Short Communication Open Access
Jerusalem Balsam Lowers Kynurenic Acid Formation: An In Vitro Study
Halina Barana1,3*, Marcelin Jan Pietryja2, Carina Kronsteinera1 and Berthold Kepplinger3
1Division of Neurophysiology, Institute of Physiology, Veterinary Medical University Vienna, Austria
2Herbarium St. Franciszka, Institute of Monastic Medicine, Monastery of Friars Minor Franciscans Katowice-Panewniki, Poland
3Karl Landsteiner Research Institute for Neurochemistry, Neuropharmacology, Neurorehabilitation and Pain Treatment, Austria
- *Corresponding Author:
- Halina Barana
Karl Landsteiner Research Institute for Neurochemistry
Neuropharmacology, Neurorehabilitation and Pain Treatment
Mauer bei Amstetten, 3362, Austria
Tel: 00436644436169
E-mail: halina.baran@neuro-lab.eu
Received date: May 16, 2017; Accepted date: June 05, 2017; Published date: June 14, 2017
Citation: Barana H, Pietryja MJ, Kronsteinera C, Kepplinger B (2017) Jerusalem Balsam Lowers Kynurenic Acid Formation: An In Vitro Study. J Tradit Med Clin Natur 6:224.
Copyright: © 2017 Barana H, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Traditional Medicine & Clinical Naturopathy
Abstract
The present study evaluates the action of Jerusalem Balsam with respect to the biosynthetic machinery of Kynurenic Acid (KYNA) synthesis e.g. the activity of the enzyme synthesizing KYNA, Kynurenine Aminotransferase II (KAT II) in the rat liver homogenate. Subsequently we compared the action of Jerusalem Balsam on KAT II activity in the rat liver homogenate with the action of Cerebrolysin and D-cycloserine, known to inhibit rat liver KAT II activities. We found that Jerusalem Balsam blocked dose-dependently and significantly KAT II activity in the rat liver homogenate. The effect of Jerusalem Balsam on KAT II activity comparing to action of Cerebrolysin or D-cycloserine was strong and significant and the inhibition was seen up to 5 hrs of assay incubation time. Obtained data suggest that lowering of KYNA synthesis by Jerusalem Balsam is notable biochemical effect since it might influence KYNA levels. Increased KYNA levels, respectively KYNA synthesis has been reported in stroke patient, in patient with respiration and cardiovascular problem and in neuropsychiatric disorders. The possible therapeutic mechanism and advantage of the remedy Jerusalem Balsam, i.e., mixture of plants might be due to modulation of KYNA synthesis and improvement of biochemical processes in the periphery and likely in the CNS.
Keywords
Kynurenine aminotransferase; Kynurenic acid; Liver; Cardiovascular disease; Dementia
Introduction
The Jerusalem Balsam was formulated in 1719 in the pharmacy of the Saint Savior monastery in the old city of Jerusalem was replicated and prepared in Europe. The Jerusalem Balsam is based on an ethanolic extract of herbal mixture. There are variations of the formula in current pharmacopoeias (B.P., 1998. The Stationary Office, London, p: 1510; Sweetman, S.C., Blake, P.S., McGlashan, J.M., Parsons, A.V., Martindale: The Extra Pharmacopeia, 33rd edition. Pharmaceutical Press, London, p: 1101). Moussaieff et al. [1] have reported about five different formulas, all referred to as “The Jerusalem Balsam”. One of the formulas, found in a manuscript form in the archive of the monastery, contains four plants: Aloe (Aloe sp.), myrrh (Commiphora spp.), olibanum (Boswellia spp.) and mastic (Pistacia lentiscus L.). Authors conducted pharmacological investigations on this four-plant formula and found its antiinflammatory, as well as anti-oxidative and anti-septic properties [1]. Recently, Kurkiewicz et al. [2] published composition of original Jerusalem Balsam and of contemporary Balsams by using analytical GC/MS methods and found an array of plants including prior described ingredients [1]. Kynurenic Acid (KYNA), an intermediate metabolite of L-kynurenine, is a competitive antagonist of ionotropic Excitatory Amino Acid (EAA) receptors and a non-competitive antagonist of 7 alpha nicotine cholinergic receptors and its involvement in memory deficit and cognition impairment has been suggested [3-5]. KYNA is altered significantly in various neuropsychiatric and immunologic disorders [6-8] and also in aging process [9]. Alterations of L-TRP metabolite levels in the blood found immediately after strokes indicate a significant activation of metabolism along the kynurenine pathway and increase the cardiovascular risk factor, as well [10-12]. In the line, we found that KYNA lowers the efficacy of mitochondria ATP synthesis of heart mitochondria [13,14].The aim of the study was to examine the Jerusalem Balsam action, whether this “natural mixture” has an ability to influence KYNA synthesis in the rat liver tissue, in an in vitro study. Subsequently we compared the effect of Jerusalem Balsam to effect of Cerebrolysin [15] and D-cycloserine [16] in respect to inhibit KAT II activity in rat liver homogenate.
