Jatropha tanjorensis Ameliorate Effects of Aspirin Induced Stomach UIcer in Wistar Rats
Received: 30-Oct-2022 / Manuscript No. JGDS-22-78651 / Editor assigned: 02-Nov-2022 / PreQC No. JGDS-22-78651 (PQ) / Reviewed: 16-Nov-2022 / QC No. JGDS-22-78651 / Revised: 09-Feb-2023 / Manuscript No. JGDS-22-78651 (R) / Published Date: 16-Feb-2023
Abstract
Background: Jatropha tanjorens is considered a potential source of medicinal agents to treat different diseases. Aim: This study investigated the comparative effects of J. tanjorensis ethanolic leaves extract and amlodipine on gastro intestinal function in aspirin induced ulcer in wistar rats.
Methods: Wistar rats of both sexes (180 g-200 g) were divided into 6 groups (n=5). Group 1 received rat chow; group 2 received (5 mg/kg) of amlodipine orally. Group 3 received (200 mg/kg) of J. tanjorensis orally group 4 received aspirin only (250 mg/kg). Group 5 received (250 mg/kg) of Aspirin+Amlodipine (5 mg/kg). Group 6 received aspirin (250 mg/kg)+J. tanjorensis (200 mg/kg). After 14 days of treatment, animals were sacrificed and blood samples were collected for biochemical analysis while the stomach was harvested for histological analysis.
Results: Stomach acid secretion, gastric pepsin secretion and ulcer score all increased significantly (p<0.001) in the aspirin treated group as compared to other treatment group and control respectively. Aspirin significantly reduced adherent mucus secretion when compared to other treatment groups and control respectively at (p<0.05). When compared to control, ulcer score in Aspirin+Amlodipine and Aspirin+J. tanjorensis treated groups both showed significant increase (p<0.05). Aspirin treated animals showed decreased goblet cell with marked inflammation that was reversed by J. tanjorensis treatment. The results suggest that J. tanjorensis ameliorates aspirin induced stomach damage and that amlodipine treatment does not compromise the integrity of the stomach.
Conclusion: It is concluded that the leaves extract of J. tanjorensis ameliorated the effect of aspirin induced ulcer in Wistar rats; and that amlodipine does not adversely affect gastric secretion and predispose to gastric ulcer.
Keywords: Aspirin; Gastric secretion; Jatropha tanjorensis; Stomach; Ulcer
Introduction
A great number of modern medicines have been derived from plants that are considered as important sources of medicinal agents to treat different diseases. For drug development, bioactive compounds like flavonoids, tannins, phenols and alkaloids in medicinal plants play a vital role [1]. Immense benefits are derived from using medicinal herbs in treatment and management of diseases. They are relatively safer, more affordable and sometimes offer better therapeutic value than synthetic drugs [2]. One of such plant is Jatropha tanjorensis.
The plant, J. tanjorensis, is a member of the Euphorbiaceae family. Its common names are catholic vegetable and hospital too far [3]. Since the leaf doesn't need any specific conditions to flourish in the Southern soil of Nigeria, it is frequently consumed there. The plant J. tanjorensis in the southern region is a well-known medical plant that is frequently used as a home medicine for diabetes mellitus, malaria, infection, hypertension and several other illnesses [4]. Ebe had stated that the roots of this plant may act as an immune and blood system booster [5]. J. tanjorensis leaves have been reported to have beta blocking effect, anti-anaemic, anti-microbial activity anti-plasmodial and anti-oxidant properties against malaria parasite induced oxidative stress [6,7]. The leaf extract of J. tanjorensis contained flavonoids, alkaloids, tannins, saponins, terpernoids, cardiac glycosides and anthroquinones, according to phytochemical research.
Amlodipine is a calcium channel blocker with the longest half-life at 30 hours to 50 hours, when compared to nifedipine and other dihydropyridine medicines. It has an advantage of lengthy half-life in the ability to perform once daily dosing [8]. Amlodipine, like other calcium channel blockers, works by preventing calcium ions from entering vascular smooth muscle and cardiac muscle cells during membrane depolarization. This action induces the smooth muscle cells in the arteries and veins to relax, resulting in arterial vasodilation and a reduction in cardiac effort and oxygen consumption [9]. In heart related chest pain (angina), the enhanced blood flow in the body relaxes the heart muscles by reducing the workload on the heart. It also improves the oxygen flow in the body, thereby, preventing any heart related chest pain [10].
Peptic ulcer is the erosion of the mucosal lining of gastrointestinal tract [11]. The esophagus, stomach and intestines are all susceptible to erosion. Peptic ulcer illness manifests as mucosal rupture in the stomach or duodenum with a noticeable depth of more than 3 mm-5 mm. It is also a lesion in the upper gastrointestinal mucosa that spreads into the deeper layers of the gut wall through the muscularis mucosa. Peptic ulcer is due to an imbalance in the stomachs protective and harmful factors. When the mucus cells, which typically release viscid, adherent mucus to protect the mucosal wall, are compromised, pepsin has the chance to digest the columnar epithelium and produce an ulcer in the presence of acid [12]. Additionally, a higher concentration of pure human pepsin can accelerate the ulcerative process by accelerating mucus barrier disintegration [13]. Infection with the bacteria Helicobacter pylori (H. pylori) and the use of anti-inflammatory drugs, such as ibuprofen or aspirin, especially when taken frequently or in high quantities, can also cause stomach ulcers [14]. Although stomach ulcer complications are generally uncommon, they can be extremely serious and even fatal. These symptoms include discomfort, bleeding at the ulcer's site, rupture of the stomach lining there and gastric obstruction, which occurs when the ulcer prevents food from passing through the digestive system [15].
