Ivermectin for Patients with Coronavirus Disease 2019: A Systematic Review and Meta-Analysis of Randomised Controlled Trials with Trial Sequential Analysis
Received: 07-Mar-2022 / Manuscript No. jidt-22-53762 / Editor assigned: 09-Mar-2022 / PreQC No. jidt-22-53762(PQ) / Reviewed: 23-Mar-2022 / QC No. jidt-22-53762 / Revised: 30-Mar-2022 / Manuscript No. jidt-22-53762(R) / Published Date: 08-Apr-2022
Abstract
Background: Studies evaluating the effectiveness of ivermectin in reducing time to recovery in patients with COVID-19 have yielded mixed results. We conducted a systematic review and meta-analysis to determine if ivermectin was effective in patients with COVID-19.
Methods: Six databases were searched for Randomised Controlled Trials (RCTs), assessing ivermectin in adults hospitalised with COVID-19 up till December 15th, 2021. Random effects meta-analyses (DerSimonian and Laird) were conducted. The risk of bias was evaluated using the Cochrane Risk-ofBias 2 tool, with certainty of evidence rated using the Grading of Recommendations, Assessment, Development, and Evaluations (GRADE) approach. Trial sequential analysis (TSA) was conducted on the reduction in time-to-recovery, as well as mortality.
Results: Twenty-three RCTs (3087 patients, 1601 ivermectin and 1486 control) were included in the meta-analysis; 5 with high risk of bias, 13 with moderate risk and 5 with low risk. Ivermectin reduced overall time-to-recovery (Hedges’ g: -0.65, 95%-CI: -1.04 to -0.27, p=0.0009, low certainty), and inhospital mortality (Risk Ratio [RR]: 0.62, 95%-CI: 0.39-0.99, p=0.046, low certainty). There were no differences in hospital length of stay (Hedges’ g: -0.49, 95%-CI: -1.16 to 0.18, p=0.15, low certainty), or the final proportion of patients with negative SARS-CoV-2 PCR (RR: 1.04, 95%-CI: 0.98-1.10, p=0.18, low certainty). TSA found that the cumulative Z-curve passed the TSA-adjusted boundary for benefit for time to recovery. The cumulative Z-curve did not pass the boundaries for benefit or futility for reduction in mortality.
Conclusion: Our meta-analysis revealed ivermectin may reduce time-to-recovery in patients with COVID-19. However, most RCTs included were limited by risk of bias in the randomisation process, reporting of outcomes and deviations from intended interventions. There was also significant heterogeneity in terms of timing, duration, and dosing of ivermectin. Thus, the apparent benefit seen in this analysis should be interpreted in this context.
Keywords: COVID-19; Severe acute respiratory syndrome coronavirus-2; Ivermectin; Mortality; Meta-analysis
Introduction
The coronavirus disease 2019 (COVID-19) pandemic has resulted in a wave of research into therapeutic targets that might be able to treat, or prevent the progression of COVID-19 disease [1]. Despite this spike in research, very few treatments have been established to reduce morbidity and mortality from COVID-19. While drugs like corticosteroids [2] and tocilizumab may reduce mortality in severe disease, [3,4] there is less convincing evidence on other therapies, such as convalescent plasma or remdesivir, which are shown to prevent or reduce disease progression and hospitalisation [5,6]. One such other therapy that lacks conclusive evidence is ivermectin, despite its use in observational and randomised control trials (RCTs) as therapeutic and/or prophylactic agent in patients with COVID-19 [7]. Initially used as an antiparasitic agent, ivermectin has in recent years received interest as an antiviral against ribonucleic acid (RNA) viruses. It was initially discovered as an inhibitor of interaction between human immunodeficiency virus-1 (HIV-1) integrase (IN) and the importin (IMP) α/β1 heterodimer for IN nuclear import, limiting HIV-1 replication by inhibiting the nuclear import of host and viral proteins [8,9]. Such functions extend to other RNA viruses such as influenza and dengue which are similarly reliant on IMP α/β1 [10,11]. With further studies highlighting the role of IMP α/β1 in severe acute respiratory syndrome coronavirus (SARS-CoV) infection, ivermectin has received considerable attention as a potential antiviral medication for patients with COVID-19.Prior studies have addressed the usage of ivermectin in COVID-19, but there is still a paucity of conclusive evidence on its effectiveness. The World Health Organisation (WHO) living guideline has recommended against using ivermectin to treat COVID-19, except in clinical trials [12]. There is also equivocal evidence between clinical studies suggesting a lack of consensus on whether ivermectin is able to provide a clinical benefit to patients with COVID-19 [13-16]. As such,we conducted a systematic review and meta-analysis with trial sequential analysis (TSA) of published RCTs to elucidate the effect ivermectin has on disease outcomes in patients with COVID-19.