Material and Methods
L-kynurenine, KYNA and pyridoxal-5’-phosphate were purchased from Sigma. All other chemicals used were of the highest commercially available purity. Original Jerusalem Balsam was provided by Dr. Marcelin Jan Pietryja, Herbarium ?w. Franciszka, Instytut Medycyny Klasztornej, Klasztor Braci Mniejszych Franciszkanów Katowice-Panewniki, Poland. The composition of the original Jerusalem Balsam was published recently by Kurkiewicz et al. [2].
Animals
Male Sprague-Dawley rats (Forschungsinstitut für Versuchstierzucht, Himberg, Austria) of 250-280 g body weight were used. The animals were housed in groups of four to five per cage, in a room with controlled light/dark cycle (12 h light/12 h dark), and were given free access to laboratory chow and tap water. Rats were sacrificed in the morning organs were immediately removed and tissue was frozen at –70°C until analysis. The number of rats used was N=5.
Assay of KAT II activities
Preparation of homogenate: The tissue samples were homogenised in an ice bath in 5 volumes (wt/vol) of 5 mM Tris-acetate buffer pH 8.0 containing 50 μM pyridoxal-5’-phosphate and 10 mM mercaptoethanol, additionally diluted with the buffer as requested in the assay procedure and the homogenates obtained were used for KAT II activity determination.
KAT assay
KAT II activity in the liver homogenate was measured using an enzymatic assay described by Baran and Kepplinger [15]. In brief, the reaction mixture contained homogenate (0.25 mg of liver tissue), 100 μM L-kynurenine, 1 mM pyruvate, 70 μM pyridoxal-5’-phosphate and 150 mM Tris-acetate buffer pH 7.4 for KAT II in a total volume of 200 μl. After incubation for 2 h at 37°C the reaction was stopped by adding 14 μl of 50% trichloroacetic acid and 1 ml of 0.1 M HCl. Denatured proteins were removed by centrifugation and the synthesized KYNA was purified on Dowex 50W cation-exchange and quantitated by High Performance Liquid Chromatography (HPLC) method. Blanks were prepared by boiling samples of homogenate for 15 min before adding the reaction mixture. The whole preparations for assay were performed on ice, before the incubation started.
HPLC method for KYNA detection
Measurement of KYNA was performed as described by Baran and Kepplinger [15]. The HPLC system consisted of the following: Merck Hitachi LaChrom Pump L-7100, Autosampler L-7200, Fluorescence Detector L 7485 and a Merck Hitachi D-7500 Integrator. The mobile phase consisted of 50 mM sodium acetate, 250 mM zinc acetate, an 4% acetonitril, pH 6.15, and was pumped through a 10 × 0.4 cm column (HR-80, C-18, Particle size 3 μM, InChrom, Austria) at flow rate of 0.7 ml/min. The fluorescence detector was set at an excitation wavelength of 340 nm and an emission wavelength of 398 nm. The injection volume was 50 μl. The retention time of KYNA was approximately 8.1 min, with a sensitivity of 50 fmol per injection (signal: noise ratio=5).
