Kinetic Studies of Ranitidine Hydrochloride Film Coated Tablets Available in Bangladesh: In vitro Study Reflection on in vivo
Received: 25-Oct-2017 / Accepted Date: 04-Nov-2017 / Published Date: 06-Nov-2017 DOI: 10.4172/2167-065X.1000178
Abstract
Background: This study deals with the comparative in vitro dissolution and in vitro disintegration characteristics of different brands of ranitidine hydrochloride film coated tablets most commonly available in Bangladesh which reflects the in vivo study. The objective of this present study was to evaluate the in vitro kinetic studies of ranitidine hydrochloride film coated tablets available in Bangladesh. Ranitidine hydrochloride is a potent H2 blocker recommended for hyperacidity related disorders and most common OTC drugs that frequently used by the common people in the world.
Methods: Twenty one brands of ranitidine hydrochloride film coated tablets available in Bangladesh drug market were assayed spectrophotometrically and their various kinetics (Zero Order, First Order, Higuchi and Hixson-Crowell) studies were performed to predict in vivo analysis.
Results: In Bangladesh, commercially available twenty one brands of ranitidine hydrochloride film coated tablets were studied. The media of the study was distilled water (pH 7.5) due to identify complete organoleptic properties of Ranitidine Hydrochloride. Nine brands (RH-3, RH-5, RH-6, RH-10, RH-11, RH-12, RH-13, RH-14 and RH-17) showed delayed disintegration time instead of quick release formulation, which often claimed by those manufacturer. Other twelve brands (RH-1, RH-2, RH-4, RH-7, RH-8, RH-9, RH-15, RH-16, RH-18, RH-19, RH-20 and RH-21) showed the moderate disintegration time. The tablets were studied for in vitro dissolution behavior for 1 h in distilled water using USP reference dissolution apparatus. The in vitro dissolution profiles of the thirteen brands (RH-1, RH-2, RH-4, RH- 7, RH-8, RH-9, RH-13, RH-15, RH-16, RH-18, RH-19, RH-20 and RH-21) were fulfilled the USP in vitro dissolution specification of 80% drug release within 45 min. On the other hand, five of the total selected brands (RH-3, RH-5, RH-10, RH-12 and RH-17) were failed to fulfill the USP in vitro dissolution specification. Rest three brands (RH-6, RH-11 and RH-14) exhibited very poor release pattern among the selected brands and their drug release rate were 23%, 11% and 19% respectively after one hour study. The drug potency, multiple coefficient (from zero order, first order, Higuchi and Hixson-Crowell cube root law), similarity and dissimilarity factors were determined in this study. The predicted steady state plasma concentration determined by comparing the in vivo pharmacokinetic data of reference brand (Zantac) using superposition principle of steady state plasma levels determination.
Conclusion: Most of the brands meet the official requirements, which are very essential parameters for the prediction of in vivo drug release by oral route. As a result the patients will get the proper therapeutic effect to combat against hyperacidity problems while they use those brands of ranitidine hydrochloride film coated tablets.
Keywords: Ranitidine hydrochloride; H2 blocker; Hyperacidity; Kinetics studies; Bangladesh
Abbreviations
OTC: Over-The-Counter; GD: Gastrointestinal Disorder; NSAID: Non-Steroidal Anti-Inflammatory Drugs; GERD: Gastroesophageal Reflux Disease; USP: United States of Pharmacopoeia; DI: Dosage Form Index
Introduction
Ranitidine hydrochloride is a histamine H2-receptor antagonist. It is widely prescribed in active duodenal ulcers, gastric ulcers, Zollinger- Ellison syndrome, gastro esophageal reflux disease (GERD) and erosive esophagitis. The effective treatment of erosive esophagitis requires administration of 150 mg of Ranitidine hydrochloride four times a day. A conventional dose of 150 mg can inhibit gastric acid secretion up to five hours, but not up to 10 h. An alternative dose of 300 mg leads to plasma fluctuations; thus a sustained release dosage form of Ranitidine hydrochloride is desirable. The short biological half-life of the drug (~2.5 3 h) also favors development of a sustained release formulation. Ranitidine is absorbed only in the initial part of the small intestine and has 50% absolute bioavailability. Moreover, colonic metabolism of Ranitidine is partly responsible for the poor bioavailability of ranitidine from the colon [1]. The gastro retentive drug delivery systems can be retained in the stomach and assist in improving the oral sustained delivery of drugs that have an absorption window in a particular region of the gastrointestinal tract. These systems help in continuously releasing the drug before it reaches the absorption window, thus ensuring optimal bioavailability [2]. Hyperacidity is a serious health care problem and almost 67% of total global population suffered by gastrointestinal disorders (GD). Thus management of hyperacidity becomes very important to improve the human physiological system. A number of no systemic antacid drugs are used; however, their therapeutic potential is very ambiguous. On the other hand, H2 blockers replaced the therapeutic discrepancies of non-systemic antacids and successfully managed the hyperacidity problems in pathological and NSAID induced condition [3].