Methodology
Search strategy and selection and criteria
This study was registered with PROSPERO (CRD42021254751), and was conducted in adherence with the Preferred Reporting Items for Systematic Reviews and Meta-analyses Statement Checklist [17]. PubMed, Embase, Cochrane, Scopus, medRXiv, and COVID- NMA databases were originally searched for publications up till 1st September 2021, using the keywords ‘COVID-19, ‘ivermectin’, and ‘randomised controlled trial’ (Supplementary Data 1). The search was then subsequently re-ran at a later date till 15th December 2021, to account for any new studies that might have been published in the meantime. The studies and citation lists obtained from these searches were then assessed for inclusion. Inclusion criteria were RCTs written in English reporting on 10 or more adults (≥ 18 years) hospitalised with COVID-19, that compared ivermectin with a control group and reporting on the following prespecified outcomes. Studies reporting on non-human populations, as well as studies where ivermectin was administered prophylactically were excluded.
Data collection
Data collection was conducted using a prespecified data extraction form and the included the following areas: Study characteristics, patient demographics, baseline characteristics of the subjects, details of their indications for ivermectin or comparator treatment, and clinically relevant patient outcomes (Supplementary Data 2).
Risk of bias assessment
Risk of bias within individual RCTs was rated using the Cochrane Risk-of-Bias 2 Tool. The certainty of evidence was rated using the Grading of Recommendations, Assessment, Development, and Evaluations (GRADE) approach. Publication bias was assessed using visual inspection of funnel plots (when studies<10) and Egger’s regression test (when studies>10). The screening of articles, data collection, and risk of bias assessment was carried out independently by three reviewers, with conflicts resolved by a fourth reviewer.
Study outcomes
The primary outcome was the time-to-recovery, which comprised time-to-negative Polymerase Chain Reaction (PCR) test, and/or symptom resolution. This was quantified using the standardized mean difference (SMD, Hedge’s g), which accounts for heterogeneity and differing variances across treatment arms in the selected studies. Secondary outcomes included the hospital length of stay (LOS), negative PCR test, hospital mortality, and presence of any ivermectin- related recorded adverse drug events.
Statistical analysis
Means and standard deviations (SD) were derived from the reported data using the methods by Wan and colleagues [18]. Statistical analyses were performed using R3.6.1. We anticipated significant heterogeneity considering the variability of patients’ conditions and pharmacological therapies used in the treatment of COVID-19. As such, random-effects meta-analyses (DerSimonian and Laird) were conducted based on the Freeman-Tukey double arcsine transformation, and 95% confidence intervals (CIs) were computed using the Clopper-Pearson method [19- 21]. A post hoc sensitivity analysis was conducted by excluding pre- print studies, and analysing only studies published in peer-reviewed journals to identify the possible causes of substantial heterogeneity.Pre-specified subgroup analysis was conducted based on the risk of bias of each study (low, moderate, high).
To further elicit the therapeutic effect of ivermectin in COVID-19, we performed TSA using TSA v0.9.5.10 (www.ctu.dk/tsa), assessing efficacy and futility based on the O’Brien-Fleming alpha and beta- spending functions respectively. Similar to group sequential monitoring boundaries in RCTs during interim analyses, TSA implements cumulative meta-analysis to evaluate the cumulative pooled effect following the inclusion of an additional trial based on the information size thus obtained.
Results
Study details and demographics
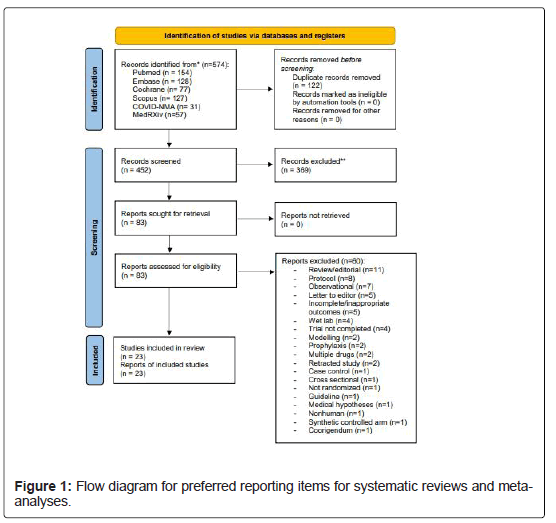
Out of the 452 potentially relevant studies across the databases, 83 were selected for full-text screening. Twenty-seven RCTs were selected for review in our study, however, four studies were excluded: two of them did not report on prespecified outcomes, [22,23] while the other two were subsequently retracted due to data discrepancies[24, 25]. Consequently, 23 studies (3087 patients, 1601 and 1486 patients in ivermectin and control, respectively) were included in the meta- analysis (Figure 1) [13-16, 26-44]. At the time of writing, there were 9 pre-prints and 14 peer-reviewed publications. Seventeen studies were from Asia and the Middle East, 3 studies were from South America, and 1 study each from Europe, Africa and Central America. The pooled mean age between the ivermectin (42.9 years, 95%-CI: 39.5 to 46.4) and control group (42.6 years, 95%-CI: 38.7 to 46.5) was similar. Ivermectin was started in variable presentations of COVID-19 (mild, moderate, severe, and critical), across a range of doses (from 6 mg to 24 mg, though many studies gave doses in mcg/kg), and drug administration timings. Study details, patient demographics, outcomes, complications, and adverse events are summarised in Supplementary Data 3 and 4, and the indications, doses, and interval from symptom onset to ivermectin administration are summarised in Supplementary Data 5.