Effect of Jerusalem Balsam on rat liver KAT II activity
To verify the Jerusalem Balsam action on rat liver KAT II activity, the homogenate of rat liver (1:100 wt/vol) was incubated in the absence and in the presence of different amounts of Jerusalem Balsam (0, 1, 5, 10 μl) under standard assay condition and the amount of KYNA formed was determined as described before. Five independent experiments were performed. Jerusalem Balsam, alcoholic plants extract was diluted (1:10 vol/vol) and used for the assay. Blanks and control samples received respectively amount of vehicle. In separate experiment the effect of Jerusalem Balsam on KAT II activity in rat liver homogenate using different doses of 1, 5 and 7.5 μl under standard assay condition was investigated.
Comparison of the effect of Jerusalem Balsam on KAT II activity to effect of Cerebrolysin and D- cycloserine in rat liver homogenate
To compare the effect of Jerusalem Balsam on KAT II activity to effect of Cerebrolysin and D-cycloserine in the same experiment the homogenate of rat liver (1:100 wt/vol) was incubated in the absence and in the presence of Jerusalem Balsam (1 and 7.5 μl), in the presence of effective dose of Cerebrolysin (15 μl) and in the presence of effective dose of D-cycloserine (168 μM) under assay condition as described in Material and Methods. The incubation period lasted for 1, 3 and 5 hrs.
Statistical Analyses
Results were expressed in means ± the standard error of the mean (SEM). For statistical analyses, the one-way ANOVA-test and Student t-test were applied, respectively. Each sample (number of sample are given in parenthesis) was determined in triplicate. Asterisks indicate a significant difference: *p<0.05; **p<0.01; ***p<0.001 compared to control.
Results
Effect of Jerusalem Balsam on rat liver KAT II activity
