Research Article Open Access
Ivabradine Significantly Decreases hsCRP and Increases the Activity of Glutathione Peroxidase in Patients with Stable Coronary Artery Disease
Jedlicková L1*, Merkovská L1, Jacková L1, Janicko M1, Fedacko J1, Chmelárová A2, and Pella D11Department of Internal Medicine, Faculty of Medicine, Pavol Jozef Šafárik University and Louis Pasteur University Hospital in Košice, Slovak Republic
2Department of Experimental Medicine, Faculty of Medicine, Pavol Jozef Šafárik University in Košice, Slovak Republic
- *Corresponding Author:
- Jedlicková L
Department of Internal Medicine, Faculty of Medicine
Pavol Jozef Šafárik University and Louis Pasteur University Hospital in Košice
Slovak Republic, Europe
Tel: 00421915961304
E-mail: lucia.jedlickova@gmail.com
Received date: December 05, 2014; Accepted date: December 14, 2014; Published date: December 17, 2014
Citation: Jedlicková L, Merkovská L, Jacková L, Janicko M, Fedacko J, et al. (2014) Ivabradine Significantly Decreases hsCRP and Increases the Activity of Glutathione Peroxidase in Patients with Stable Coronary Artery Disease. Interdiscip J Microinflammation 1:125. doi: 10.4172/2381-8727.1000125
Copyright: © 2014 Jedlicková L, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at International Journal of Inflammation, Cancer and Integrative Therapy
Abstract
Introduction: Ivabradine treatment is considered to be a new approach in the coronary artery disease treatment based on the principle of the heart rate lowering. The aim of this project was to determine whether the heart rate decrease achieved by the ivabradine is accompanied by the improvement of antioxidant activity and reduction of the vascular inflammation, which can lead to the enhancement of the endothelial function.
Patients and methods: There were 30 patients with coronary artery disease in this study. They received treatment with the daily 2x5 mg dose of ivabradine in addition to their long- term treatment. Plasmatic level of hsCRP and activity of glutathione peroxidase was assessed at the beginning of the study and after three months (± one week) of the treatment.
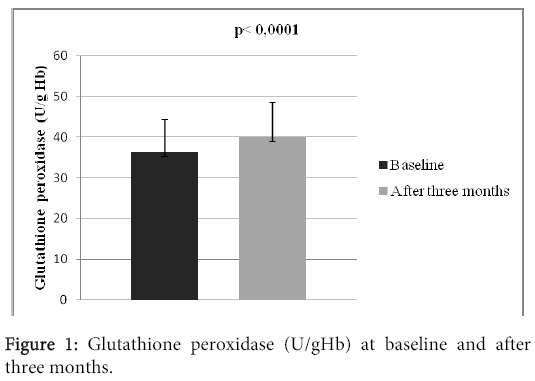
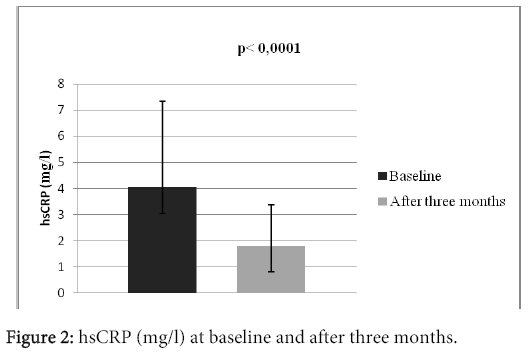
Results: There were 25 (83.3%) men and 5 (16.7%) women in the study. Average age of the patients was 65.4 ± 6.7 years. 28 (93.3%) patients survived myocardial infarction (STEMI or NSTEMI), 23 (76.6%) experienced revascularization (PCI or CABG), 16 (53.3%) patients suffered from diabetes mellitus of the 2. type, 29 (96.6%) patients experienced arterial hypertension. The mean heart rate at the moment of patient’s admission was 77 ± 7 beats in a minute. There was observed a significant increase in the activity of glutathione peroxidase, which increased from 36.31 ± 8.02 U/gHb to 39.96 ± 8.56 U/gHb (p?0.0001). We also observed statistically significant decrease of the hsCRP level, from 4.05 ± 3.28 mg/l to 1.82 ± 1.54 mg/l, p?0.0001.