Assessment of study quality
The risk of bias for the included studies are summarised in Supplementary Data 6. 5 studies were deemed to have overall high risk of bias, 13 studies had moderate risk of bias, while the remaining 5 studies had an overall low risk of bias. The main risks of bias were in the randomisation process, the selection of reported results, and deviations from intended interventions. The GRADE assessment of evidence was summarized in Supplementary Data 7. Primary meta-analysis
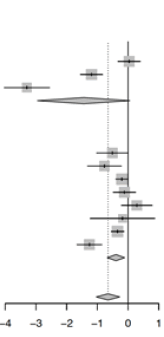
Eleven studies reported on the time-to-recovery between both groups, ivermectin significantly reduced the time-to-recovery in patients with COVID-19 (Hedge’s g: -0.65, 95%-CI: -1.04 to -0.27, p=0.0009, lowcertainty, Table 1) with no significant evidence for publication bias (pegger=0.21, Supplementary Data 8). A post-hoc sensitivity analysis, excluding pre-print studies was conducted, only analysing studies which were published in peer-reviewed journals. The pooled estimate remained relatively similar (g: -0.65, 95%-CI: -1.10 to -0.21, p=0.0037). Subgroup analysis by the risk of bias found that improvements in time-to-recovery with ivermectin usage reported by studies with some concerns of bias (8 studies, g: -0.39, 95%-CI: -0.68 to 9-0.11, p=0.0068) did not significantly differ (pinteraction=0.18) from those with high risks of bias (3 studies, g: -1.45, 95%-CI: -2.96 to 0.06, p=0.060). No studies with low risk of bias were included in the subgroup analysis.
| Ivermectin | Control | Time to recovery | SMD | 95% CI | Weight | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | Total | Mean | SD | Total | Mean | SD | |||||||||
| Bias=High |
|
||||||||||||||
| Chowdhury 2021 | 60 | 9.98 | 3.7900 | 56 | 9.78 | 7.6090 | 0.03 | [-0.33; 0.40] | 9.7% | ||||||
| Hashim 2020 | 70 | 10.61 | 5.3000 | 70 | 17.90 | 6.8000 | -1.19 | [-1.55; -0.83] | 9.8% | ||||||
| Shahbaznejad 2021 | 35 | 4.20 | 0.3000 | 34 | 5.20 | 0.3000 | -3.30 | [-4.03; -2.56] | 7.7% | ||||||
| Random effects model | 250 | 247 | -1.45 | [-2.96; 0.06] | 27.2% | ||||||||||
| Heterogeneity: I 2=97%, t2=1.7097, p<0.01 | |||||||||||||||
| Bias=Some | |||||||||||||||
| Ahmed 2021 | 45 | 10.62 | 4.3250 | 23 | 12.70 | 3.3530 | -0.51 | [-1.02; 0.00] | 9.0% | ||||||
| Babalola 2021 | 42 | 5.53 | 3.1200 | 20 | 9.15 | 7.4200 | -0.77 | [-1.32; -0.22] | 8.8% | ||||||
| Lopez-Medina 2021 | 200 | 10.70 | 2.9870 | 198 | 11.30 | 2.9870 | -0.20 | [-0.40; 0.00] | 10.4% | ||||||
| Mohan 2021 | 80 | 4.26 | 2.5300 | 45 | 4.58 | 2.9400 | -0.12 | [-0.48; 0.25] | 9.7% | ||||||
| Podder 2020 | 32 | 6.33 | 4.2300 | 30 | 5.31 | 2.4800 | 0.29 | [-0.21; 0.79] | 9.0% | ||||||
| Pott-Junior 2021 | 27 | 5.17 | 2.7200 | 4 | 5.67 | 2.1831 | -0.18 | [-1.23; 0.87] | 6.0% | ||||||
| Mahmud 2021 | 183 | 7.00 | 4.4830 | 180 | 8.67 | 5.2310 | -0.34 | [-0.55; -0.14] | 10.4% | ||||||
| Aref 2021 | 57 | 8.30 | 2.8000 | 57 | 12.90 | 4.3000 | -1.26 | [-1.66; -0.86] | 9.6% | ||||||
| Random effects model | 763 | 652 | -0.39 | [-0.68; -0.11] | 72.8% | ||||||||||
| Heterogeneity:Not applicable | |||||||||||||||
| Random effects model | 831 | 717 | -0.65 | [-1.04; -0.27] | 100.0% | ||||||||||
| Heterogeneity: I2=91%, t2=0.3634, p<0.01 Residual heterogeneity: I 2=91%, p<0.01 |
|||||||||||||||
Table 1: Standardised mean difference in time to recovery among patients with COVID-19 treated with ivermectin or standard of care (control).