Jerusalem Balsam significantly and dose-dependently (0, 1, 5, 10 μl) lowered the rat liver KAT II activity, comparing to controls (Table 1). KAT II was 49.6, 29.6 and 10.1% of control, P<0.001, respectively. Oneway ANOVA analysis of variance between 4 groups revealed the means of KAT activities levels statistically different (F=344.55, P=9.6589E-15, Table 1).
| KYNA (pmol/mg wet tissue weight/h) (% of control) | ||
|---|---|---|
| Control (CO) | 2212.8 ± 63.2 (5) | 100 % |
| Jerusalem Balsam 1 µl | 1097.4 ± 63.0*** (5) | 49.6 % of CO |
| Jerusalem Balsam 5 µl | 654.6 ± 17.0*** (5) | 29.6 % of CO |
| Jerusalem Balsam 10 µl | 224 ± 15.1*** (5) | 10.1 % of CO |
Table 1: Effect of Jerusalem Balsam on KAT II activity in rat liver homogenate.
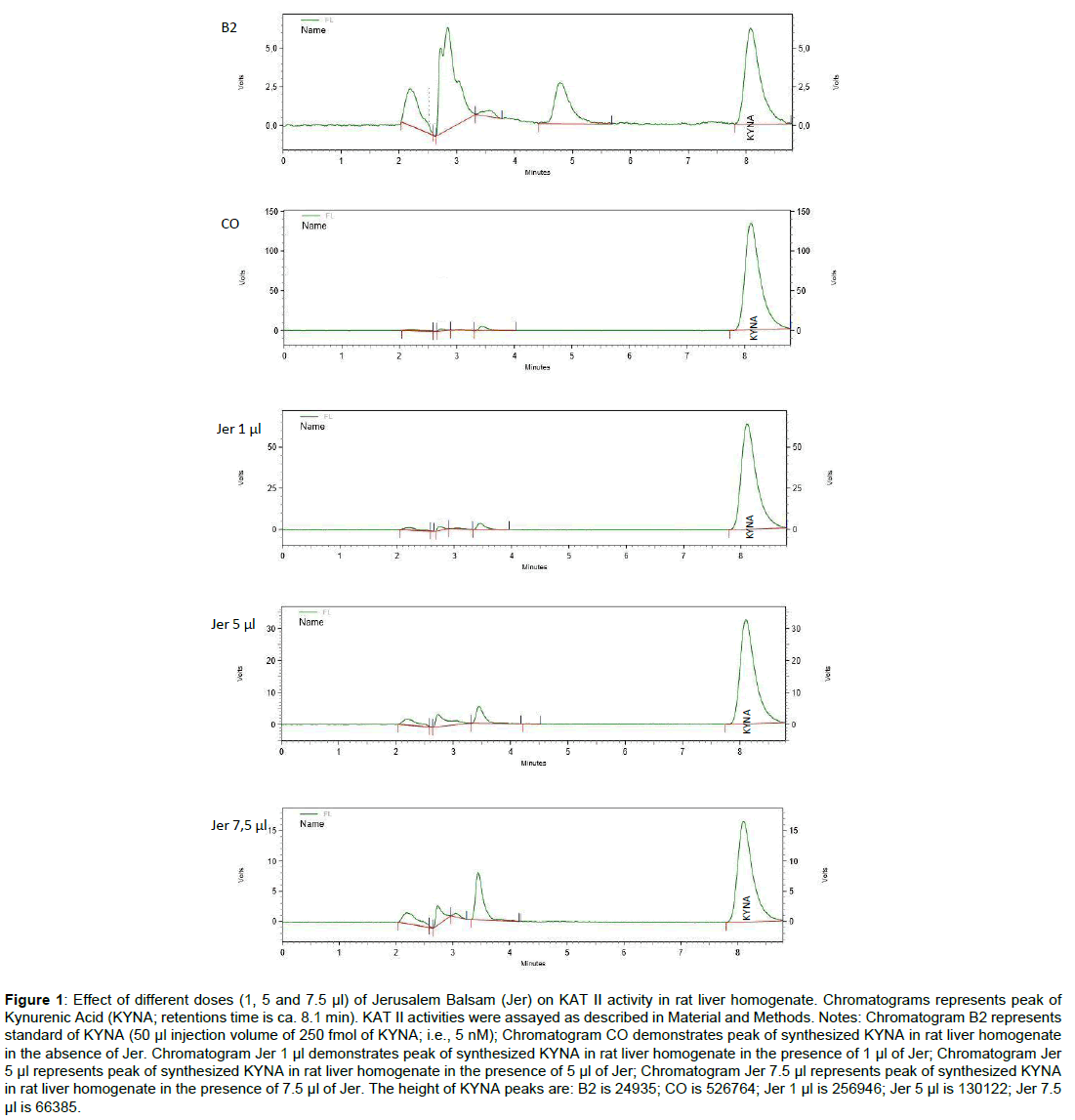
The chromatograms of determined synthesized KYNA in the absence and presence of Jerusalem Balsam (1, 5 and 7.5 μl) are shown in Figure 1. Chromatograms indicate that Jerusalem Balsam dose-dependently lowered KYNA synthesis in rat liver homogenate, comparing to control.
Figure 1: Effect of different doses (1, 5 and 7.5 μl) of Jerusalem Balsam (Jer) on KAT II activity in rat liver homogenate. Chromatograms represents peak of Kynurenic Acid (KYNA; retentions time is ca. 8.1 min). KAT II activities were assayed as described in Material and Methods. Notes: Chromatogram B2 represents standard of KYNA (50 μl injection volume of 250 fmol of KYNA; i.e., 5 nM); Chromatogram CO demonstrates peak of synthesized KYNA in rat liver homogenate in the absence of Jer. Chromatogram Jer 1 μl demonstrates peak of synthesized KYNA in rat liver homogenate in the presence of 1 μl of Jer; Chromatogram Jer 5 μl represents peak of synthesized KYNA in rat liver homogenate in the presence of 5 μl of Jer; Chromatogram Jer 7.5 μl represents peak of synthesized KYNA in rat liver homogenate in the presence of 7.5 μl of Jer. The height of KYNA peaks are: B2 is 24935; CO is 526764; Jer 1 μl is 256946; Jer 5 μl is 130122; Jer 7.5 μl is 66385.