The process of in vitro disintegration and in vitro dissolution played vital roles in liberating a drug from the tablet matrix and marking whether it is available for subsequent gastrointestinal absorption. The in vitro dissolution of the drug from the tablet matrix depends on many factors, which include not only the physicochemical properties of drug, rather the nature of formulation and manufacturing process [4]. Hence in vitro dissolution analysis of pharmaceutical dosage form has emerged a very important parameter that ensures product quality as well as for differentiating among formulations of the same therapeutic agent [5]. In vitro dissolution studies is an important tool in the evaluation of the best formulation and also in the understanding of possible risks related to specific gastrointestinal environment, dose dumping, food effects on bioavailability and interaction with other drugs [6]. In continuation of our research on different finished pharmaceutical dosage form [7] here we further focused on in vitro evaluations of different brands of ranitidine hydrochloride film coated tablets in Bangladesh.
Materials And Methods
Chemicals
USP references standard of ranitidine hydrochloride (Merk, Germany).
Reference brand
Zantac (Glaxco Smith Kline, Bangladesh).
Equipment
UV-visible Spectrometer (UV mini-1700, Shimadzu Corporation, Kyoto, Japan with 1 cm quartz cells), HANNA HI 2211 PH meter ( Romania), Automated eight basket tablet dissolution tester UDP- 80 USP Standard (Veego, India), Electronic balance AL 204 (Mettler Toledo, Japan), Hot air oven Labtech (Daihan, Korea), Sartorius electronic balance, Thickness gauge (Campbell Electronics, India), Monsanto hardness tester (Campbell Electronics, India), Roche friabilator (Campbell Electronics, India).
Dosages forms
Twenty one national brands of marketed (production date not more than four months ago from the time of purchase) ranitidine hydrochloride film coated tablets of the test drug were purchased from various stores. The samples were properly checked for their manufacturing license number, batch number and date of manufacture and expiry dates before collection. They were randomly coded (RH- 1, RH-2, RH-3, RH-4, RH-5, RH-6, RH-7, RH-8, RH-9, RH-10, RH- 11, RH-12, RH-13, RH-14, RH-15, RH-16, RH-17, RH-18, RH-19, RH-20, RH-21). The labeled active ingredient containing ranitidine hydrochloride was 150 mg and packaged in strip or in blister packing. The strip or blister packs were stored at 25 ± 2°C for four weeks before the dissolution study in order to evaluate any organoleptic changes.
In vitro dissolution study
The release rate of ranitidine hydrochloride film coated tablets was determined by United States Pharmacopoeia (USP) referred dissolution testing apparatus 2 (paddle method). The dissolution test was performed in distilled water (900 ml) at 37 ± 0.5ºC and at 50 rpm paddle speed. The medium was preheated to 37°C and then added to the vessels. The paddle rotation was started after the medium was placed in the vessels and the system was allowed to equilibrate for 15 min. Each vessel, vessel position and corresponding tablet result were assigned the same number. Thus, for each sub sample of six tablets tested simultaneously, every individual tablet result was identified with a particular vessel and position. The in vitro release profile of those formulations were studied in distilled water (pH 7.5) continued for 1 h using USP reference dissolution apparatus. Six tablets from each formulation were weighed and placed in the baskets. At every ten minutes interval sample (10 ml) of the solution was withdrawn from the dissolution medium and immediately replaced with equal volumes of dissolution medium. The withdrawn sample (10 ml) was then filtered, diluted and analyzed at 314nm for ranitidine hydrochloride by UV spectrophotometer (Shimadzu, Model UV-160A, Kyoto, Japan). The amount of drug present in the samples was calculated from calibration curves constructed from the standard solution of USP reference standard of the test drugs [8-10].
Drug release kinetics
The in vitro drug release kinetic data were tested with the following mathematical model:
Zero order equation: The equation assumes that the cumulative amount of drug release vs. time. The equation may be as follows [11]:
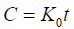 (1)
(1)
Where, K0 is the zero order rate constant expressed in unit concentration/time and t is the time in hours. A graph of concentration vs. time would yield a straight line with a slope equal to K0 and intercept the origin of the axes.
First order equation: The release behavior of first order equation expressed as log cumulative percentage of drug remaining vs. time. The equation may as follows [12]:
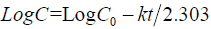 (2)
(2)
Where, C is the amount of drug undissolved at t time, the C0 is drug concentration at t = 0, k corresponding release rate constant.