Conclusion: Not only is the addition of ivabradine to the treatment of patients with coronary artery disease lowering the heart rate, it also positively affects the antioxidant activity and reduces vascular component of inflammation. However, it is necessary to conduct broader clinical, randomized, double-blind, placebo controlled trials to support the findings.
Keywords
Ivabradine; Coronary artery disease; hsCRP; Glutathione peroxidase
Introduction
Stable angina pectoris is the most frequent symptom or form of the chronic coronary artery disease. Current pharmacotherapy of the stable angina pectoris is limited. Developing the ivabradine, selective and specific inhibitor of the If current in the sinus node [1], has provided us with the new possibilities in its management. Its antianginal properties were tested in many randomized, placebo-controlled studies [2-4]. Nowadays, it is indicated as a second-line therapy for the patients, for whom the beta blocker therapy is contraindicated, unacceptable due to intolerance or it is not enough to reach adequate heart rate [5]. The aim of this work was to determine whether the decrease of the heart rate by ivabradine is accompanied by the improvement of antioxidant activity and reduction of the vascular inflammation, which could possibly lead to the improvement of the endothelial function.
Patients
Patients for our project were selected from January 2012 to May 2014, based on the inclusion and exclusion criteria. They were either ambulatory patients of the 1st Department of Internal Medicine at the Faculty of Medicine at Pavol Jozef Šafárik University and Louis Pasteur University Hospital in Košice, or patients hospitalized in the 1st Department of Internal Medicine (former 3rd Department of Internal Medicine) at the Faculty of Medicine at Pavol Jozef Šafárik University and Louis Pasteur University Hospital in Košice. Patients who fulfilled the inclusion criteria were accepted into our research after they were informed about the process of clinical observation and provided us with signature to acknowledge their participation. The local ethics committee also approved the research in advance.
Inclusion criteria:
•Patient with documented coronary artery disease
•Ongoing angina pectoris symptoms despite the treatment according to current valid recommendations
•Sinus rhythm
•Resting heart rate above 70 beats per minute measured by standard 12- lead ECG
•Patient previously not treated with ivabradine.
Exclusion criteria:
•Patient indicated to undergo invasive examination/intervention
•Patient whose previous treatment has to be modified
•Patient with contraindication with ivabradine treatment: severe hepatic insufficiency, sinusnode dysfunction, sinoatrial block, third-degree atrioventricular block, pacemaker dependency, supraventricular tachydysrhythmias, gravidity, lactation
Design of the study and methods
Once the patients signed and agreed with the research, they were included into a prospective non-randomized clinical study, which consisted of two visits. The first visit at the beginning of the study contained: anamnesis, physical examination including the blood pressure and heart rate measurement, anthropometric data – height and weight, BMI (body mass index) calculation based on the pattern: BMI=weight (kg)/height (m)2, standard 12 lead ECG and blood samples were obtained. All patients received treatment with 2x5 mg daily dose of ivabradine in addition to their treatment. Patients had to follow the recommended therapy during the whole period of our study and attend the planned visits and examination. After three months (± 1 week), the second visit took place, during which we performed the same examination and measurements.
Laboratory methods
Blood samples were obtained in every patient in the morning (between 7:00–9:00AM) after 12 hours of fasting by antecubital venipuncture. Blood samples were assessed immediately after the procedure in the local laboratory (despite assessment of the level of hsCRP, activity of glutathione peroxidase). Blood samples for the determination of glutathione peroxidase activity were collected into tubes containing heparin and stored in the freeze (-20°C) until their assessment and evaluation. Samples for the assessment of the hsCRP level were centrifuged (3000 rpm) and blood serum was frozen until the time of the assessment. hsCRP level and activity of glutathion peroxidase were assessed by the Daytona analyser (Randox Laboratoires, Great Britain) by the spectrophotometric turbidimetric method. Activity of the enzyme was measured indirectly and the results were recalculated to U/gHb with the programme based on Microsoft Excel.