Aspirin has been used for many years to treat a variety of illnesses and it has a long history going back to antiquity [16]. Its significance in medical therapy strategies is still growing nowadays [17,18]. Pain, fever and inflammation are frequently treated with aspirin (acetylsalicylic acid), an efficient non-steroidal anti-inflammatory medication (NSAID) [19,20]. It is frequently used to avoid cardiovascular thrombosis and is frequently given for the treatment of inflammatory illnesses like rheumatoid arthritis [21]. Despite these benefits; aspirin has a number of harmful side effects, including allergies, nausea and vomiting, dyspepsia and stomach discomfort. The mucosa of the digestive tract can suffer significant damage from aspirin, which raises the risk of ulcer development [22]. Gastric mucosal erosions, ulceration, bleeding and perforation have all been linked to long term usage of NSAIDs [23].
Due to the fact that both disorders are made worse by stress, food and personality type, hypertension and peptic ulcer disease frequently coexist in the adult population [24,25]. Gastric injury resulting in peptic ulcer has posed a great deal of threat to the world’s population with a high morbidity and mortality [26]. Currently, the treatment for gastric ulcers involves pharmacological medications that block the production of gastric acid, antacids that neutralize the acid or cytoprotective treatments that prevent cell death [27,28]. However, the majority of these medications have undesirable side effects, including systemic alkalosis, gynecomastia, impotence and joint discomfort.
Nowadays, more research is being done on the use of natural products to create medications with fewer side effects [29]. J. tanjorensis is one of such natural products with medicinal potentials. This plant has received a wide attention due to its nutritional, hypotensive, hypolipodermic, antioxidant, antiplasmodial and hypoglycemic properties among others [30]. The goal of the current study was to assess the antiulcer effects of Jatropha tanjorensis and amlodipine in aspirin induced ulcer utilizing albino Wistar rats as the model.
Materials and Methods
Chemicals
Aspirin, as acetyl salicylic was obtained from Micro Labs Limited, India. Amlodipine tablet 10 mg was obtained from Dr. Reddy's Laboratories (UK) Ltd and stored below 25°C.
Plant materials
J. tanjorensis fresh leaves (6 kg) were bought from Watt market in Calabar South, Cross River State, Nigeria. The Chief Botanist of the University of Calabar, Department of Botany in Cross River State, Nigeria, verified the authenticity of the leaves. The University of Calabar, Physiology Laboratory dried the leaves after washing them under running water to get rid of the dirt. And was later homogenized using the manual blender to get powder. It spent 48 hours submerged in 4500 ml of 99.5% ethanol at 35°C. Whatman's No. 1 filter paper was used to filter the homogenate. Using a vacuum rotatory evaporator at 30°C, the filtrate was allowed to dry out. Until usage, the extract was kept in a refrigerator at 4°C.
Experimental animals
The PAMO University of Medical Sciences' animal facility in Nigeria provided thirty adult Wistar rats (both sexes), weighing 180 g-200 g, which were kept in the animal facility under conditions of 27.2°C and 12 hour light/dark cycles. Before the start of the trial, the animals were acclimated for a week. All animals had unrestricted access to food pellets and water. The PAMO university of medical sciences ethical guidelines accepted the experimental methods (Approval number: PUMA-AREC/2021/027), which were carried out in strict adherence to the national research council's regulation for the care and use of laboratory animals (2011).
Experimental design
The test animals were weighed and divided into six equal groups at random. Each rat in group I (the control group) received 0.5 mL of distilled water orally every day for 14 days in a row. Animals in group 2 (amlodipine) received the drug orally once daily for 14 days at a dose of 5 mg/kg BW [31]. Animals in Group 3 (the J. tanjorensis group) received J. tanjorensis (200 mg/kg BW) orally once daily for 14 days while it was dissolved in water [32]. Group 4 (Aspirin): Rats received 250 mg/kg of aspirin via oral route [33]. Group 5 (Aspirin+Amlodipine) received 250 mg/kg) of aspirin via oral dose and 200 mg/kg of J. tanjorensis daily via oro-gastric intubation and Group 6 (Aspirin+J. tanjorensis): Rats were orally administered 250 mg/kg for 14 days. Treatment lasted for 14 days after which stomach was harvested for biochemical and histopathological analysis respectively.
Aspirin induced gastric ulcer and ulcer score
Using the method first reported by Hawthorne and modified by Umoren, gastric ulceration was caused in the animals by giving groups (4, 5) and (6) a single oral dosage of acetyl salicylic acid (250 mg/kg body weight), respectively [34,35]. Group 5 and 6 were additionally treated with J. tanjorensis and amlodipine respectively for 14 days.
The animal was fasted for 18 h after the last administration. Each rat was euthanized and to reveal the stomach, the linea alba of the abdomen was cut. The stomach was then separated and removed by the larger curvature. After that, it was cleaned with regular saline. The tissues were fixed in place with pins to allow for optimal visibility. The ulcers were measured using a Vernier caliper and a magnifying glass. According to Macallister methodology, ulcer scores were calculated.
Assay of pepsin
The method described by Krishnan was used to measure the pepsin activity in the gastric juice [36]. Pepsin was applied to denatured hemoglobin (the substrate) for 19 minutes. All undigested protein was precipitated by 10% trichloroacetic acid at the conclusion of the incubation time. Tyrosine, tryptophan and phenylalanine are soluble peptides with phenolic amino acids that were present in the filtrate after this was processed through filtering. Under alkaline conditions, the phenolic amino acids were produced to produce a blue hue with the Folin-Ciocaltreau reagent. A red filter was used to measure the amount of color produced on a photometer. Each activity tube contained a blank tube and the absorbance was measured at 700 nm. The amount of pepsin activity per milliliter of gastric juice was stated.
Extraction of adherent mucus
The Tan method was used to calculate the weight of adherent mucus [37]. Briefly, the mucus coating the stomach wall of each experimental and control animal was gently scraped into a tiny sample tube containing 1 ml of water with a predefined weight using a glass slide. A digital electronic balance was used to weigh the container and mucus, with the difference representing the weight of the mucus.