Comparison of effects of Jerusalem Balsam, Cerebrolysin and D-cycloserine on KAT II activity in rat liver homogenate
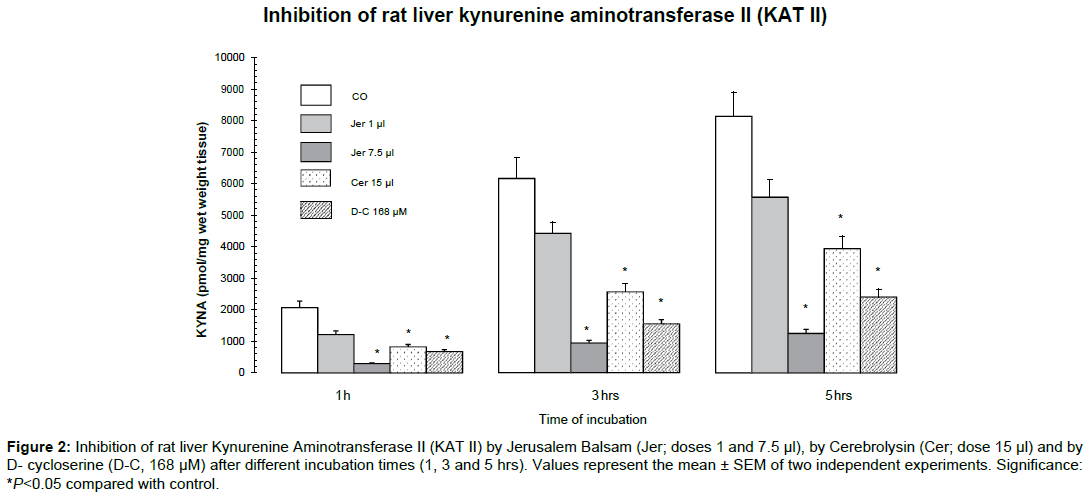
The effects of Jerusalem Balsam, Cerebrolysin and D-cycloserine on KAT II activity in rat liver homogenate after 1, 3 and 5 hrs incubation time are shown in Figure 2. Jerusalem Balsam dose-dependently and significantly lowered KAT II activity in the rat liver homogenate up to 5 hrs of incubation time. The action of Jerusalem Balsam to block KAT II activity was remarkably effective, similar to effects of Cerebrolysin or D-cycloserine.
Figure 2: Inhibition of rat liver Kynurenine Aminotransferase II (KAT II) by Jerusalem Balsam (Jer; doses 1 and 7.5 μl), by Cerebrolysin (Cer; dose 15 μl) and by D- cycloserine (D-C, 168 μM) after different incubation times (1, 3 and 5 hrs). Values represent the mean ± SEM of two independent experiments. Significance: *P<0.05 compared with control.
Discussion
Jerusalem Balsam is widely used because of good reputation as a natural remedy. It is a mixture of certain plants, which supposes to have antibacterial and anti-oxidative properties. Jerusalem Balsam is used to improve liver and lung diseases, as for example bronchopneumonia [1,2]. Further, Jerusalem Balsam is suggested to be helpful as an adjuvant treatment of various tumour burdens [1,2]. But there are no studies which confirm its positive effect and indicate its pharmacological actions. Beside that the mechanism of action might be complex since it is a mixture of several plants. Nevertheless, it is interesting to search if this mixture, as an adjuvant treatment, might exert special properties. Central and peripheral organs of mammalians are excessively involved in tryptophan metabolisms to synthesize the neurotransmitter serotonin and along kynurenine pathway to form several neuroactive compounds including KYNA. Our data for the first time demonstrate that Jerusalem balsam lower KYNA synthesis by blocking KAT II activities in the rat liver homogenate. Revealed data suggest that application of Jerusalem Balsam might modulate KYNA in the periphery and probably in the brain. Similar effect to lower KYNA synthesis we have observed with anti-dementia drug Cerebrolysin, a piglet’s brain extract [15] and with the tuberculostaticum D-cycloserine [16]. In the present study we could confirm our previous findings and in addition we could show a high capacity of natural plant product to affect KYNA synthesis. Jerusalem Balsam has also ability to block KAT III activity in rat liver homogenate (data not shown), similarly as we have seen by Cerebrolysin and D-cycloserine [15,16]. Interestingly, the inhibitory effect of Jerulasem Balsam lasted until 5 hrs, at least in vitro, similar to Cerebrolysin or D-cycloserine, what means that this remedy offers long lasting effects. Revealed observation is important since Jerusalem Balsam is used since centuries and this without reported side effects. Gottlieb et al. described that D-cycloserine enhanced learning significantly [17]. Furthermore, it has been shown that D-cycloserine improved memory consolidation and facilitation of behavioural therapy for delusions by schizophrenia [17,18]. On the other hand, meta-analysis provided evidence that Cerebrolysin has an overall beneficial effect and a favorable benefit-risk ratio in