Ketohexokinase (KHK) Physiology and Treatments in Obesity and Fructose Metabolism
Received: 01-Nov-2023 / Manuscript No. JOWT-23-119054 / Editor assigned: 03-Nov-2023 / PreQC No. JOWT-23-119054 (PQ) / Reviewed: 17-Nov-2023 / QC No. JOWT-23-119054 / Revised: 24-Nov-2023 / Manuscript No. JOWT-23-119054 (R) / Published Date: 01-Dec-2023
Introduction
Obesity is defined as a disproportionate body weight for height with excessive accumulation of subcutaneous and visceral adipose tissue. Over the past decades, number of obesity patient multiplied. Obesity has turned into a medical issue worldwide. According to a survey from WHO in 2016, more than 1.9 billion adults were overweight or obese [1]. By 2030, there will be 3.3 billion people who have a BMI greater than 25 kg/m2 [2]. Obesity is associated with the development of type 2 diabetes mellitus, cardiovascular infection, systemic inflammation and particular kinds of cancer [3]. Some of these obesity-associated diseases belong to metabolic syndrome [4]. In recent years, several factors leading to obesity have been disclosed. Among these, excessive intake of fructose may contribute heavily to the epidemic of obesity, type 2 diabetes mellitus, Non-Alcoholic Fatty Liver Disease (NAFLD) and other metabolic disease [5-7]. Herein, we review the biochemistry, physiology of fructose metabolism and generalize the current treatment progress.
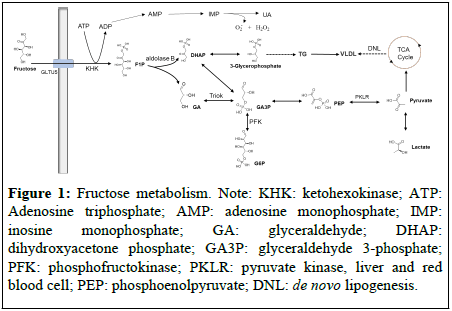
As a simple ketohexose, fructose is derived mostly from fruits, honey, and vegetables. It is the sweetest natural sugar. Sucrose composed of 50% fructose and 50% glucose, and High-Fructose Corn Syrup (HFCS) are commonly used as sweeteners in many processed foods and carbonated beverages. Worryingly, in recent decades, the fructose consumption has increased dramatically. Upon oral ingestion, fructose is absorbed in the small intestine and transported into enterocytes and hepatocytes via Glucose transporter5 (Glut5). Upon entering the cells, fructose is initially metabolized to Fructose-1- Phosphate (F1P) by Ketohexokinase (KHK) with Adenosine Triphosphate (ATP) depletion [8]. The consuming of ATP leads to the production of Reactive Oxygen Species (ROS) and Uric Acid (UA) which is a major etiologic factor in gout. F1P could be converted by aldolase B to GA and DHAP. GA is phosphorylated by Triose kinase (Triok) to GA3P which could be resynthesized into glucose via gluconeogenesis. Both GA3P and DHAP could be metabolized into lactate and pyruvate which are used for lipogenesis. Unlike the glucose metabolism pathway, there is no negative feedback regulation of fructokinase to prevent it from metabolizing fructose [9-11]. Compared to glucose, fructose significantly elevates de novo Lipid (DNL) synthesis [12]. According to our knowledge, there are several potential mechanisms for leading to obesity or other metabolic syndrome associated with fructose: (1) High doses of fructose can induce both hepatic and peripheral insulin resistance [13]; (2) Excessive consuming fructose lead to hyper energy intake; (3) Dietary fructose improves the survival of intestinal cells and increases intestinal villus length resulting to the promotion of the nutrient absorption [5] (Figure 1).
Figure 1: Fructose metabolism. Note: KHK: ketohexokinase; ATP: Adenosine triphosphate; AMP: adenosine monophosphate; IMP: inosine monophosphate; GA: glyceraldehyde; DHAP: dihydroxyacetone phosphate; GA3P: glyceraldehyde 3-phosphate; PFK: phosphofructokinase; PKLR: pyruvate kinase, liver and red blood cell; PEP: phosphoenolpyruvate; DNL: de novo lipogenesis.
KHK is the significant fructose metabolic enzyme that initiates the phosphorylation of fructose on position C1 utilizing ATP as a cofactor [14-16]. KHK is expressed as two distinct isoforms (KHK-A and KHK-C) from a single gene [17]. Although the affinity of KHK-A for fructose is more potent than KHK-C, KHK-C is the primary isoform because of that KHK-C is expressed at high levels in key metabolic tissues including liver and small intestine while KHK-A is expressed on low levels. Additionally, KHK knockout mice were fully protected from fructose-induced increases in body weight, serum lipid and serum insulin [18]. In especial, liver-specific knockout or knockdown of KHK can protect against fructose-induced metabolic disease including obesity [19]. These results support that fructose is metabolized by KHK especially in the liver.