Statistical analysis
Data are expressed as quantity, mean values ± standard deviation and in percentage. Student’s paired t-test comparing the parameter values at the beginning and at the end of the treatment was used to determine the effects of the treatment. Wilcoxon’s test was used for non-parametrical variables. The level of statistical significance during the hypothesis testing was 0.05.
Results
There were 30 patients in the study, who were accepted based on the inclusion criteria. All of them participated in the clinical trial until the end. There were 25 (83.3%) men and 5 (16.7%) women in the study. Mean age of the patients was 65.4 ± 6.7 years; with the age diversity from 54 to 78 years. The mean BMI was 29.8 ± 3.9. 28 (93.3%) patients survived myocardial infarction (STEMI or NSTEMI), 23 (76.6%) experienced revascularization (PCI or CABG), 16 (53.3%) patients suffered from diabetes mellitus type 2. 29 (96.6%) patients experienced arterial hypertension, 5 (16.6%) patients had previous stroke, 2 (6.6%) had peripheral artery disease and 2% (6.6%) patients were current smokers. The mean left ventricular ejection fraction was 44.7 ± 8.7%, systolic blood pressure 127 ± 13 mmHg, diastolic blood pressure 78 ± 10 mmHg. The mean heart rate at the beginning of the study was 77 ± 7 beats per minute (Table 1). At the beginning of the study, the patients were long- term users of drug treatment depicted in the table 2. There were no statistically significant differences in the basic biochemistry parametres (Table 3). After three months long treatment, we observed statistically significant increase in the activity of tested antioxidant enzyme glutathione peroxidase, which increased from 36.31 ± 8.02 U/gHb to 39.96 ± 8,56 U/gHb (p<0.0001) (Table 3, Figure 1). We also observed statistically significant decrease of the hsCRP level, from 4.05 ± 3.28 mg/l to 1.82 ± 1.54 mg/l, p<0.0001 (Table 3 and Figure 2).
| Number of the patients | 30 |
|---|---|
| Sex: male/female | 25 (83.3%) / 5 (16.7%) |
| Age (years) | 65.4 ± 6.7 |
| Age diversity (years) | 54 – 78 |
| BMI (kg/ m2) | 29.8 ± 3.9 |
| Arterial hypertension | 29 (96.6%) |
| Diabetes mellitus type 2 | 16 (53.3%) |
| Previous stroke | 5 (16.6%) |
| Peripheral artery disease | 2 (6.6%) |
| Current smoker | 2 (6.6%) |
| NSTEMI/STEMI | 28 (93.3%) |
| Previous coronary revascularization | 23 (76.6%) |
| Left ventricular ejection fraction (%) | 44.7 ± 8.7 |
| Systolic blood pressure (mmHg) | 127 ± 13 |
| Diastolic blood pressure (mmHg) | 78 ± 10 |
| Heart rate (beats per minute) | 77 ± 7 |
Table 1: Characteristics of the patients at baseline.
| Number of patients | |
|---|---|
| Clopidogrel | 13 (43.3%) |
| Aspirin | 27 (93.3%) |
| Statin | 29 (96.6%) |
| Beta-blocker | 30 (100%) |
| ACE inhibitor | 24 (80%) |
| Angiotensin II-receptor blocker | 4 (13.3%) |
| Dihydropyridine calcium- channel blocker | 12 (40%) |
| Verapamil or diltiazem | 0 |
| Nitrate | 7 (23.3%) |
| Trimetazidine | 17 (56.6%) |
| Antidiabetic agent | 12 (40%) |
Table 2: Concomitant treatment.
