21 expression levels in the PCa cell lines DU 145 and LNcap and human prostatic stromal cell line (WPMY). Based on the finding that NUDT21was up-regulated in the PCa cell lines, it will also be interesting to test whether its expression contributes to progression the by modulating cell proliferation and/or migration. In order to verify this conjecture, we designed three target sequences of a short hairpin RNA for NUDT21 and then constructed the stable silenced NUDT21 PCa cell lines by using the best effect sequence. We further verified the effectiveness of the shRNA knockdown strategy in their corresponding cell lines DU 145 and LNcap by PCR and WB. These data indicated that the successful transfection of NUDT21 and could be used for loss-of-function assays. Interestingly, we found that NUDT21 silencing inhibited proliferation and induced cell apoptosis in both PCa cell lines through in vitro experiments. Further experimental results revealed that NUDT21 silencing induced DU 145 cell cycle arrest at G1 phases while promoted G2 cell cycle arrest in LNcap cells. These results suggested that the growth of PCa cells were inhibited by reducing the expression of NUDT21. Additionally, the wound healing assay and transmigration assay verified that NUDT21 suppressed the migration of PCa cells. In a sense, this study provides a theoretical basis for follow-up research. Nevertheless, the functions of NUDT21 in PCa progression remain mostly unknown, additional mechanistic studies are needed to understand the contribution of NUDT21 to the pathogenesis of PCa.
In conclusion, our findings demonstrated that NUDT21 was highly expressed in PCa cells. Moreover, we successfully constructed the shNUDT21 vector, which efficiently knocks down NUDT21 expression. Further in vitro results indicated that targeted knockdown of NUDT21 may suppress PCa progression by inhibiting proliferation and metastasis and promoting apoptosis. Overall, the results of the present study may facilitate further efforts to explore the possibility of NUDT21 as a therapeutic target for PCa treatment.
Conflict of Interest
The authors declare no conflict of interest.
Availability of Data and Materials
The data generated in this study are available within the article and its supplementary data files.
Author Contributions
Wei Wang designed this research. Yu Sun operated the cell and animal experiments. Yu Sun, Tao Sun and Yu Yuan conducted the data processing and analysis. Wei Wang drafted the manuscript which was reviewed by all the authors. All the authors have confirmed the submission of this manuscript.
References
- Tai Z, Ma J, Ding J, Pan H, Chai R, et al. (2020) Aptamer-Functionalized Dendrimer Delivery of Plasmid-Encoding lncRNA MEG3 Enhances Gene Therapy in Castration-Resistant Prostate Cancer. Int J Nanomedicine 15: 10305-10320.
- Wang L, Liu X, Liu Z, Wang Y, Fan M, et al. (2022) Network models of prostate cancer immune microenvironments identify ROMO1 as heterogeneity and prognostic marker. Sci Rep 12: 192.
- Lu L, Li K, Mao Y, Qu H, Yao B, et al. (2017) Gold-chrysophanol nanoparticles suppress human prostate cancer progression through inactivating AKT expression and inducing apoptosis and ROS generation in vitro and in vivo. Int J Oncol 51: 1089-1103.
- Omabe K, Paris C, Lannes F, Taïeb D, Rocchi P (2021) Nanovectorization of Prostate Cancer Treatment Strategies: A New Approach to Improved Outcomes. Pharmaceutics 13: 591.
- Zachovajeviene B, Siupsinskas L, Zachovajevas P, Venclovas Z, Milonas D (2019) Effect of diaphragm and abdominal muscle training on pelvic floor strength and endurance: results of a prospective randomized trial. Sci Rep 9: 19192.
- Bilusic M, Madan RA, Gulley JL (2017) Immunotherapy of Prostate Cancer: Facts and Hopes. Clin Cancer Res 23: 6764-6770.
- Xiao Q, Sun Y, Dobi A, Srivastava S, Wang W, et al. (2018) Systematic analysis reveals molecular characteristics of ERG-negative prostate cancer. Sci Rep 8: 12868.
- Widjaja L, Werner R, Ross T, Bengel F, Derlin T (2021) PSMA Expression Predicts Early Biochemical Response in Patients with Metastatic Castration-Resistant Prostate Cancer under Lu-PSMA-617 Radioligand Therapy. Cancers 13: 2938.
- Zhu Y, Zhang R, Zhang Y, Cheng X, Li L, et al. (2021) NUDT21 Promotes Tumor Growth and Metastasis Through Modulating SGPP2 in Human Gastric Cancer. Frontiers Onc 11: 670353.
- Xiong M, Chen L, Zhou L, Ding Y, Kazobinka G, et al. (2019) NUDT21 inhibits bladder cancer progression through ANXA2 and LIMK2 by alternative polyadenylation. Theranostics 9: 7156-7167.