Measurement of gastric acid secretion by continuous perfusion method
Another set of experiments used the previously reported modified continuous perfusion approach to quantify stomach acid secretion [38]. Each rat was given 6 ml/kg of 25% urethane intraperitoneally after a fast of 18 hours. To make breathing easier, the trachea was cut open and an esophageal tube was hooked to a 60 cc syringe that was attached to a peristaltic pump (Harvard Apparatus, MA, USA). To administer sterile saline perfusion, the tube was inserted through the mouth and into the stomach. Along the linea alba, the abdomen was cut open, and the pyloric end of the stomach was cut off, cannula inserted and ligated to collect stomach liquid. To remove the food particle, the stomach was flushed with regular saline using the esophageal cannula. After that, 1 ml/min of normal saline with a pH of 7.0 was infused into the stomach at a temperature of 37°C. The perfusate was titrated with 0.01N NaOH solution using phenolphthalein as an indicator, with a pink tint that marked the end point. The stomach juice was collected every 10 minutes. Histamine (100 mg/kg) and cimetidine (5 mg/kg) were given subcutaneously once a stable basal output was achieved. Gastric juice was then taken every 10 minutes and examined as previously described.
Determination of body weight
An animal weighing balance was used to determine the animal's body weight before the experiment began. They were weighed initially before being divided into groups at random. Each week, their weight was recorded and the weight differences were calculated. Each rat had its stomach removed and the weight of the organ was recorded using a weighing balance.
Histological preparation of gastric tissue
Histological preparation was done using Ismail technique [39]. The animals were briefly sacrificed in a desiccator with chloroform. The stomach and liver were swiftly removed and preserved at 10% in buffered formalin. Hematoxylin and eosin was used to stain the tissues after they had been divided into sections with a thickness of 5 m (H and E). Slices were examined using a light microscope while photomicrographs were taken.
Statistical analysis
Results were given as mean ± Standard Error of Mean (SEM). The statistical analysis was done with SPSS 16.0 (SPSS Inc. Chicago II, USA). One way Analysis of Variance (ANOVA) was used to evaluate group differences, followed by the Tukey test when the F-value was significant. P<0.05 was considered statistically significant.
Results
Effects of amlodipine, aspirin and J. tanjorensis on stomach acid output after histamine and cimetidine administration in rats among experimental groups
Following the administration of histamine and cimetidine to rats, the effects of amlodipine, aspirin and J. tanjorensis on stomach acid output are illustrated in. Comparing the aspirin treated group to the other treatment groups and the control, there was a significant rise in basal production (P<0.001). When compared to the control, there was no discernible difference in basal output between the J. tanjorensis and amlodipine alone treated groups. But when compared to control group, aspirin+amlodipine and aspirin+J. tanjorensis treated groups both showed a substantial increase (p<0.001) in basal production.
Following the administration of histamine, the maximal acid output was significantly higher (p<0.001) in the aspirin treated group compared to the control and other experimental groups. But in the control and all treatment groups, the administration of cimetidine after the injection of histamine considerably (p<0.001) lowered the peak acid output (Figure 1).
Effect of amlodipine, aspirin and J. tanjorensis on gastric pepsin impact and ulcer score
The results of the experimental groups' responses to amlodipine, aspirin and J. tanjorensis on stomach pepsin secretion in rats are displayed in (Table 1). When compared to the control group and other treatment groups, the aspirin treated group's secretion of pepsin increased significantly (P<0.001). When compared to the control, there was no discernible difference in pepsin secretion between the J. tanjorensis and amlodipine only treated groups. Aspirin+Amlodipine and Aspirin+J. tanjorensis treated groups' stomach pepsin levels, however, significantly increased (p<0.001) as compared to the control group.
When compared to the other treatment groups and the control group, the ulcer score in the aspirin treated group increased significantly (P<0.05). When compared to the control group, there was no discernible change in ulcer score between the J. tanjorensis and amlodipine alone treated groups. However, when compared to the control group, the ulcer score was shown to have increased significantly (p<0.05) in both the Aspirin+ Amlodipine and Aspirin+J. tanjorensis treated groups.
| Group | Pepsin secretion (mg/mL) | Ulcer score |
|---|---|---|
| Control | 32.05 ± 1.53 | 0.2 ± 0.20 |
| Amlodipine | 33.93 ± 0.21 | 0.2 ± 0.16 |
| J. tanjorensis | 31.76 ± 0.18 | 0.2 ± 0.20 |
| Aspirin | 69.73 ± 2.01*ab | 5.2 ± 0.37*ab |
| Aspirin+Amlodipine | 63.19 ± 1.12*a | 2.2 ± 0.13*a |
| Aspirin+J. tanjorensis | 61.85 ± 0.67*b | 1.9 ± 0.42*b |
| Values are expressed as mean ± SEM, n=5; *=P<0.001 vs. control; J. tanjorensis; b=P<0.001 vs. Amlodipine; ab=P<0.001 vs. control; J. tanjorensis; Amlodipine; Aspirin+J. tanjorensis; Aspirin+Amlodipine groups respectively. | ||
Table 1: Effect of amlodipine, aspirin and J. tanjorensis on gastric pepsin activity and ulcer score.
Effect of J. tanjorensis, amlodipine and aspirin on adherent mucus in the treatment groups
The Effect of amlodipine, aspirin and J. tanjorensis on adherent mucus in rats among the treatment groups is represented in (Table 2). When compared to other treatment groups and the control, the aspirin treated group's stomach mucus output was significantly higher (p<0.001). When compared to the control group, there was no discernible change in mucus secretion between the J. tanjorensis and amlodipine alone treated groups. But compared to the control group, the aspirin+amlodipine and aspirin+J. tanjorensis treated groups both showed a substantial increase (p<0.001) in stomach mucus secretion.
| Group | Adherent mucus (mg/g) tissue |
|---|---|
| Control | 0.014 ± 0.013 |
| Amlodipine | 0.011 ± 0.021 |
| J. tanjorensis | 0.015 ± 0.014 |
| Aspirin | 0.006 ± 0.012c# |
| Aspirin+Amlodipine | 0.013 ± 0.013*a |
| Aspirin+J. tanjorensis | 0.012 ± 0.013b |
| Values are expressed as mean ± SEM, n=5; *=P<0.001 vs. control; J. tanjorensis; b=insignificant vs. control; c =P<0.05 vs. Aspirin+J. tanjorensis; #=P<0.05 vs. Aspirin+Amlodipine groups respectively. | |
Table 2: Effect of amlodipine, aspirin and J. tanjorensis on adherent mucus among the experimental groups.