Trial sequential analysis
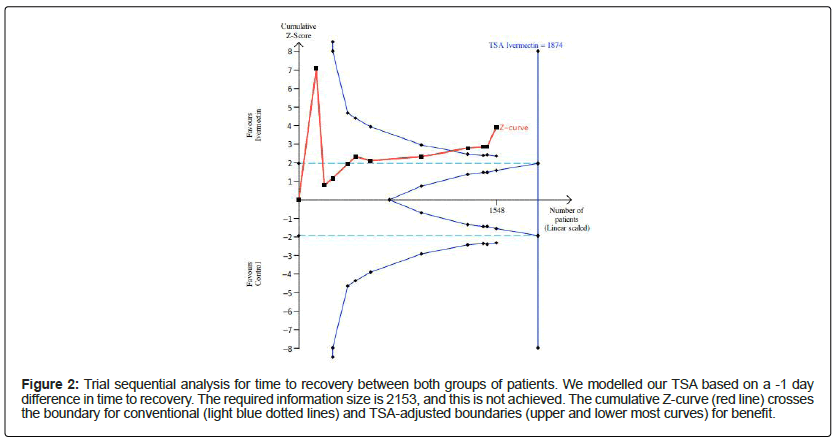
We conducted an efficacy analysis based on the RCTs that reported on the time-to-recovery and mortality between both groups of patients. In this model, we specified a type I error 0.05 and a power of 0.80 and estimated the required information size from the pooled effects of our meta-analysis, namely (1) a reduction in time-to-recovery by -1.0 day, and (2) a 37.9% RR reduction in mortality form a baseline in-hospital mortality rate of 7%. For the time-to-recovery, the required information size was 1874 patients. The cumulative Z-curve passed the conventional boundary and TSA-adjusted boundary for benefit (Figure 2). The required information size to estimate the pooled reduction in hospital mortality was 2870. While the cumulative Z-curve crossed the conventional boundary of benefit, it did not pass the TSA-adjusted boundaries for benefit or harm, (Supplementary Data 9). Given that efficacy was not demonstrated for mortality, we deemed the hypothesis of futility to be relevant. Thus, futility analysis was conducted to see whether ivermectin was completely futile in treating COVID-19. The cumulative Z-curve did not cross the boundary for futility either.
Figure 2: Trial sequential analysis for time to recovery between both groups of patients. We modelled our TSA based on a -1 day difference in time to recovery. The required information size is 2153, and this is not achieved. The cumulative Z-curve (red line) crosses the boundary for conventional (light blue dotted lines) and TSA-adjusted boundaries (upper and lower most curves) for benefit.
Secondary outcomes
Several secondary outcomes were also measured during this study. Ivermectin significantly reduced the risk of hospital mortality (11 studies, RR: 0.62, 95%-CI: 0.39 to 0.99, p=0.046, pegger=0.24, low certainty). There were no differences in hospital length of stay (5 studies, g: -0.49, 95%-CI: -1.16 to 0.18, p=0.15, lowcertainty), or in the final proportion of patients with negative SARS-CoV-2 PCR at the end of data collection (11 studies, RR: 1.04, 95%-CI: 0.98 to 1.10, p=0.18, low certainty). The details of the other secondary outcomes, including the forest plots and SMDs of individual’s studies, are summarised in Supplementary Data 9.
Data was also collected for several other outcomes that were eventually not meta-analyzed due to insufficient data across all the studies and the heterogenous way in which outcomes were defined and assessed. These details are also found in Supplementary Data 4.
We also looked at disease progression, which was measured as the need for invasive mechanical ventilation in 4 studies, ICU admission in 3 studies, or recorded simply as disease progression in 3 studies. One study recorded a decrease in WHO ordinal scale score to track disease progression. (Supplementary Data 4).