patients with mild-to-moderate Alzheimer’s disease [19]. Accumulated observations suggest that lowering of KYNA by Jerusalem Balsam might be a significant approach for many pharmacological strategies. Endogenous component “Glia depressing factor” which we found in human cerebrospinal fluid and in serum can also lower KYNA content [20]. We belief that our approach to measure the inhibitory capacity of biological materials, in respect to block KATs activities, might be useful to describe the inhibitory properties of biological materials used for the therapy reason as an anti-dementia drug by various disorders. The approach to measure the inhibition capacity of biological material has been patented [21]. In summary, our data demonstrate that Jerusalem Balsam, a mixture of special plants significantly lowered KYNA formation in rat liver homogenate in an in vitro study. Today pharmacological researches are looking for oral application of tolerable compounds which show the ability to lower KYNA content and which are well tolerable by patients. We suggest that Jerusalem Balsam could have therapeutic application due to ability to modulate KYNA synthesis. Further studies on Jerusalem Balsam and on others formulas of Jerusalem Balsam particularly on certain plants in respect to block KATs activities and to influence KYNA synthesis need to be performed.
Acknowledgements
This work was supported in part by SeneCura Austria and in part by a grant given to Halina Baran, National Bank Austria.
Ethical Considerations
The procedures of the research proposal from Halina Baran have been approved b Austrian Ethical Regulations Veterinary University Vienna.
Conflict of Interest
The authors declare no conflict of interest. The idea for the article was conceived by Halina Baran. The investigations were performed by Halina Baran and Carina Kronsteiner. The data were analyzed by Halina Baran and Berthold Kepplinger. The article was written by Halina Baran and Berthold Kepplinger and was read, corrected and accepted by all authors.
References
- Moussaieff A, Fride E, Amar Z, Lev E, Steinberg D, et al. (2005) The Jerusalem Balsam: from the Franciscan Monastery in the old city of Jerusalem to Martindale 33. J Ethnopharmacol 10: 16-26.
- Kurkiewicz S, Dzier??ga-L?cznar A, Pietryja MJ, St?pie? K (2017) Study of historical composition of Jerusalem Balsam and modern monastic lotions by GC/MS technique. XI Franciscan Herbal-Pharmaceutical Conference on May 27, 2017. Wydawca Herbarium ?w. Franciszka.
- Hilmas C, Pereira ER, Alkondon M, Rassoulpour A, Schwarcz R, et al. (2001) The brain metabolite kynurenic acid inhibits α7 nicotinic receptor activity and increases non-α7 nicotinic receptor expression: Physiopathological implications. J Neurosci 21: 7463-7473.
- Birch PJ, Grossman CJ, Hayesm AG (1988) Kynurenic acid antagonizes responses to NMDA via an action at the strychnine-insensitive glycine receptor. Eur J Pharmacol 154: 85-87.
- Chess AC, Simoni MK, Alling TE, Bucci DJ (2007) Elevations of kynurenic acid produce working memory deficits. Schizophr Bull 33: 797-804.
- Baran H, Jellinger K, Deecke L (1999) Kynurenine metabolism in Alzheimer’s disease. J Neural Transm 106: 165-181.
- Baran H, Kepplinger B (2012) Kynurenic acid metabolism in various types of brain pathology in HIV-1 infected patients. Int J Tryptophan Res 5: 49 -64.