- Lang X, Zhao W, Huang D, Liu W, Shen H, et al. (2019) The role of NUDT21 in microRNA-binging sites of EZH2 gene increases the of risk preeclampsia. J Cell Mol Med 23: 3202-3213.
- Gao C, Xu Q, Xiao F, Wang H, Wu C, et al. (2020) NUDT21 suppresses the growth of small cell lung cancer by modulating GLS1 splicing. Biochem Biophys Res Commun 526: 431-438.
- Zheng Y, Chen M, Lei W, Zhu S, You X, et al. (2020) NUDT21 knockdown inhibits proliferation and promotes apoptosis of pancreatic ductal adenocarcinoma through EIF2 signaling. Exp Cell Res 395: 112182.
- Xing Y, Chen L, Gu H, Yang C, Zhao J, et al. (2021) Downregulation of NUDT21 contributes to cervical cancer progression through alternative polyadenylation. Oncogene 40: 2051-2064.
- Wang X, Zhang Y, Xiao J, Zhang K, Li Q, et al. (2018) 18 beta-glycyrrhetinic acid ameliorates the cognitive functions and decreases the recurrence rate of pituitary adenomas patients. EXCLI J 17: 753-761.
- Chen L, Li W, Li Z, Song Y, Zhao J, et al. (2021) circNUDT21 promotes bladder cancer progression by modulating the miR-16-1-3p/MDM2/p53 axis. Mol Ther Nucleic Acids 26: 625-636.
- Lou J, Lan Y, Gao J, Ma B, Yang T, et al. (2017) Silencing NUDT21 Attenuates the Mesenchymal Identity of Glioblastoma Cells via the NF-κB Pathway. Front Mol Neurosci 10: 420.
- Suh S, Chen Y, Zaman M, Hirata H, Yamamura S, et al. (2011) MicroRNA-145 is regulated by DNA methylation and p53 gene mutation in prostate cancer. Carcinogenesis 32: 772-778.
- Josson S, Xu Y, Fang F, Dhar S, St Clair D, et al. (2006) RelB regulates manganese superoxide dismutase gene and resistance to ionizing radiation of prostate cancer cells. Oncogene 25: 1554-1559.
- Yu J, Sun L, Hao T, Zhang B, Chen X, et al. (2019) Restoration of FKBP51 protein promotes the progression of castration resistant prostate cancer. Ann Transl Med 7: 729.
- Liu Y, Jiang J, Liu C, Zhao W, Ma Y, et al. (2021) Synergistic anti-tumor effect of anti-PD-L1 antibody cationic microbubbles for delivery of the miR-34a gene combined with ultrasound on cervical carcinoma. Am J Transl Res 13: 988-1005.
- Díaz de la Guardia-Bolívar E, Barrios-Rodríguez R, Zwir I, Jiménez-Moleón J, Del Val C (2022) Identification of novel prostate cancer genes in patients stratified by Gleason classification: role of antitumoral genes. Int J Cancer.
- Wang B, Liu D, Guo Q, Han X, Bi X, et al. (2020) NUDT21 Suppresses Breast Cancer Tumorigenesis Through Regulating CPSF6 Expression. Cancer Manag Res 12: 3069-3078.
- Gennarino V, Alcott C, Chen C, Chaudhury A, Gillentine M, et al. (2015) NUDT21-spanning CNVs lead to neuropsychiatric disease and altered MeCP2 abundance via alternative polyadenylation. Elife 4: e10782.
- Chu Y, Elrod N, Wang C, Li L, Chen T, et al. (2019) Nudt21 regulates the alternative polyadenylation of Pak1 and is predictive in the prognosis of glioblastoma patients. Oncogene 38: 4154-4168.
- Brumbaugh J, Di Stefano B, Wang X, Borkent M, Forouzmand E, et al. (2018) Nudt21 Controls Cell Fate by Connecting Alternative Polyadenylation to Chromatin Signaling. Cell 172: 106-120.
- Tan S, Li H, Zhang W, Shao Y, Liu Y, et al. (2018) NUDT21 negatively regulates PSMB2 and CXXC5 by alternative polyadenylation and contributes to hepatocellular carcinoma suppression. Oncogene 37: 4887-4900.
- Szczepanek J, Styczyński J, Tretyn A, Pogorzała M, Wysocki M (2012) Identification of the genes expression profile associated with the ex vivo resistance to etoposide in childhood acute leukemias. Postepy Hig Med Doswy 66: 401-408.
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Citation: Sun Y, Sun T, Yu Y, Wang W (2022) Knockdown of NUDT21 Inhibits Cell Proliferation and Cell Migration, Promotes Apoptosis in Prostate Cancer. Cell Mol Biol, 68: 230. DOI: 10.4172/1165-158X.1000230
Copyright: © 2022 Sun Y. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 2877
- [From(publication date): 0-2022 - Nov 15, 2025]
- Breakdown by view type
- HTML page views: 2268
- PDF downloads: 609