Discussion
This systematic review and meta-analysis quantitatively summarised the evidence for outcomes. The composite time-to- recovery for ivermectin was shorter when compared to the control. TSA was concordant with the primary meta-analysis and suggested that ivermectin may significantly reduce the time-to-recovery. studies establishing ivermectin’s antiviral effects against SARS-CoV-2 were concordant with results obtained from recently published RCTs, which showed significantly reduced time-to-recovery when using ivermectin or ivermectin combinations as opposed to placebo or non-ivermectin combinations [13,28]. Additionally, TSA for reduction in mortality showed that the use of ivermectin did not cross the boundary for futility, highlighting that more RCTs looking at this outcome should be performed before arriving at a meaningful conclusion.
Although several prior studies have been published, the research into effectiveness of ivermectin in COVID-19 has been plagued by controversies. Elgazzar et al. (2020) demonstrated the benefits of ivermectin in patients with COVID-19 in a large RCT, but the publication has been withdrawn due to methodological issues, and discrepancies in data presented [45]. Additionally, another study by Kory et al. (2021) was also removed pre-publication for unsubstantiated claims [46]. While there are concerns with regards to the risk of bias of the included studies, there is some evidence that suggests ivermectin might not be futile in COVID-19. While early reviews have suggested a significant mortality benefit, [47] other reviews did not find any significant improvements in any clinical benefits [48]. Nonetheless, smaller sample sizes in the aforementioned meta-analyses may have affected the quality and reliability of the data. More recent reviews of larger sample sizes have found concordant results with our review [49,50]. Additionally, the TSA found that the cumulative Z-curve had crossed TSA-adjusted boundaries for benefit, lending weight that ivermectin might be useful in treating patients with COVID-19. Furthermore, while the cumulative Z-curve did not pass the boundary for benefit with regards to hospital mortality, it more importantly did not cross the futility boundary. Combining these two analyses together implied that there was sufficient statistical evidence to suggest that ivermectin reduced the time-to-recovery in patients with COVID-19, however there was insufficient evidence to confirm whether it improved mortality or not in patients with COVID-19.
Currently the evidence base favouring ivermectin is plagued with inconsistent data and risks of bias, making the effectiveness of ivermectin in COVID-19 unclear. Rather, more high-quality RCTs should be conducted to determine its effectiveness more conclusively, and the target population where it yields the most meaningful benefit. With a surge of cases once again in the second half of 2021, this holds immense public health implications, particularly so as ivermectin is still commonly used in several countries as a routine therapeutic drug for COVID-19. The Platform Randomised Trial of Treatments in the Community for Epidemic and Pandemic Illnesses (PRINCIPLE), is a large scale RCT currently being conducted by the University of Oxford which aims to shed more light on the efficacy of ivermectin, and its methodological quality must be subsequently assessed [51]. Furthermore, initial results from the recently concluded Early Treatment of COVID-19 with Repurposed Therapies (TOGETHER) trial with more than 1,300 patients has found non-significant decreases in the risk of extended hospitalisation (RR: 0.91, 95%-CI: 0.69 to 1.19) and mortality (RR: 0.82, 95%-CI: 0.44 to 1.52) in the ivermectin group [52].
Strengths and Limitations
The meta-analysis and TSA are particularly apposite in the context of the uncertainty regarding ivermectin for COVID-19. While several meta-analyses have been conducted, the added value of our meta-analysis lies in the use of TSA, which was able to evaluate the cumulative pooled effect in relation to the information size for the time-to-recovery and in-hospital mortality. Through this, we were able to show that ivermectin may not be futile in patients with COVID-19. Moreover, most of the studies were conducted in a wide range of centres across the world with differing healthcare resource capacities. If proven to be effective, ivermectin would be beneficial in treating mild COVID-19 and avoiding hospitalisations in nations where resources to treat COVID-19 may already be stretched thin. Nevertheless, we still note that this may still have its limitations, with trials in Asia being disproportionately frequent compared to other nations. We also recognise several limitations of our study. Firstly, and most importantly, the quality of the studies included in our analysis ranged from some concerns to high risks of bias, and this reduces our certainty in the pooled effect estimate to some extent. We have acknowledged this risk of bias and accordingly downgraded the certainty of evidence for our effect estimates via GRADE. Secondly, there was a wide range of heterogeneity in the reported outcomes between studies. Reported timings to recovery vary greatly between studies, and the secondary outcomes and the way in which they were measured also differ greatly based on the protocols and practices of individual institutions. There was also no consensus of the dosing regimen of ivermectin or other standard regimens. Taken together, the results of the analysis should hence be interpreted with caution, and the true effect of ivermectin is yet to be definitively determined. Nonetheless, our systematic review and meta-analysis represents the most updated data in the current literature and suggests a possibility that ivermectin might be effective in patients with COVID-19. More wellconducted and well-powered RCTs are urgently needed to clarify the effectiveness of ivermectin better.