Based on the above evidence, KHK inhibitors or KHK expression downregulation appeared to be potential therapeutic strategy for fructose metabolic diseases. Pharmaceuticals have paid considerable attention to discover novel KHK inhibitors. In 2011, Johnson & Johnson Pharmaceutical reported a KHK inhibitor with high potency but in low exposure due to a high metabolic clearance [20]. PF-06835919, discovered by Pfizer in 2015, is currently in two Phase 2a studies (NCT06089265, NCT05463575). Preclinical studies showed dose-dependent (p.o., 75 mg/kg and 300 mg/kg) downregulation of HOMA-IR, Hs-CRP, triglyceridemia, uric acid, and upregulation of adiponectin level under a high-fructose diet after PF-06835919 was administrated for six weeks [21]. The results indicated that KHK inhibition may be helpful for treatment of fructose-induced metabolic diseases. Compared to PF-06835919, Eli Lilly and Company reported a novel KHK inhibitor with high potency on enzyme and cell in 2020 [22]. Shandong Xuanzhu Pharma, TuoJie Biotech (Shanghai), and LG Chem etc. also disclosed KHK inhibitors, respectively [23-25].
Except for KHK inhibitors, KHK expression downregulation via siRNA technology has been developed in recent years. Compared to wild type group, KHK knockout mice on 8 weeks assay revealed lower TG level, liver weight, insulin resistance with normal glucose level [26]. These results inspired the development of KHK siRNA technology. Alnylam Pharmaceuticals reported their siRNA candidates (AD-1613400 and AD-1613243) in 2022 [27]. Results showed that single dose administration (s.c., 3 mg/kg) to cynomolgus monkeys led to durable and potent inhibition of KHK mRNA expression and KHK protein. In the same year, Boehringer Ingelheim reported a siRNA product (KHK-1334) for reducing the expression of KHK. Administration of KHK-1334 (s.c., 6 mg/kg) to cynomolgus monkeys resulted in significant knockdown of KHK mRNA and KHK protein expression in the liver [28].
Conclusion
Over the past decades, number of obesity patient multiplied. Obesity has turned into a medical issue worldwide. Obesity is associated with the development of type 2 diabetes mellitus, cardiovascular infection, systemic inflammation and particular kinds of cancer. Excessive intake of fructose may contribute heavily to the epidemic of obesity, type 2 diabetes mellitus, NAFLD and other metabolic disease. Some promising KHK inhibitors or KHK siRNA silencers have been developed with attractive results.
Acknowledgement
None
Conflict of Interest
None
References
- World Health Organization (2021) Obesity and overweight.
- Kelly T, Yang W, Chen CS, Reynolds K, He J (2008) Global burden of obesity in 2005 and projections to 2030. Int J Obes 32:1431-1437.
[Crossref] [Google Scholar] [PubMed]
- Williams EP, Mesidor M, Winters K, Dubbert PM, Wyatt SB (2015) Overweight and obesity: Prevalence, consequences, and causes of a growing public health problem. Curr Obes Rep 4:363-370.
[Crossref] [Google Scholar] [PubMed]
- Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, et al. (2009) Harmonizing the metabolic syndrome: a joint interim statement of the international diabetes federation task force on epidemiology and prevention; national heart, lung, and blood institute; American heart association; world heart federation; international atherosclerosis society; and international association for the study of obesity. Circulation 120:1640-1645.
[Crossref] [Google Scholar] [PubMed]
- González-Muniesa P, Mártinez-González MA, Hu FB, Després JP, Matsuzawa Y, et al. (2017) Obesity (Primer). Nat Rev Dis Primers 3:17034.
[Crossref] [Google Scholar] [PubMed]
- Liu L, Li T, Liao Y, Wang Y, Gao Y, et al. (2020) Triose kinase controls the lipogenic potential of fructose and dietary tolerance. Cell Metab 32:605-618.
[Crossref] [Google Scholar] [PubMed]
- Herman MA, Birnbaum MJ. (2021) Molecular aspects of fructose metabolism and metabolic disease. Cell Metab 33:2329-2354.
[Crossref] [Google Scholar] [PubMed]
- Elliott SS, Keim NL, Stern JS, Teff K, Havel PJ (2002) Fructose, weight gain, and the insulin resistance syndrome. Am J Clin Nutr 76:911-922.
[Crossref] [Google Scholar] [PubMed]
- Stanhope KL, Schwarz JM, Keim NL, Griffen SC, Bremer AA, et al. (2009) Consuming fructose-sweetened, not glucose-sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans. J Clin Invest 119:1322-1334.
[Crossref] [Google Scholar] [PubMed]
- Swarbrick MM, Stanhope KL, Elliott SS, Graham JL, Krauss RM, et al. Consumption of fructose-sweetened beverages for 10 weeks increases postprandial triacylglycerol and apolipoprotein-B concentrations in overweight and obese women. Br J Nutr 100:947-952.