Higuchi square root law: The Higuchi release models describe as cumulative percentage of drug release vs. square root of time. The equation may as follows [13].
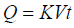 (3)
(3)
Where, Q = (100-C) the amount of drug dissolved at time t. K is the constant reflecting the design variables of the system. Hence, drug release rate is proportional to the reciprocal of the square root of time.
Hixson-Crowell cube root law: It is the law that presenting idea about the evaluation of drug release pattern changes with the surface area and the diameter of the particles/tablets. It is mentioned as the cube root of the percentage of drug remaining in the matrix vs. time. The equation may as follows [14]:
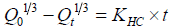 (4)
(4)
Where Q0 is initial amount of the drug in the tablets, Qt is the amount of drug release in time t and kHC is rate constant for the Hixson- Crowell cube root law.
Distinguish the similarity and dissimilarity factors: Dissolution profile were compared by fit factors (similarity and dissimilarity factor) using the dissolution profile of reference brand. In vitro drug release parameters (R0= rate of input and tdel= time of drug release) of similar release profile (based on f2 value) to that of the reference product were fit to in vivo drug concentrations using reported pharmacokinetic properties. Method of superposition was used for steady state prediction concentration. The values of Cmax and Cmin obtained by comparing the actual plasma level of reference brand [15].
The goodness of modified release formulations were evaluated from calculated dosage form index,
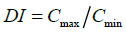 (5)
(5)
And fluctuation 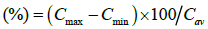 (6)
(6)
Where Cmax and Cmin are maximum and minimum blood concentration. Cav=average blood concentration at steady state.
Results And Discussion
Commercially available twenty one brands (RH-1, RH-2, RH-3, RH-4, RH-5, RH-6, RH-7, RH-8, RH-9, RH-10, RH-11, RH-12, RH- 13, RH-14, RH-15, RH-16, RH-17, RH-18, RH-19, RH-20 and RH- 21) of ranitidine hydrochloride film coated tablet were thoroughly evaluated for their physical properties and found Hardness (kg/ cm2) 3.19 ± 0.01 to 4.35 ± 0.03, Friability (%); 00 to 0.12 ± 0.02, Thickness (mm); 2.51 ± 0.02 to 3.19 ± 0.12 and Weight Variation Test (%); 1.132 ± 0.02 to 2.903 ± 0.23 parameters are in acceptable range. The potency of those selected brands was estimated and found four brands (RH-6, RH-11 and RH-14) did not meet the declared potency (Table 1).
| Code | Potency (%) | Code | Potency (%) |
|---|---|---|---|
| RH-1 | 96.4 | RH-12 | 99.7 |
| RH-2 | 94.8 | RH-13 | 92.3 |
| RH-3 | 98.4 | RH-14 | 69.7 |
| RH-4 | 96.6 | RH-15 | 97.6 |
| RH-5 | 97.9 | RH-16 | 93.2 |
| RH-6 | 72.8 | RH-17 | 92.8 |
| RH-7 | 93.4 | RH-18 | 99.1 |
| RH-8 | 94.8 | RH-19 | 94.1 |
| RH-9 | 95.4 | RH-20 | 96.7 |
| RH-10 | 94.7 | RH-21 | 98.2 |
| RH-11 | 85.4 | Reference | 98.7 |
Table 1: The amount of drug present in different commercial brands of ranitidine hydrochloride film coated tablets.
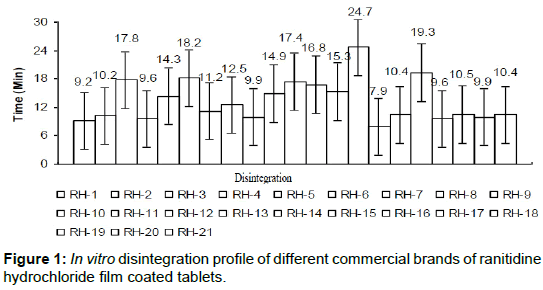
The in vitro disintegration of the selected brands were studied and compared with reference brand (Zantak) as shown in Figure 1.
The declared disintegration time of brands (RH-3, RH-5, RH-6, RH-10, RH-11, RH-12, RH-13, RH-14 and RH-17) showed delayed disintegration time instead of exhibited quick release formulation that often claimed by those manufacturers. Moreover, other brands (RH-1, RH-2, RH-4, RH-7, RH-8, RH-9, RH-15, RH-16, RH-18, RH-19, RH- 20 and RH-21) showed the moderate disintegration time which was similar to that of reference (9.8 min).