- Schwarcz R, Bruno JP, Paul J, Muchowski PJ, Wu HQ (2012) Kynurenines in the mammalian brain: when physiology meets pathology. Nat Rev Neurosci 13: 465-477.
- Kepplinger B, Baran H, Kainz A, Ferraz-Leite H, Newcombe J, et al. (2005) Age- related increase of kynurenic acid in human cerebrospinal fluid - IgG and beta2- microglobulin changes. Neurosignals 14: 126-135.
- Urba?ska EM, Luchowski P, Luchowska E, Pnieski J, Wo?niak R, et al. (2006) Serum kynurenic acid positively correlates with cardiovascular disease risk factor, homocysteine: a study in stroke patients. Pharmacol Rep 58: 507-511.
- Darlington LG, Mackay GM, Forrest CM (2007) Altered kynurenine metabolism correlates with infarct volume in stroke. Eur J Neurosci 26: 2211-2221.
- Kepplinger B, Sedlnitzky-Semler B, Eigner S, Kalina P, Berger P, et al. (2014) Stroke patients after repetitive Transcranial Magnetic Stimulation (rTMS)–alterations of tryptophan metabolites in the serum. Int J Neurorehab Eng 1: 1-11.
- Baran H, Staniek K, Kepplinger B, Stur I, Draxler M, et al. (2003) Kynurenines and the respiratory parameters in rat heart mitochondria. Life Sci 72: 1103-1115.
- Baran H, Staniek K, Bertignol-Spörr M, Attam M, Kronsteiner C, et al. (2016) Effects of various kynurenine metabolites on respiratory parameters of rat brain, liver and heart mitochondria. Int J Tryptophan Res 9: 17-29.
- Baran H, Kepplinger B (2006) Cerebrolysin lowers kynurenic acid formation-an in vitro study. Eur Neuropsychopharmacol 19: 161-168.
- Baran H, Kepplinger B (2014) D-Cycloserine lowers kynurenic acid formation-new mechanism of action. Eur Neuropsychopharmacol 24: 639-644.
- Gottlieb JD, Cather C, Shanahan M, Creedon T, Macklin EA, et al. (2011) D-cycloserine facilitation of cognitive behavioural therapy for delusions in schizophrenia. Schizophrenia Res 131: 69-74.
- Goff DC (2012) D-cycloserine: an evolving role in learning and neuroplasticity in schizophrenia. Schizophr Bull 38: 936-941.
- Gauthier S, Proaño JV, Jia J, Froelich L, Vester JC, et al. (2015) Cerebrolysin in mild-to-moderate Alzheimer's disease: a meta-analysis of randomized controlled clinical trials. Dement Geriatr Cogn Disord 39: 332-347.
- Baran H, Kepplinger B, Draxler M (2010) Endogenous kynurenine aminotransferases inhibitor is proposed to act as “Glia Depressing Factor” (GDF). Int J Tryptophan Res 3: 13-22.
- Baran H, Kepplinger B (2007) Measurement of the activity of a kynurenine-converting enzyme and/or of a kynurenic acid, anthranilic acid and/or 3-hydoxykynurenine-producing enzyme. Patent.
Relevant Topics
- Acupuncture Therapy
- Advances in Naturopathic Treatment
- African Traditional Medicine
- Australian Traditional Medicine
- Chinese Acupuncture
- Chinese Medicine
- Clinical Naturopathic Medicine
- Clinical Naturopathy
- Herbal Medicines
- Holistic Cancer Treatment
- Holistic health
- Holistic Nutrition
- Homeopathic Medicine
- Homeopathic Remedies
- Japanese Traditional Medicine
- Korean Traditional Medicine
- Natural Remedies
- Naturopathic Medicine
- Naturopathic Practioner Communications
- Naturopathy
- Naturopathy Clinic Management
- Traditional Asian Medicine
- Traditional medicine
- Traditional Plant Medicine
- UK naturopathy
Recommended Journals
Article Tools
Article Usage
- Total views: 4052
- [From(publication date):
August-2017 - Apr 04, 2025] - Breakdown by view type
- HTML page views : 3143
- PDF downloads : 909