Effect of amlodipine, aspirin and J. tanjorensis on mean body weight change and stomach weight among the treatment groups
The results of the experimentation with amlodipine, aspirin and J. tanjorensis on mean body weight change and stomach weight are displayed in (Table 3). When compared to the other groups, there was a significant (p<0.05) decline in the mean body weight change in the aspirin treated group. A similar pattern in the change in mean stomach weight was seen. When compared to the control group, there was no discernible difference between the groups treated with aspirin and J. tanjorensis, aspirin and amlodipine and J. tanjorensis, respectively. However, as compared to the control, amlodipine treated and aspirin+J. tanjorensis treated groups, there was a significant increase in the mean body weight change in the J. tanjorensis treated (p<0.05), control and amlodipine treated groups, respectively.
| Group | Initial body weight (g) | Final body weight (g) | Mean body weight change (g) | Mean stomach weight (g/g) |
|---|---|---|---|---|
| Control | 180.5 ± 3.12 | 190.5 ± 4.25 | 10.1 ± 1.13 | 1.41 ± 0.124 |
| Amlodipine | 187.2 ± 0.81 | 199.4 ± 3.32 | 12.2 ± 2.51 | 1.12 ± 0.152* |
| J. tanjorensis | 190.4 ± 2.55 | 210.7 ± 1.33 | 20.3 ± 0.78 | 1.41 ± 0.097* |
| Aspirin | 200.1 ± 0.13 | 106.6 ± 0.02*abcd | -93.5 ± 0.11 | 0.48 ± 0.037*ab |
| Aspirin+ Amlodipine | 195.7 ± 6.15 | 165.5 ± 3.41 | -30.2 ± 2.74 | 0.97 ± 0.066*a |
| Aspirin+J. tanjorensis | 198.7 ± 8.05 | 175.6 ± 2.73 | -23.1 ± 5.32 | 1.02 ± 0.121*b |
| Values are expressed as mean ± SEM, n=5; *=P<0.05 vs. control; ab=P<0.05 vs. J. tanjorensis and Amlodipine; c=P<0.01 vs. Aspirin+J. tanjorensis; d=P<0.01 vs. Aspirin+Amlodipine groups respectively. | ||||
Table 3: Effect of amlodipine, aspirin and J. tanjorensis on body weight change and stomach weight among the experimental groups.
Amlodipine, aspirin and J. tanjorensis impact on stomach histology and gross morphology
The stomach's gross morphology and histology are presented in (Figure 2).
The impact of J. tanjorensis, aspirin and amlodipine on the general shape of rat gastrointestinal mucosa: (A) Control group presented no lesion and micro bleeding lesion. (B) J. tanjorensis group depicted no lesion of gastric mucosa. (C) Amlodipine group displaying no stomach mucosal lesions. (D) The number of ulcer lesions increased significantly (p<0.05) in the aspirin treated group. Keep an eye out for hyperemia and linear mucosal sores. (E) Treatment with aspirin and amlodipine resulted in a substantial (p<0.01) decrease in the number of lesions. Observe Groups (E and F) for mild hyperemia and absence of any hemorrhagic mucosal lesions, respectively. (F) A significant decrease in the number of lesions was observed (p<0.01) in the group treated with aspirin and J. tanjorensis.
Photomicrograph showing the effect of amlodipine, aspirinandJ. tanjorensis on stomach sections stained with hematoxylin-eosin.
Photomicrographs showing the effect of amlodipine, aspirin and J. tanjorensis on stomach sections stained with haematoxylin-eosin are presented in (Figure 3). The stomach in the (A) control group was visible, displaying typical gastric architecture with prominent mucosa and villi. In the (D) Aspirin treated group, the glandular cells appear congested, goblet cells however few in number appear larger in size, with more necrosis observed (red circle). In the (F) Aspirin+J. tanjorensis treated group there appeared minimal gaps in the mucosae and minimal cell congestion (yellow circle), the goblet cells became prominent with few necrosis signifying a recovery response (red circle).
Discussion
The effects of amlodipine, aspirin and Jatropha tanjorensis leaves extract on stomach and body weight in albino Wistar rats were studied. At the end of 2 weeks of treatment, results obtained showed increased gastric acid output, increased pepsin secretion, increased ulcer score, decreased mucus secretion, decreased body weight and decreased stomach weight in aspirin administered group as compared with control. Treatment with J. tanjorensis leaves extract reversed the adverse trend similar to amlodipine treatment.
Aspirin induced mucosal damage is caused by a COX dependent mechanism [40]. Gastric mucosal homeostasis and integrity are preserved by COX-1 [41,42]. Additionally, its suppression thins the mucosal layer. As a result of decreased angiogenesis and increased leukocyte adhesion caused by COX-2 inhibition, there is microvascular blockage, which impairs mucosal defence, induces oxidative stress and damages the mucosa. Additionally, aspirin lyses phospholipids of mucosal epithelial cells, which results in increased mucosal permeability and allows gastric acid and aspirin to intrude the mucosal barrier and cause inflammation.
By blocking the action of natural Prostaglandins (PGs), aspirin induces stomach damage. Two isoforms of cyclooxygenase produce PGs from arachidonic acid, which defend the stomach mucosa against a variety of attacks (COX) [43]. A protein known as COX-1, which is normally expressed in the stomach, is crucial for the production of PGs, which defend the mucosa. According to, COX-2 is an inducible isoform involved in inflammation and in the production of PGs that help heal gastric ulcers. The mucosal blood flow, which typically provides epithelial cells with enough nutrients and oxygen to create mucus and bicarbonate, is decreased by COX-1 inhibition. In addition, COX-1 inhibition decreases the formation of bicarbonate and mucus while increasing the output of stomach acid, leading to damage to the gastric mucosa and ulcer. Reactive Oxygen Species (ROS), such as the superoxide anion radical (O2), hydrogen peroxide (H2O2) and hydroxyl radical (OH), are crucial in the etiology of peptic ulcer [44].