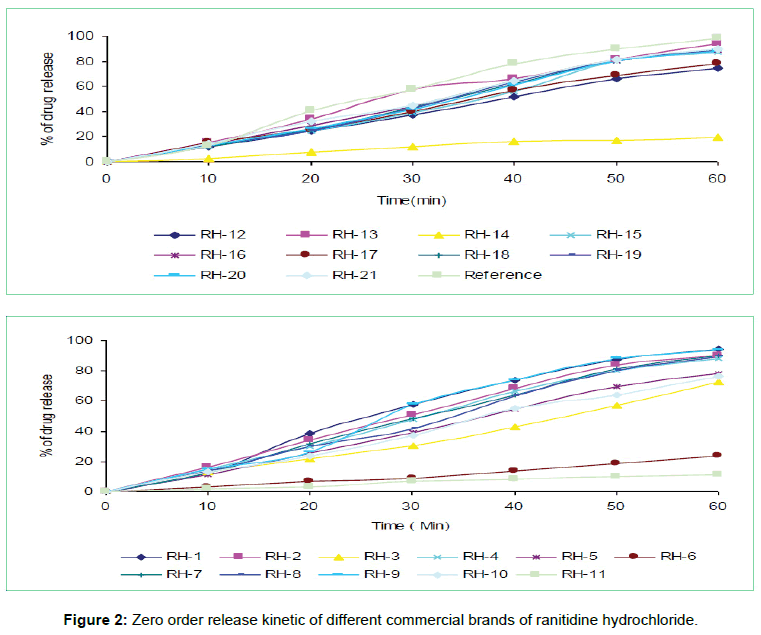
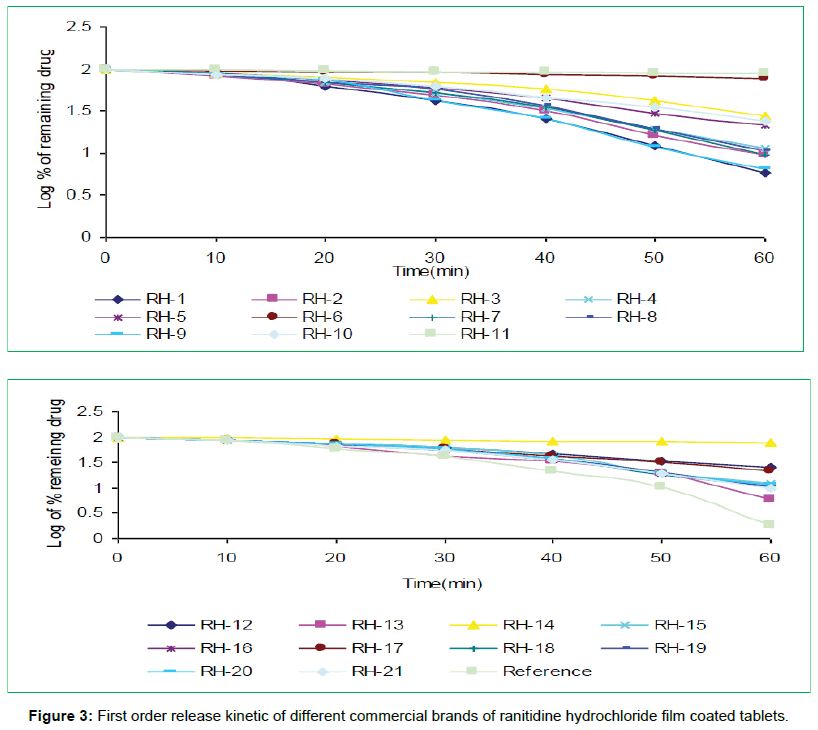
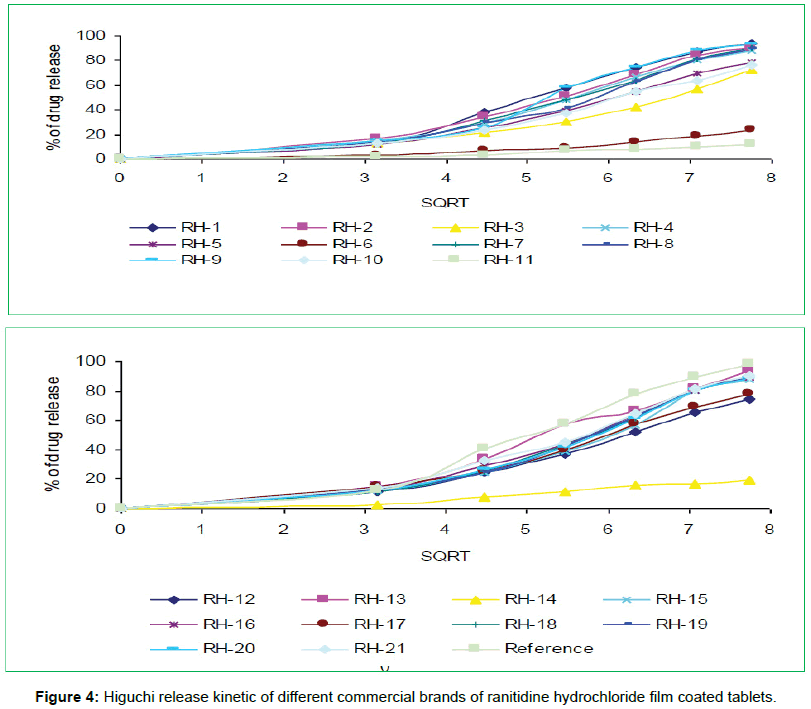
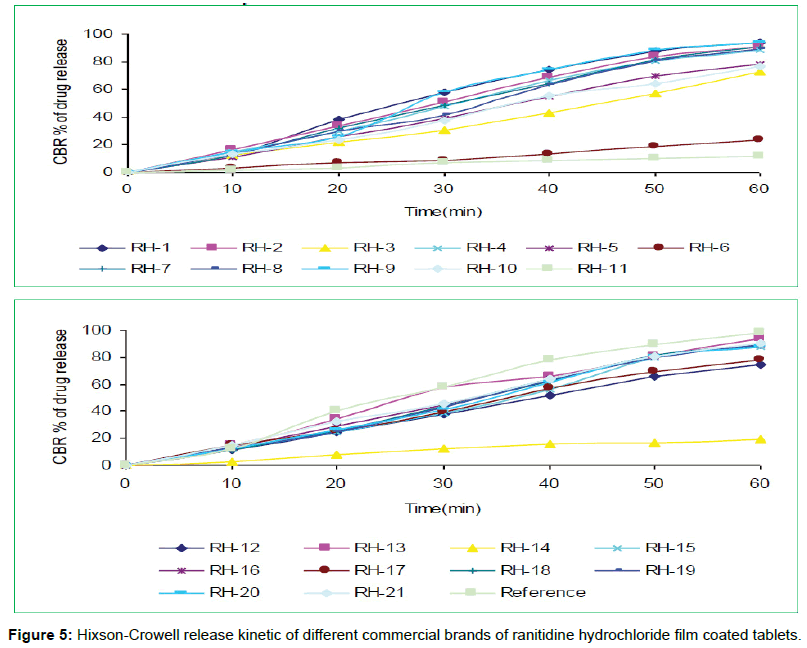
The tablets were studied for in vitro dissolution behavior for 1 h time period in distilled water using USP reference dissolution apparatus as shown in table and figure (Table 2, Figures 2 to 5).
A: Code: RH-1 to RH-11
| Time | RH-1 | RH-2 | RH-3 | RH-4 | RH-5 | RH-6 | RH-7 | RH-8 | RH-9 | RH-10 | RH-11 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 10 | 12.6 | 16.4 | 12.8 | 14.9 | 11.3 | 3.2 | 12.7 | 13.5 | 14.9 | 13.1 | 1.6 |
| 20 | 38.5 | 33.9 | 21.5 | 29.9 | 25.4 | 6.8 | 31.7 | 29.6 | 25.8 | 23.8 | 3.2 |
| 30 | 57.8 | 51.1 | 30.2 | 47.8 | 38.9 | 8.7 | 48.5 | 41.4 | 57.8 | 37.5 | 6.8 |
| 40 | 74.2 | 68.4 | 42.6 | 66.2 | 54.7 | 13.6 | 64.1 | 63.2 | 74.2 | 55.1 | 8.3 |