Conclusion
While current clinical sentiment is that ivermectin is ineffective in treating COVID-19, our systematic review and meta-analysis of the current literature suggests that it is worthwhile to further explore its use in patients with COVID-19, as ivermectin may reduce time-to-recovery. While trial sequential analysis demonstrated that ivermectin treatment might be effective, the current evidence is limited by very serious risk of bias, as well as significant statistical and clinical heterogeneity. Further RCTs directed at the timing and dose of ivermectin in patients with COVID-19 would be needed to better assess clinical benefit.
Acknowledgements
CJWL and KR had full access to all the data in the study and take responsibility for the integrity of the data and accuracy of the data- analysis.
Declaration of Interests
All authors declare no competing interests.
All authors declare no competing interests.
Data Sharing Statement
All data generated or analysed during this study are included in the published studies and their supplementary information files.
References
- Zhaori G, Lu L, Liu C, Guo Y (2020) Progresses in clinical studies on antiviral therapies for COVID-19-experience and lessons in design of clinical trials. Pediatr Investig 4:263-274.
[Crossref] [Google Scholar] [PubMed]
- Group TR (2020) Dexamethasone in hospitalized patients with Covid-19-preliminary report. N Engl J Med.
- Ling RR, Ramanathan K, Tan WQ, Yeo LH, Poon WH (2021) Interleukin-6 receptor antagonists for severe coronavirus disease 2019: A meta-analysis of reconstructed individual participant data from randomised controlled trials.
- Soin AS, Kumar K, Choudhary NS, Sharma P, Mehta Y, et al. (2021) Tocilizumab plus standard care versus standard care in patients in India with moderate to severe COVID-19-associated cytokine release syndrome (COVINTOC): An open-label, multicentre, randomised, controlled, phase 3 trial. Lancet Respir Med 9:511-521.
[Crossref] [Google Scholar] [PubMed]
- Janiaud P, Axfors C, Schmitt AM, Gloy V, Ebrahimi F, et al. (2021) Association of convalescent plasma treatment with clinical outcomes in patients with COVID-19:A systematic review and meta-analysis. Jama 325:1185-1195.
[Crossref] [Google Scholar] [PubMed]
- Rochwerg B, Agarwal A, Siemieniuk RA, Agoritsas T, Lamontagne F, et al. A living WHO guideline on drugs for covid-19. BMJ 370:3379.
[Crossref] [Google Scholar] [PubMed]
- Garegnani LI, Madrid E, Meza N (2021) Misleading clinical evidence and systematic reviews on ivermectin for COVID-19. BMJ Evid Based Med.
[Crossref] [Google Scholar] [PubMed]
- Caly L, Druce JD, Catton MG, Jans DA, Wagstaff KM (2020) The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antivir Res 178:104787.
[Crossref] [Google Scholar] [PubMed]
- Wagstaff KM, Rawlinson SM, Hearps AC, Jans DA (2011) An AlphaScreen®-based assay for high-throughput screening for specific inhibitors of nuclear import. J Biomol Screen 16:192-200.
[Crossref] [Google Scholar] [PubMed]
- Tay MY, Fraser JE, Chan WK, Moreland NJ, Rathore AP, et al. (2013) Nuclear localization of dengue virus (DENV) 1-04 non-structural protein 5; protection against all 4 DENV serotypes by the inhibitor Ivermectin. Antivir Res 99:301-306.
[Crossref] [Google Scholar] [PubMed]
- Götz V, Magar L, Dornfeld D, Giese S, Pohlmann A, et al. (2016) Influenza A viruses escape from MxA restriction at the expense of efficient nuclear vRNP import. Sci Rep 6(1):1-5.
[Crossref] [Google Scholar] [PubMed]
- Rochwerg B, Agarwal A, Siemieniuk RA, Agoritsas T, Lamontagne F, et al. A living WHO guideline on drugs for covid-19. BMJ 370: m3379.
[Crossref] [Google Scholar] [PubMed]
- Ahmed S, Karim MM, Ross AG, Hossain MS, Clemens JD, et al. (2021) A five-day course of ivermectin for the treatment of COVID-19 may reduce the duration of illness. Int J Infect Dis 103:214-216.
[Crossref] [Google Scholar] [PubMed]
- Babalola OE, Bode CO, Ajayi AA, Alakaloko FM, Akase IE, et al. (2021) Ivermectin shows clinical benefits in mild to moderate COVID19: A randomized controlled double-blind, dose-response study in Lagos. QJM: Int J Med 114:780-788.
[Crossref] [Google Scholar] [PubMed]
- Galan LEB, Santos NMD, Asato MS, Araújo JV, de Lima Moreira, et al. (2021) Phase 2 randomized study on chloroquine, hydroxychloroquine or ivermectin in hospitalized patients with severe manifestations of sars-cov-2 infection. Pathog Glob Health 115:235-242.
[Crossref] [Google Scholar] [PubMed]
- López-Medina E, López P, Hurtado IC, Dávalos DM, Ramirez O, et al. (2021) Effect of ivermectin on time to resolution of symptoms among adults with mild COVID-19: A randomized clinical trial. Jama 325:1426-1435.