Among the many substances that the stomach normally secretes are gastric acid, pepsin, and gastric mucus. Gastric mucus shields the epithelial cells from harm caused by gastric acid and pepsin while stomach acid and pepsin aid in the digestion of food that has been consumed [45]. However, a high gastric acid concentration worsens peptic ulcer mucosal damage [46]. Therefore, it is imperative that excessive gastric acid output be inhibited when treating peptic ulcers [47]. Plant phytochemicals like flavonoids, which control gastrointestinal hormones and decrease H+K+-ATPase activity, are useful for reducing the production of stomach acid and preventing additional harm [48]. Pepsin is another endogenous aggressor in gastric juice in addition to gastric acid. Pepsin overuse may result in significant mucosal damage characterized by punctate ulcers, bleeding into the lumen and isolated areas of discontinuity in the adherent mucus gel layer without any indication of re-epithelialization or mucus cap formation [49]. Prostaglandin controls the production of bicarbonate and mucus, which shield stomach epithelial cells from pepsin and acid. The initial line of mucosal defense against luminal acid is provided by bicarbonate, which establishes a pH gradient with epithelial surfaces in the stomach and duodenum that is almost neutral in pH. The underlying mucosa is shielded from proteolytic digestion by the continuous adherent mucus layer, which acts as a barrier to luminal pepsin.
A calcium channel blocker amlodipine acts by relaxing blood vessels to lower the pressure and restore normal blood pressure in cases of high blood pressure, thereby enhancing the body's blood flow. In Amlodipine treated group, the observed increase in body weight as compared to control may be attributable to the relaxing effect of amlodipine on blood vessels thereby allowing for easy flow and increased transport of nutrients with blood to cells and tissues along the vessels. This also impacted positively on the stomach mucosa with attendant reduction in the level of ulceration. According to, who reported increased anti-ulcer activity of amlodipine as well as increased volume of gastric secretions as compared to ranitidine, the observed increase in gastric secretion in amlodipine treated group as well as (aspirin+amlodipine) treated group compared to control is consistent with those findings [50]. Increases in mucus, bicarbonate ions, or secretions other than hydrochloric acid can all contribute to an increase in the volume of gastric secretion. Amlodipine may be directly responsible for this effect or an increase in stomach mucosal blood flow, or both [51]. Despite the fact that the results of the current investigation indicated lower mucus secretion, photomicrograph of the stomach revealed that aspirin induced damage group had fewer but larger goblet cells than treatment groups. This result corroborates the work of Diem-Phuong that goblets cells are responsible for the production of mucus [52]. Thus, the aspirin induced damage to the stomach mucosa is explained by the fewer goblet cells and increased necrosis in ulcer group.
Second, the increased volume of pepsin secretion from the aspirin+amlodipine treated animal suggests that subcutaneous histamine stimulates copious secretion of acid in a rat's stomach through H2 receptor and the cellular mechanism involves the activation of cyclic adenosine phosphate, a process that is fueled by H+/K+ ATPase [53]. Aspirin and amlodipine may potentially increase stomach acid output by activating and acting on histaminergic H2 receptors. This was demonstrated by the findings, which showed that the aspirin+amlodipine treated group enhanced the effect of histamine induced stomach acid secretion.
Cimetidine was also seen to lessen the amount of acid that histamine induced groups of experimental subjects produced. This is consistent with claims that cimetidine is a drug that prevents histamine from binding to the H2 receptors [54].
In the absence of the viscid and adherent mucus that the mucus cells normally secrete to protect the mucosal wall, pepsin will have the chance to digest the columnar epithelium and lead to the development of an ulcer. A higher concentration of pure human pepsin, according to Pearson, can accelerate the ulcerative process by accelerating mucus barrier disintegration. This was clear from the study's findings, which indicated that the treatment groups produced more acid and pepsin than control groups did. Another well-known aggressive substance that can solubilize stomach mucus and promote the development of ulcers is pepsin.
The observed significant increase in mean body weight change in J. tanjorensis administered group, control and amlodipine treated group when compared with Aspirin+J. tanjorensisand Aspirin+amlodipine treated groups respectively could be attributable to the rich nutritional content and antioxidative properties of J. tanjorensis leaves that could effectively ameliorate oxidative stress and confer some health benefits by improving feeding amongst the J. tanjorensis test groups. Jatropha contain phytochemicals such as flavonoids and alkaloids which plays gastroprotective role [55]. The reduction in body weight change observed in aspirin administered group may be due to impaired digestive activity caused by disruption of the gastric mucosa by aspirin.
The observed increase in relative stomach weight from (Amlodipine; aspirin+ amlodipine; and aspirin+J. tanjorensis) treated groups respectively when compared to the group that received aspirin, maybe due to the antioxidant, anti-inflammatory, anti-nociceptive properties of J. tanjorensis leaves extract as well as the dilating properties of amlodipine, a calcium channel blocker on the gastric lumen thereby improving body and stomach weight [56].
In this study, J. tanjorensis (200 mg/kg) was found to lessen the impact of an aspirin induced ulcer by improving the histology, mucus layer and gross morphology of the ulcerated gastric tissues. The ameliorating effect of J. tanjorensis could be attributable to its rich content in phytochemicals present in the plant extract. The photomicrograph of stomach sections showed normal gastric architecture with prominent mucosa and villi. In the (Aspirin+J. tanjorensis) treated group, the goblet cells became prominent with few necrosis signifying a recovery response (red circle). This outcome corroborates the works of previous studies that ulcerated animals exhibited typical histological abnormalities such as a loss of mucosal integrity, submucosal edema, inflammatory cell infiltration and weakening of the mucosal layer [57]. Both ethanol and aqueous leaf extracts of J. tanjorensis have been found to include tannins, alkaloids, phenols, flavonoids, saponins, terpenoids, glycosides and anthraquinones according to studies.