| 50 | 87.6 | 83.9 | 57.3 | 80.3 | 69.8 | 18.5 | 81.1 | 80.4 | 88.1 | 64.1 | 9.9 |
| 60 | 94.1 | 90.3 | 72.4 | 88.5 | 78.3 | 23.3 | 90.3 | 89.4 | 93.5 | 76.4 | 11.4 |
B: RH-12 to Reference
| Time | RH-12 | RH-13 | RH-14 | RH-15 | RH-16 | RH-17 | RH-18 | RH-19 | RH-20 | RH-21 | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 10 | 11.8 | 14.9 | 2.5 | 12.3 | 13.2 | 14.9 | 11.6 | 13.4 | 12.4 | 14.6 | 12.6 |
| 20 | 24.7 | 33.9 | 7.8 | 24.7 | 28.9 | 25.8 | 25.3 | 24.5 | 26.3 | 32.4 | 40.5 |
| 30 | 37.5 | 57.8 | 11.9 | 38.7 | 44.3 | 39.7 | 43.5 | 42.7 | 41.5 | 45.2 | 57.8 |
| 40 | 51.9 | 66.2 | 15.8 | 56.3 | 64.3 | 57.2 | 62.1 | 63.2 | 61.3 | 64.3 | 78.2 |
| 50 | 65.7 | 81.1 | 17.1 | 80.2 | 80.4 | 68.9 | 81.2 | 80.1 | 80.9 | 81 | 89.6 |
| 60 | 74.7 | 94.1 | 19.6 | 87.3 | 89.3 | 78.2 | 89 | 89.3 | 88.1 | 90.1 | 98.1 |
Table 2: Release profile of different commercial brands of ranitidine hydrochloride film coated tablets.