[Crossref] [Google Scholar] [PubMed]
- Mayes PA (1993) Intermediary metabolism of fructose. Am J Clin Nutr 58:754-765.
[Crossref] [Google Scholar] [PubMed]
- Stanhope KL, Schwarz JM, Keim NL, Griffen SC, Bremer AA, et al. (2009) Consuming fructose-sweetened, not glucose-sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans. J Clin Invest 119:1322-1334.
[Crossref] [Google Scholar] [PubMed]
- Debray FG, Seyssel K, Fadeur M, Tappy L, Paquot N, et al. (2021) Effect of a high fructose diet on metabolic parameters in carriers for hereditary fructose intolerance. Clin Nutr 40: 4246-4254.
[Crossref] [Google Scholar] [PubMed]
- Bonthron DT, Brady N, Donaldson IA, Steinmann B (1994) Molecular basis of essential fructosuria: Molecular cloning and mutational analysis of human ketohexokinase (fructokinase). Hum Mol Genet 3: 1627-1631.
[Crossref] [Google Scholar] [PubMed]
- Raushel FM, Cleland WW (1977) Determination of the rate-limiting steps and chemical mechanism of fructokinase by isotop exchange, isotope partitioning, and pH studies. Biochemistry 16:2176-2181.
[Crossref] [Google Scholar] [PubMed]
- Raushel FM, Cleland WW (1973) The substrate and anomeric specificity of fructokinase. J Biol Chem 248: 8174-8177.
[Crossref] [Google Scholar] [PubMed]
- Hayward BE, Bonthron DT (1998) Structure and alternative splicing of the ketohexokinase gene. Eur J Biochem 257: 85-91.
[Crossref] [Google Scholar] [PubMed]
- Ishimoto T, Lanaspa MA, Le MT, Garcia GE, Diggle CP, et al. (2012) Opposing effects of fructokinase C and A isoforms on fructose-induced metabolic syndrome in mice. Proc Natl Acad Sci USA 109: 4320-4325.
[Crossref] [Google Scholar] [PubMed]
- Andres-Her nando A, Orlicky DJ, Kuwabara M, Ishimoto T, Nakagawa T, et al. (2020) Deletion of fructokinase in the liver or in the intestine reveals differential effects on sugar-induced metabolic dysfunction. Cell Metab 32: 117-127.
[Crossref] [Google Scholar] [PubMed]
- Maryanoff BE, O'Neill JC, McComsey DF, Yabut SC, Luci DK, et al. (2011) Inhibitors of ketohexokinase: Discovery of pyrimidinopyrimidines with specific substitution that complements the ATP-binding site. ACS Med Chem Lett 2:538-543.
[Crossref] [Google Scholar] [PubMed]
- Calle R, Bergman A, Somayaji V, Chidsey K, Kazierad D (2019) PS-110-Ketohexokinase inhibitor PF-06835919 administered for 6 weeks reduces whole liver fat as measured by magnetic resonance imaging-proton density fat fraction in subjects with non-alcoholic fatty liver disease. J Hepatol 70:69-70.
- Coates DA, Durham TB, Johnston RD, Massey SM, Spinazze PG, et al. (2020) Disubstituted pyrazole compounds as ketohexokinase inhibitors. Pat WO2020257171.
- Liu B, Chen B (2020) Hexone glucokinase inhibitor and use theroef. Pat WO 2020156445.
- Zhu G, Li J, Lin X, Zhang Z, Hu T et al. (2023) Discovery of a Novel Ketohexokinase Inhibitor with Improved Drug Distribution in Target Tissue for the Treatment of Fructose Metabolic Disease. J Med Chem.
[Crossref] [Google Scholar] [PubMed]
- Lee SB, Kim CH, Moon HJ, Hur JM (2022) Oxadiazole compound and pharmaceutical composition comprising same Patent.
- Softic S, Gupta MK, Wang G-X, Fujisaka S, O’Neill BT, et al. (2017) Divergent effects of glucose and fructose on hepatic lipogenesis and insulin signaling. J Clin Invest 127: 4059-4074.
[Crossref] [Google Scholar] [PubMed]
- Noetzli L, Mcininch J, Tremblay F, Schlegel M, Castoreno A (2022) Ketohexokinase (KHK) iRNA compositions and methods of use thereof. Pat WO2022182574.
- Brown BD, Dudek HT, Saxena U, Park J, Abrams M, et al. (2022) Compositions and methods for inhibiting ketohexokinase (KHK). Pat WO2022218941.
Citation: Li Y, Zhu G (2023) Ketohexokinase (KHK) Physiology and Treatments in Obesity and Fructose Metabolism. J Obes Weight Loss Ther S6:002.
Copyright: © 2023 Li Y, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricteduse, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Usage
- Total views: 1301
- [From(publication date): 0-2023 - Apr 05, 2025]
- Breakdown by view type
- HTML page views: 1024
- PDF downloads: 277