Phytochemicals have antioxidant property and protective effect in the body including the gastrointestinal tract. Flavonoids exhibit anti-ulcer effects by boosting protective factors such as mucus, bicarbonate, prostaglandins, antioxidant enzymes, etc. and resisting aggressive factors. Hence the protective properties of the leave extract of J. tanjorensis from oxidative damage on the mucosal layer when challenged with aspirin induced lesions.
Studies on natural substances having gastro protective properties, such as flavonoids, alkaloids, terpenes and terpenoids, saponins, phenolic acids, tannins and fatty acids, have been more prevalent in recent years [58]. Flavonoids exhibit multiple pharmacological effects, such as antioxidative, anti-inflammatory, anti-diabetic properties. Studies have shown that flavonoids have protective effects on the intestinal epithelium, including maintaining the function of the intestinal barrier.
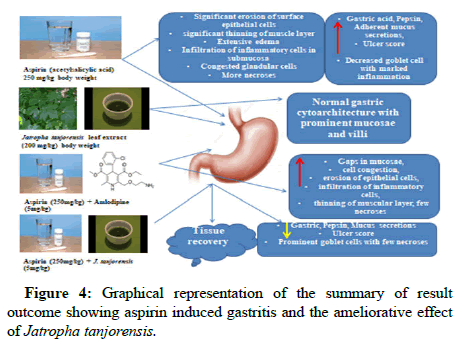
In conclusion, this study’s findings revealed no differences in ulcer scores between control and amlodipine administered groups respectively. There was however, an increased ulcer scores in the group treated with (Aspirin+J. tanjorensis). The score was significantly reduced when compared with the (Aspirin+Amlodipine) treated group. Interestingly, gross morphology of stomach wall of rats showed no injury in J. tanjorensis treated group. There was significant increase in relative stomach weight in J. tanjorensis treated as compared to amlodipine treated. This could possibly be attributed to flavonoids found in the leaves of J. tanjorensis plants, which have anti-inflammatory and antioxidant characteristics. The overview of the outcome is shown visually in (Figure 4).
Conclusion
The results from the present study have shown that gastric ulcers occur in aspirin administration accompanied with elevated gastric juice and pepsin secretion. The leaves extract of J. tanjorensis and amlodipine could militate against the incidence of peptic ulcer by reducing gastric acid secretion and pepsin secretion. These effects are attributed to the inhibition of calcium ion activity and the phytochemicals present in the leaves of J. tanjorensis which offer gastro protective effect. It is concluded that amlodipine does not adversely affect gastric secretion and that the leaves extract of J. tanjorensis offer gastro-protection to aspirin induced gastritis.
References
- Khan MSA, Khundmiri SUK, Khundmiri SR, Al-Sanea MM, Mok PL (2018) Fruit derived polysaccharides and terpenoids: Recent update on the gastroprotective effects and mechanisms. Front Pharmacol 9: 569.
[Crossref] [Google Scholar] [PubMed]
- Danborno AM, Tarfa F, Toryila JE, Awheela EU, Shekarau VT (2019) The effects of Jatropha tanjorensis aqueous leaf extract on haematological parameters in Wistar rats. J Afr Assoc Physiol Sci 7: 133-137.
- Iwalewa EO, Adewunmi CO, Omisore NO, Adebanji OA, Azike CK (2005) Pro and antioxidant effects and cytoprotective potentials of nine edible vegetables in Southwest Nigeria. J Med Food 8: 539-544.
[Crossref] [Google Scholar] [PubMed]
- Olayiwola G, Iwalewa EO, Omobuwajo OR, Adeniyi AA, Verspohi EJ (2004) The antidiabetic potential of Jatropha tanjorensis leaves. Nig J Nat Prod Med 8: 55-58.
- Ebe NU, Effiong, GS, Ikot AE (2019) Qualitative phytochemical analysis of ethanol leaves extract of Jatropha tanjorensis and its effects on liver function of male albino Wistar. Int J Biochem Bioinfo Biotech Stud 4: 1-11.
- Vijayakumar AR, Daniel EP, Ilavarasan R, Venkatarama S, Vijayakumar S (2016) Ulcer protective activity of Jatropha gossypiifolia Linn. in Wistar rats. Pharmacognosy Res 8: S61-S66.
[Crossref] [Google Scholar] [PubMed]
- Ochulor OC, Njoku OU, Uroko RI, Egba SI (2018) Nutritional composition of Jatropha tanjorensis leaves and effects of its aqueous extract on carbon tetrachloride induced oxidative stress in male Wistar albino rats. Biol Res 29: 3569-3576.
- Brozovich FV, Nicholson CJ, Degen CV, Yuan ZG, Aggarwal M, et al. (2016) Mechanisms of vascular smooth muscle contraction and the basis for pharmacologic treatment of smooth muscle disorders. Pharmacol Rev 68: 476–532.
[Crossref] [Google Scholar] [PubMed]
- Opie LH (2013) Calcium channel blockers. Drugs for the Heart 8: 64-92.
- Sverden E, Agreus L, Dunn J, Lagergren J (2019) Peptic ulcer disease. BMJ 367: l5495.
[Crossref] [Google Scholar] [PubMed]
- Macallister CG, Andrews FM, Deegan E (2010) A scoring system for gastric ulcers in the horse. Equine Vet J 29: 430–433.
[Crossref] [Google Scholar] [PubMed]
- Pearson JP, Allen A, Ward R (1986) Mucus degradation by pepsin: Comparison of mucolytic activity of human pepsin 1 and pepsin 3: Implications in peptic ulceration. Gut 27: 243–248.
[Crossref] [Google Scholar] [PubMed]
- Musa D (2017) Peptic ulcer disease and non-steroidal anti-inflammatory drugs. Aust Prescr 40: 91-93.