The in vitro dissolution profile of the thirteen brands (RH-1, RH- 2, RH-4, RH-7, RH-8, RH-9, RH-13, RH-15, RH-16, RH-18, RH-19, RH-20 and RH-21) fulfill the USP in vitro dissolution specification of 80% drug release within 45 min. On the other hand, five of the brands (RH-3, RH-5, RH-10, RH-12 and RH-17) failed to fulfill the USP in vitro dissolution specification that was very much related with error in formulation. Rest three brands (RH-6, RH-11 and RH-14) exhibited very poor release pattern among the selected brands and their drug release rate were 23%, 11% and 19% respectively after 1hour study.
In vitro dissolution studies of all the brands (RH-1 to RH-21) studied throughout the stipulated period and theoretical release profiles of the drug analyzed (Table 3) by multiple coefficients (r2), zero order release kinetics, first order release kinetics, Higuchi release kinetics and Hixson-Crowell release kinetics.
| Code | Zero order | First order | Higuchi | Hixson-Crowell |
|---|---|---|---|---|
| RH-1 | 0.8873 | 0.9839 | 0.8331 | 0.7295 |
| RH-2 | 0.9836 | 0.9506 | 0.8997 | 0.6893 |
| RH-3 | 0.9748 | 0.885 | 0.9203 | 0.7195 |
| RH-4 | 0.9379 | 0.9714 | 0.9621 | 0.6872 |
| RH-5 | 0.2212 | 0.6419 | 0.8682 | 0.4661 |
| RH-6 | 0.7265 | 0.8902 | 0.9871 | 0.6865 |
| RH-7 | 0.9998 | 0.9057 | 0.9856 | 0.8704 |
| RH-8 | 0.9816 | 0.9993 | 0.913 | 0.6225 |
| RH-9 | 0.978 | 0.893 | 0.8267 | 0.4561 |
| RH-10 | 0.8584 | 0.9464 | 0.8977 | 0.4961 |
| RH-11 | 0.9833 | 0.9269 | 0.8558 | 0.4271 |
| RH-12 | 0.9865 | 0.9105 | 0.8474 | 0.7691 |
| RH-13 | 0.8822 | 0.9789 | 0.9125 | 0.8524 |
| RH-14 | 0.9823 | 0.9142 | 0.8524 | 0.7165 |
| RH-15 | 0.9836 | 0.9252 | 0.8453 | 0.6661 |
| RH-16 | 0.8835 | 0.9788 | 0.9254 | 0.689 |
| RH-17 | 0.9846 | 0.9387 | 0.8824 | 0.7244 |
| RH-18 | 0.9809 | 0.9387 | 0.8721 | 0.7052 |
| RH-19 | 0.9697 | 0.959 | 0.9158 | 0.4724 |
| RH-20 | 0.8949 | 0.9563 | 0.8559 | 0.7195 |
| RH-21 | 0.873 | 0.9139 | 0.8649 | 0.6145 |
| Reference | 0.9836 | 0.893 | 0.9267 | 0.6872 |
Table 3: Drug release mechanism (Multiple coefficients [r2]) of different commercial brands ranitidine hydrochloride film coated tablets.