[Crossref] [Google Scholar] [PubMed]
- Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, et al. (2015) Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Systematic reviews. 4(1):1-9.
[Crossref] [Google Scholar] [PubMed]
- Wan X, Wang W, Liu J and Tong T (2014) Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC 14:135.
[Crossref] [Google Scholar] [PubMed]
- Clopper CJ, Pearson ES (1934) The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika 26:404-413.
- DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Controlled clinical trails 7:177-188.
[Crossref] [Google Scholar]
- Miller JJ (1978) The inverse of the Freeman-Tukey double arcsine transformation. Am Stat 32:138.
[Crossref] [Google Scholar] [PubMed]
- Chahla RE, Ruiz LM, Mena T, Brepe Y, Terranova P, et al. (2021) Ivermectin reproposing for COVID-19 treatment outpatients in mild stage in primary health care centers. medRxiv.
- Krolewiecki A, Lifschitz A, Moragas M, Travacio M, Valentini R, et al. Antiviral effect of high-dose ivermectin in adults with COVID-19:A pilot randomised, controlled, open label, multicentre trial.
- Elgazzar A, Eltaweel A, Youssef SA, Hany B, Hafez M, et al. (2020) Efficacy and Safety of Ivermectin for Treatment and prophylaxis of COVID-19 Pandemic.
- Samaha AA, Mouawia H, Fawaz M, Hassan H, Salami A, et al. (2021) Effects of a single dose of ivermectin on viral and clinical outcomes in asymptomatic SARS-CoV-2 infected subjects: A pilot clinical trial in Lebanon. Viruses 13:989.
[Crossref] [Google Scholar] [PubMed]
- Chaccour C, Casellas A, Blanco-Di Matteo A, Pineda I, Fernandez-Montero A, et al . (2021) The effect of early treatment with ivermectin on viral load, symptoms and humoral response in patients with non-severe COVID-19:A pilot, double-blind, placebo-controlled, randomized clinical trial. EClin Med. 32:100720.
[Crossref] [Google Scholar] [PubMed]
- Chachar AZ, Khan KA, Asif M, Tanveer K, Khaqan A, et al. (2020) Effectiveness of ivermectin in SARS-CoV-2/COVID-19 patients. Int J Sci 9:31-35.
- Chowdhury AT, Shahbaz M, Karim R, Islam J, Dan G, et al. (2021) A comparative study on ivermectin-doxycycline and hydroxychloroquine-azithromycin therapy on COVID-19 patients. EJMO 5:63-70.
- Gonzalez JLB, González Gámez M, Enciso EAM, Maldonado RJE, Hernández Palacios D. Efficacy and safety of ivermectin and hydroxychloroquine in patients with severe covid-19. A randomized controlled trial. medRxiv.
- Hashim HA, Maulood MF, Rasheed AM, Fatak DF, Kabah KK, et al. (2020) Controlled randomized clinical trial on using Ivermectin with Doxycycline for treating COVID-19 patients in Baghdad, Iraq. medRxiv.
- Kishoria N, Mathur SL, Parmar V, Kaur RJ, Agarwal H, et al. (2020) Ivermectin as adjuvant to hydroxychloroquine in patients resistent to standard treatment for SARS-CoV-2:Results of an open-label randomized clinical study. Indian J Res 9(8).
- Mahmud R, Rahman MM, Alam I, Ahmed KG, Kabir AH, et al. (2021) Ivermectin in combination with doxycycline for treating COVID-19 symptoms:A randomized trial. Int J Med Res 49:03000605211013550.
[Crossref] [Google Scholar] [PubMed]
- Mohan A, Tiwari P, Suri TM, Mittal S, Patel A, et al. (2021) Single-dose oral ivermectin in mild and moderate COVID-19 (RIVET-COV):A single-centre randomized, placebo-controlled trial. J Infect Chemother 27:1743-1749.
[Crossref] [Google Scholar] [PubMed]
- Morteza Shakhsi N, Nematollah G, Peyman N, Abbas A, Leila Z, et al. (2020) Ivermectin as an adjunct treatment for hospitalized adult covid-19 patients: A randomized multi-center clinical trial. Research Square.
- Okumuş N, Demirtürk N, Çetinkaya RA, Güner R, Avcı İY, et al. (2021) Evaluation of the effectiveness and safety of adding ivermectin to treatment in severe COVID-19 patients. BMC 21:1-1.
[Crossref] [Google Scholar] [PubMed]
- Podder CS, Chowdhury N, Sina MI, Haque WM (2020) Outcome of ivermectin treated mild to moderate COVID-19 cases:A single-centre, open-label, randomised controlled study. IMC 14:11-8.