[Crossref] [Google Scholar] [PubMed]
- Gibson JB; Behrman SW, Fabian TC, Britt LG (2000) Gastric outlet obstruction resulting from peptic ulcer disease requiring surgical intervention is infrequently associated with Helicobacter pylori infection. J Am Coll Surg 191: 32-37.
[Crossref] [Google Scholar] [PubMed]
- Fürstenwerth H (2011) Aspirin: A historical and contemporary therapeutic overview. Circulation 124: e332-e333.
[Crossref] [Google Scholar] [PubMed]
- Rose PW, Watson EK, Jenkins LS (2011) Aspirin for prevention of cancer and cardiovascular disease. Br J Gen Prac 61: 412-415.
[Crossref] [Google Scholar] [PubMed]
- Rothwell PM, Fowkes FGR, Belch JF, Ogawa H, Warlow CP, et al. (2011) Effect of daily aspirin on long term risk of death due to cancer: Analysis of individual patient data from randomized trials. Lancet 377: 31-41.
[Crossref] [Google Scholar] [PubMed]
- Simmons DL, Botting RM, Hla T (2004) Cyclooxygenase isoenzymes: The biology of prostaglandin synthesis and inhibition. Pharmacol Rev 56: 387-437.
[Crossref] [Google Scholar] [PubMed]
- Fornai M, Natale G, Colucci R, Tuccori M, Carazzina G, et al. (2005) Mechanisms of protection by pantoprazole against NSAID induced gastric mucosal damage. Naunyn Schmiedebergs Arch Pharmacol 372: 79-87.
[Crossref] [Google Scholar] [PubMed]
- Wan Z, Hasegawa J, Wang X, Matsuda A, Tokuda T, et al. (2011) Protective effects of ginger against aspirin induced gastric ulcer in rats. Yonago Acta Med 54: 11-19.
[Google Scholar] [PubMed]
- Zhang Z, Dong X, Shang J, Zhou Y (2019) Research progress of traditional Chinese medicine in the treatment of gastric mucosa injury caused by long term use of low dose aspirin in patients with coronary heart disease. Longhua Chin Med 2: 9.
[Crossref]
- Wallace JL (2001) Pathogenesis of NSAID induced gastroduodenal mucosal injury. Best Pract Res Clin Gastroenterol 15: 691-703.
[Crossref] [Google Scholar] [PubMed]
- Smirnov IV, Oslopov VN, Bilich IL, Mendelevich VD (1990) The epidemiological aspects of combined arterial hypertension and peptic ulcer. Ter Arkh 62: 48–50.
[Google Scholar] [PubMed]
- Ramakrishnan K, Salinas RC (2007) Peptic ulcer disease. Am Fam Physician 6: 1005-1012.
[Google scholar] [PubMed]
- Prabhu V, Shivani A (2014) An overview of history, pathogenesis and treatment of perforated peptic ulcer disease with evaluation of prognostic scoring in adults. Ann Med Health Sci Res 4: 22–29.
[Crossref] [Google Scholar] [PubMed]
- Halabi MF, Shaki RM, Bardi DA, Al-Wajeeh NS, Ablat A, et al. (2014) Gastroprotective activity of ethyl-4-[(3,5-di-tert-butyl-2-hydroxybenzylidene) amino] benzoate against ethanol induced gastric mucosal ulcer in rats. PLoS One 9: 95908.
[Crossref] [Google Scholar] [PubMed]
- Hand O, Naito Y, Pukui A, Omatsu T, Yoshikawa T (2014) The impact of non-steroidal anti-inflammatory drugs on small intestinal epithelium. J Clin Biochem Nutr 54: 2-6.
[Crossref] [Google Scholar] [PubMed]
- Rasool MM, Sabina EP, Lavanya B (2006) Anti-inflammatory effect of Spirulina fusiformis on adjuvant induced arthritis in mice. Biol Pharm Bull 29: 2483-2487.
[Crossref] [Google Scholar] [PubMed]
- Nwachukwu CN (2018) Nutrient, phytochemical and anti-nutrient evaluation of Jatropha tanjorensis leaf (hospital too far). J Agric Food Sci 16: 36-46.
- van Zwieten PA (1994) Amlodipine: An overview of its pharmacodynamic and pharmacokinetic properties. Clin Cardiol 17: III3-6.
[Google Scholar] [PubMed]
- Ebenyi LN, Yongabi KA, Ali FU, Ominyi MC, et al. (2021) Effect of aqueous leaf extract of Jatropha tanjorensis on parasitaemia and haematological parameters in mice infected with Plasmodium berghei. Niger J Biotechnol 38: 146-153.
[Crossref]
- Rothwell PM, Cook NR, Gaziano JM, Price JF, Belch JF, et al. (2018) Effects of aspirin on risks of vascular events and cancer according to bodyweight and dose: Analysis of individual patient data from randomised trials. Lancet 392: 387-399.
[Crossref] [Google Scholar] [PubMed]
- Hawthorne AB, Mahida YR, Cole AT, Hawkey CJ (1991) Aspirin induced gastric mucosal damage: Prevention by enteric coating and relation to prostaglandin synthesis. Br J Clin Pharmac 32: 77-83.
[Crossref] [Google Scholar] [PubMed]
- Umoren EB, Obembe AO, Osim EE (2013) Ulcerogenic and intestinal motility/transit stimulating actions of nevirapine in albino Wistar rats. J Physiol Biochem 69: 547–557.
[Crossref] [Google Scholar] [PubMed]
- Krishnan U, Bohane TD, Day AS, Messina I, Mitchell JD (2002) Assay of tracheal pepsin as a marker of reflux aspiration. J Pediatr Gastroenterol Nutr 35: 303–308.
[Crossref] [Google Scholar] [PubMed]
- Tan PV, Dimo T, Enow-Orock GE, Kimbu SF, Nyasse B (2006) Evaluation of the antiulcer and toxicity profile of Aloe buettneri in laboratory animals. Afr J Tradit Complement Altern Med 3: 8–20.