The result obtained indicated the highest linearity of all analyzed formulations and the fit factors found (f1 and f2) discussed (Table 4). Considering the dissolution profile, similar value of f2 should lie 50 to 100 and dissimilarity factor f1 was also calculated to approximate the percentage error between different release profiles. The value of f1 is zero when the test and reference formulation profiles are identical and increase proportionally with the dissimilarity between different profiles. If the value of f2 is 50, 90% similarity in the profile was indicated and if it is 40 then 80% similarity may be indicated. Eight brands (Code: RH-1, RH-5, RH-9, RH-10, RH-16, RH-20 and RH-21) showed dissimilarity between their different release profiles (Table 4). On the other hand, eleven brands (Code: RH-2, RH-3, RH-4, RH-7, RH-11, RH-12, RH-13, RH-14, RH-15, RH-17and RH- 18) showed 80% similarity and two brands (Code: RH-8and RH- 19) showed 90% similarity between their different release profiles (Table 4).
| Code | f1 | f2 |
|---|---|---|
| RH-1 | 108.9 | 24.5 |
| RH-2 | 28.5 | 48.5 |
| RH-3 | 31.3 | 41.3 |
| RH-4 | 17.2 | 47.2 |
| RH-5 | 98.3 | 12.8 |
| RH-6 | 78.6 | 18.9 |
| RH-7 | 25.6 | 46.3 |
| RH-8 | 23.1 | 56.3 |
| RH-9 | 85.6 | 16.2 |
| RH-10 | 102 | 12.6 |
| RH-11 | 25.8 | 40.1 |
| RH-12 | 14.6 | 42.9 |
| RH-13 | 21.3 | 41.2 |
| RH-14 | 23.5 | 40.2 |
| RH-15 | 21.7 | 40.1 |
| RH-16 | 78.9 | 25.6 |
| RH-17 | 27.3 | 42.6 |
| RH-18 | 24.1 | 42.7 |
| RH-19 | 21 | 50.6 |
| RH-20 | 89.6 | 19.9 |
| RH-21 | 101.3 | 21.8 |
| Reference | 28.3 | 45.6 |
Table 4: Similarity and dissimilarity factor values for drug release profile of different commercial brands of ranitidine hydrochloride film coated tablets.
Using superposition principle study, a steady state plasma level was calculated (Table 5). The predicted steady state plasma drug concentration of all brands showed some dissimilarity compared to reference brand (Zantac). The calculated plasma drug concentration of six brands (Code: RH-5, RH-6, RH-10, RH-11, RH-12 and RH-14) were in below therapeutic level and other fifteen brands (Code: RH-1, RH-2, RH-3, RH-4, RH-7, RH-8, RH-9, RH-13, RH-15, RH-16, RH- 17, RH-18, RH-19, RH-20 and RH-21) lied within the therapeutically acceptable range compare to the reference brand. The dosage form index (DI) and fluctuation (%) determined by using zero order, first order and Higuchi release profile (Table 5).
| Code | Dose | k | Cmax (ng/ml) | Cmin (ng/ml) | DI | Fluctuation |
|---|---|---|---|---|---|---|
| (mg) | (%) | |||||
| RH-1 | 100 | 0.345a | 435 a | 418 a | 1.04 | 3.82 |
| RH-2 | 100 | 1.976b | 427 b | 411 b | 1.04 | 3.596 |
| RH-3 | 100 | 1.356 b | 408 b | 201 b | 2.03 | 46.52 |
| RH-4 | 100 | 0.894 a | 445 a | 412 a | 1.08 | 7.416 |
| RH-5 | 100 | 1.458 b | 324 b | 86 b | 3.77 | 53.48 |
| RH-6 | 100 | 24.218c | 368 c | 314 c | 1.17 | 12.13 |
| RH-7 | 100 | 1.147 b | 418 b | 392 b | 1.07 | 5.843 |
| RH-8 | 100 | 0.387 a | 432 a | 411 a | 1.05 | 4.719 |
| RH-9 | 100 | 1.156 b | 441 b | 410 b | 1.08 | 6.966 |
| RH-10 | 100 | 0.142 a | 389 a | 100 a | 3.89 | 64.94 |
| RH-11 | 100 | 1.261 b | 359 b | 130 b | 2.76 | 51.46 |
| RH-12 | 100 | 1.487 b | 289 b | 40 b | 7.23 | 55.96 |
| RH-13 | 100 | 18.341 c | 426 c | 405 c | 1.05 | 4.719 |
| RH-14 | 100 | 1.132 b | 289 b | 240 b | 1.2 | 11.01 |
| RH-15 | 100 | 1.325 b | 415 b | 395 b | 1.05 | 4.494 |
| RH-16 | 100 | 0.621 a | 422 a | 398 a | 1.06 | 5.393 |
| RH-17 | 100 | 1.213 b | 389 b | 165 b | 2.36 | 50.34 |
| RH-18 | 100 | 1.712 b | 440 b | 422 b | 1.04 | 4.045 |
| RH-19 | 100 | 1.167 b | 429 b | 410 b | 1.05 | 4.27 |
| RH-20 | 100 | 0.371 a | 421 a | 390 a | 1.08 | 6.966 |
| RH-21 | 100 | 0.154 a | 425 a | 410 a | 1.04 | 3.371 |
| Reference | 100 | 1.214 b | 445 b | 412 b | 1.08 | 7.416 |
Table 5: Predicted steady state plasma concentration level of different commercial brands of ranitidine hydrochloride film coated tablets determined from in vitro drug release profile by comparing with in vivo pharmacokinetic data of references brands available in Bangladesh.