- Pott-Junior H, Paoliello MM, Miguel AD, da Cunha AF, de Melo Freire CC, et al. (2021) Use of ivermectin in the treatment of COVID-19: A pilot trial. Toxicol Rep 8:505-510.
[Crossref] [Google Scholar] [PubMed]
- Roy R, Pattadar C, Raj R, Agarwal N, Biswas B, et al. (2021) Ivermectin as a potential treatment for mild to moderate COVID-19–a double blind randomized placebo-controlled trial. medRxiv.
[Crossref] [Google Scholar] [PubMed]
- Shah Bukhari KH, Asghar A, Perveen N, Hayat A, Mangat SA, et al. (2021) Efficacy of ivermectin in covid-19 patients with mild to moderate disease. medRxiv.
- Shahbaznejad L, Davoudi A, Eslami G, Markowitz JS, Navaeifar MR, et al. (2021) Effects of ivermectin in patients with covid-19: A multicenter, double-blind, randomized, controlled clinical trial. Clin Ther. 43:1007-1019.
[Crossref] [Google Scholar] [PubMed]
- Abd-Elsalam S, Noor RA, Badawi R, Khalaf M, Esmail ES, et al. (2021) Clinical study evaluating the efficacy of ivermectin in covid-19 treatment:A randomized controlled study. J Med Virol.
[Crossref] [Google Scholar] [PubMed]
- Aref ZF, Bazeed SE, Hassan MH, Hassan AS, Rashad A, et al. (2021) Clinical, biochemical and molecular evaluations of ivermectin mucoadhesive nanosuspension nasal spray in reducing upper respiratory symptoms of mild COVID-19. Int J Nanomedicine 16:4063.
[Crossref] [Google Scholar] [PubMed]
- Vallejos J, Zoni R, Bangher M, Villamandos S, Bobadilla A, et al. (2021) Ivermectin to prevent hospitalizations in patients with COVID-19 (IVERCOR-COVID19) a randomized, double-blind, placebo-controlled trial. BMC 21(1):1-1.
- Biber A, Mandelboim M, Harmelin G, Lev D, Ram L, et al. (2021) Favorable outcome on viral load and culture viability using Ivermectin in early treatment of non-hospitalized patients with mild COVID-19-A double-blind, randomized placebo-controlled trial. medRxiv.
- Davey M (2021) Huge study supporting ivermectin as Covid treatment withdrawn over ethical concerns. The Guardian.
- Khandelwal A, Singh GP, Jamil S. (2021) Ivermectin as a multifaceted drug in COVID-19: Current insights. Med J Armed Forces India 77:254.
[Crossref] [Google Scholar] [PubMed]
- Padhy BM, Mohanty RR, Das S, Meher BR (2020) Therapeutic potential of ivermectin as add on treatment in COVID 19: A systematic review and meta-analysis: Ivermectin in COVID-19: A meta-analysis. J Pharm Pharm Sci 23:462-469.
[Crossref] [Google Scholar] [PubMed]
- Diaz AV, Rivera JA, Román YM, Burela PA, Pasupuleti V, et al. (2021) Ivermectin for the treatment of COVID-19: A systematic review and meta-analysis of randomized controlled trials. Clin Infect Dis.
[Crossref] [Google Scholar] [PubMed]
- Bryant A, Lawrie TA, Dowswell T, Fordham EJ, Mitchell S (2021) Ivermectin for prevention and treatment of COVID-19 infection: a systematic review, meta-analysis, and trial sequential analysis to inform clinical guidelines. Am J Ther 28(4):434.
[Crossref] [Google Scholar] [PubMed]
- Hariyanto TI, Halim DA, Rosalind J, Gunawan C, Kurniawan A. (2021) Ivermectin and outcomes from Covid‐19 pneumonia: A systematic review and meta‐analysis of randomized clinical trial studies. Rev Med Virol 2265.
- Boven AG, Kenyon C, Colebunders R (2021) Ivermectin Should Not Be Recommended to Treat Severe Acute Respiratory Syndrome 2 Infection. In Open Forum Infectious Diseases US: Oxford University Press.
- Reis G, Mills E, Guyatt G, Thabane L, Lenze E, et al. (2021) Early treatment of covid-19 with repurposed therapies: The together adaptive platform trial. NIH Collaboratory.
Citation: Low CJW, Ling RR, Tham GY, Subramaniam A, Tai BC, et al. (2022) Ivermectin for Patients with Coronavirus Disease 2019: A Systematic Review and Meta-Analysis of Randomised Controlled Trials with Trial Sequential Analysis. J Infect Dis Ther S2:002.
Copyright: © 2022 Low CJW, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Usage
- Total views: 1690
- [From(publication date): 0-2022 - Nov 21, 2024]
- Breakdown by view type
- HTML page views: 1377
- PDF downloads: 313