- Owu DU, Obembe AO, Nwokocha CR, Edoho IE, Osim EE (2012) Gastric ulceration in diabetes mellitus: Protective role of vitamin c. ISRN Gastroenterology 2012: 362805.
[Crossref] [Google Scholar] [PubMed]
- Ismail OI, El-Meligy MMS (2022) Curcumin ameliorated low dose Bisphenol A induced gastric toxicity in adult albino rats. Sci Rep 12: 10201.
[Crossref] [Google Scholar] [PubMed]
- Blandizzi C, Fornai M, Colucci R, Natale G, Lubrano V, et al. (2005) Lansoprazole prevents experimental gastric injury induced by non-steroidal anti-inflammatory drugs through a reduction of mucosal oxidative damage. World J Gastroenterol 11: 4052-4060.
[Crossref] [Google Scholar] [PubMed]
- Sen S, Chakratborty R, De B, Mazumder J (2009) Plants and phytochemicals for peptic ulcer: An overview. Phcog Rev 3: 270-279.
- Mahmoud YI, Abd El-Ghffar EA (2019) Spirulina ameliorates aspirin induced gastric ulcer in albino mice by alleviating oxidative stress and inflammation. Biomed and Pharma 109: 314-321.
[Crossref] [Google Scholar] [PubMed]
- Ota K, Takeuchi T, Nouda S, Ozaki H, Kawaguchi S, et al. (2016) Determination of the adequate dosage of rebamipide, a gastric mucoprotective drug, to prevent low dose aspirin induced gastrointestinal mucosal injury. J Clin Biochem Nutr 59: 231-237
[Crossref] [Google Scholar] [PubMed]
- Salim A (1989) Scavenging free radicals to prevent stress induced gastric mucosal injury. Lancet 2: 1390.
[Crossref] [Google Scholar] [PubMed]
- Engel E, Guth PH, Nishizaki Y, Kaunitz JD (1995) Barrier function of the gastric mucus gel. Am J Physiol 269: G994–G999.
[Crossref] [Google Scholar] [PubMed]
- Shamburek RD, Schubert ML (1993) Pharmacology of gastric acid inhibition. Baillieres Clin Gastroenterol 7: 23–54.
[Crossref] [Google Scholar] [PubMed]
- Schubert ML (1999) Regulation of gastric acid secretion. Curr Opin Gastroenterol 15: 457-462.
[Crossref] [Google Scholar] [PubMed]
- Zhang W, Lian Y, Li Q, Sun L, Chen R, et al. (2020) Preventative and therapeutic potential of flavonoids in peptic ulcers. Molecules 25: 4626.
[Crossref] [Google Scholar] [PubMed]
- Allen A, Flemstrom G (2005) Gastroduodenal mucus bicarbonate barrier: Protection against acid and pepsin. Am J Physiol Cell Physiol 288: C1–C19.
[Crossref] [Google Scholar] [PubMed]
- Amol N, Patil M, Advani G, Mali SN, Sudhir P, et al. (2012) Evaluation of anti-ulcer effect of amlodipine in gastric ulcer models in rats. Indian J Pharmacol 44: 387–389.
[Crossref] [Google Scholar] [PubMed]
- Brunton LL (1996) Agents for control of Gastric acidity and treatment of peptic ulcer. McGraw Hill, 9th ed, New York, 901–915.
- Garrison JC (1992) Histamine, bradykinin, 5 hydroxytryptamine and their antagonist. Goodman and Gilman's the pharmacological basis of therapeutics. 8th edn. McGraw-Hill, New York, 234–323. [Crossref]
- Silverman RA (2004) The organic chemistry of drug action. Elsevier, Netherlands, 159.
- Vijayakumar AR, Daniel EP, Ilavarasan R, Venkataraman S, Vijayakumar S (2016) Ulcer protective activity of Jatropha gossypiifolia Linn. in Wistar rats. Pharmacognosy Res 8: S61–S66.
[Crossref] [Google Scholar] [PubMed]
- Song SC, An YM, Shin JH, Chung MJ, Seo JG, et al. (2017) Beneficial effects of a probiotic blend on gastrointestinal side effects induced by leflunomide and amlodipine in a rat model. Beneficial Microbes 8: 801-808.
[Crossref] [Google Scholar] [PubMed]
- Salga MS, Ali HM, Abdulla MA, Abdelwahab SI, ElhassanTaha MM, et al. (2010) Synthesis and gastroprotective activities of some zinc (II) complexes derived from (E)-2-(1-(2-piperazin-1-yl) ethylimino) ethyl) phenol and (E)-4-(1-2-(piperazin-1-yl) ethylimino) ethyl) benezene-1, 3-diol Schiff bases against aspirin induced ulceration. Arabian J Chem 10: 1578-1589.
- Prochazkova D, Bousova I, Wilhelmova N (2011) Antioxidant and prooxidant properties of flavonoids. Fitoterapia 82: 513–523.
[Crossref] [Google Scholar] [PubMed]
- Hussain T, Tan B, Murtaz G, Liu G, Rahu N, et al. (2020) Flavonoids and type 2 Diabetes: Evidence of efficacy in clinical and animal studies and delivery strategies to enhance their therapeutic efficacy. Pharmacol Res 152: 104629.
[Crossref] [Google Scholar] [PubMed]
- Oteiza PI, Fraga CG, Mills DA, Taft DH (2018) Flavonoids and the gastrointestinal tract: Local and systemic effects. Mol Aspects Med 61: 41–49.
[Crossref] [Google Scholar] [PubMed]
Citation: Bassey UE, Asuquo OI, Idabie BP, Udofia OD, Lawrence BA, et al. (2023) Jatropha tanjorensis Ameliorate Effects of Aspirin Induced Stomach Ulcer in Wistar Rats. J Gastrointest Dig Syst 13: 732
Copyright: © 2023 Bassey UE, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Usage
- Total views: 2544
- [From(publication date): 0-2023 - Oct 21, 2025]
- Breakdown by view type
- HTML page views: 2165
- PDF downloads: 379