Table 5 Predicted steady state plasma concentration level of different commercial brands of ranitidine hydrochloride film coated tablets determined from in vitro drug release profile by comparing with in vivo pharmacokinetic data of references brands available in Bangladesh.
Conclusion
The present study indicated that most of the brands meet the official requirements, which are very essential parameters for the prediction of in vivo drug release by oral route. As a result the patients will get the proper therapeutic effect to combat against hyperacidity problems while they use those brands of ranitidine hydrochloride film coated tablets. This investigation was performed to evaluate quality of drug products, ensure patient’s health at home and promote export of medication from Bangladesh.
Authors’ Contributions
MKI, MDS and MHK designed the concept of the study. MKI, MDS and RH performed the laboratory works. MDS and TS drafted the manuscript. MHK critically reviewed and revised the manuscript. All authors read and approved the final manuscript.
Conflict of Interests
The authors declare that they have no conflict of interest.
Funding
No Funding.
Acknowledgement
The authors wish to thank, Biopharmaceuticals Laboratory, Department of Pharmacy, State University of Bangladesh for providing laboratory facilities to carry out the experiments.
References
- Abdul WB, Larry FL (2001) Colonic metabolism of ranitidine, implications for its delivery and absorption. Int J Pharm 227: 157-165.
- Jain S, Srinath MS, Narendra C, Reddy SN, Sindhu A (2010) Development of a floating dosage form of ranitidine hydrochloride by statistical optimization technique. J Young Pharm 2: 342-349.
- Goodman and Gilman’s The Pharmacological Basis of Therapeutics (2001) McGrew-Hill Medical publishing division. J Pharm Sci 1:971-972.
- Augsburger LL, Shangraw RF, Giannini RP, Shah VP, Prasad VK, et al. (1983) Thiazides VIII: Dissolution survey of marketed hydrochlorothiazide tablets. J Pharm Sci 72: 876-881.
- Ayres JW, Huang H, Albert K (1984) Effect of polymer in sustained release matrix tablet. J Pharm Sci 73: 1629.
- Sungthongjeen S, Pitaksuteepong T, Somsiri A, Sriamornsak P (1999) Studies on pectins as potential hydrogel matrices for controlled-release drug delivery. Drug Dev Ind Pharm 25: 1271-1276.
- Addnan A, Sukumar B, Abu SSR (2008) In vitro dissolution study of different brands of sustained release diclofenac sodium matrix tablet available in Bangladesh. Pak J Pharm Sci 21: 70-77.
- Marshall K, Lachman N, Liberman HA (1987) The theory and practice of industrial pharmacy. Varghese Publishing House 1: 66-69.
- Ministry of Health and Family Welfare, Government of India, New Delhi (1996) Controller of Publications. Indian Pharmacopoeia 1: 736-737.
- Bouman J, Belton P, Venema P, Linden EV, Vries R, et al. (2016) Interplay of drug hydrophobicity and pH. Pharm Res 33: 673-685.
- Hadjiioannou TP, Christian GD, Koupparis MA (1993) Quantitative calculation in pharmaceutical practice and research. VCH 25: 345-348.
- Bourne DW (2002) Pharmacokinetics. In: Banker GS, Rhodes CT, eds. Modern Pharmaceutics. Marcel Dekker Inc 6: 67-92.
- Higuchi T (1963) Mechanism of sustained action medication, theoretical analysis of rate of release of solid drugs dispersed in solid matrices. J Pharm Sci 52: 1145-1149.
- Hixson AW, Crowell JH (1931) Dependence of reaction velocity upon surface and agitation: I-theoretical consideration. Ind Eng Chem 23: 923-931.
- Gladziwa U, Klotz U (1993) Pharmacokinetics and Pharmacodynamic of H2 receptor antagonists with renal insufficiency. Clin Pharmacokinet 24: 319-332.
Citation: Islam MDK, Sohel MDD, Hossain R, Sultana T, Kawsar MDH (2017) Kinetic Studies of Ranitidine Hydrochloride Film Coated Tablets Available in Bangladesh: In vitro Study Reflection on in vivo. Clin Pharmacol Biopharm 6: 178. DOI: 10.4172/2167-065X.1000178
Copyright: © 2017 Islam MDK, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 5474
- [From(publication date): 0-2017 - Jan 31, 2025]
- Breakdown by view type
- HTML page views: 4796
- PDF downloads: 678