| Baseline | After threemonths | P | |
|---|---|---|---|
| hsCRP(mg/l) | 4.05 ± 3.28 | 1.82 ± 1.54 | ˂0.0001 |
| Glutathion peroxidase(U/gHb) | 36.31 ± 8.02 | 39.96 ± 8.56 | ˂0.0001 |
| Creatinine(μmol/l) | 98.4 ± 11.4 | 101.1 ± 13.5 | NS |
| Total cholesterol(mmol/l) | 3.91 ± 1.20 | 3.77 ± 1.24 | NS |
| HDLcholesterol(mmol/l) | 1.17 ± 0.37 | 1.16 ± 0.30 | NS |
| LDL cholesterol(mmol/l) | 2.02 ± 1.02 | 1.85 ± 097 | NS |
| Triglycerides(mmol/l) | 1.57 ± 0.75 | 1.62 ± 0.57 | NS |
| Glycemia (mmol/l) | 7.4 ± 2.5 | 7.4 ± 2.8 | NS |
Table 3: Laboratory results at baseline and after three months.
Limitations
Low number of patients as well as the fact that the study was not randomized or placebo controlled are considered to be the greatest limitations. As a result, we have to take these limitations into consideration when assessing the results, although they are statistically significant.
Discussion
In the study of Drouin et al. [6], ivabradine prevented deterioration of the endothelial dilator function of renal and cerebral arteries associated with dyslipidemia in mice expressing human ApoB-100. Custodis et al. [7] observed in dyslipidemic mice that cholinergic, endothelial dependent vasodilatation was renewed by ivabradine and the activity of arterial NADPH oxidase, free radicals production and the production of aterosclerotical lesions were reduced. Schirmer et al. [8] demonstrated, that ivabradine enhanced arteriogenesis and increased endothelial nitric oxide synthase (eNOS) expression, NO availability and endothelial-dependent relaxation in a murine hindlimb model of endothelial dysfunction.
Elevation of the hsCRP level is a marker of the subclinical inflammation, as vascular inflammation [9-11]. Anti- inflammatory effect of ivabradine was studied by Dominguez- Rodriguez, et al. [12] in the pilot prospective, randomized, double- blind and placebo- controlled study in patients with acute coronary syndrome. Higher hsCRP levels are associated with adverse outcomes and subsequent vascular events in these patients [13]. The use of ivabradine from the first 24 hours after symptoms onset and for 30 days reduced not only baseline heart rate, but also the inflammatory response assessed by hsCRP. hsCRP levels assessed at 30 days of follow-up were significantly reduced with the administration of ivabradine compared to placebo [12]. There data suggest that ivabradine can reduce the vascular component of inflammation in line with observations of previous studies in animal models [14-16].
Generally, as far as atherosclerosis and vascular diseases are concerned, role of the If currents and potential effects of ivabradine remains unclear. Studies dealing with the effect of ivabradine to the endothelial function which have been published so far, are controversial. One group of authors [17,18] claims, that ivabradine has more functions, which are not yet described and identified, besides the reduction of the heart rate. On the other hand, other authors [19,20] believe that there are no such ‘pleiotropic’ effects of ivabradine.
Results of our study point out that ivabradine added to standard treatment in patients suffering from stable angina pectoris may have a positive effect on the endothelial function in addition to the heart rate reduction. However, it is necessary to conduct broader clinical, randomized, double blind, placebo controlled trials to support the findings.
Conclusion
There was observed positive effect of ivabradine treatment on the activity of glutathione peroxidase, which is the antioxidant enzyme. Results of our study show, that ivabradine treatment in patients with coronary artery disease is connected not only with the heart rate reduction, but may also lead to the improvement of antioxidant activity and reduction of the vascular inflammation, which can lead to the enhancement of the endothelial function in such patients. Considering design of our study and size of observed population, it is necessary to conduct further clinical research in this area, which may most probably lead us to interesting findings.
References
- Thollon C, Cambarrat C, Vian J(1994) Electrophysiological effects of S 16257, a novel sino-atrial node modulator, on rabbit and guinea-pig cardiac preparations: comparison with UL-FS 49. Br J Pharmacol 112: 37-42.
- Tardif JC, Ford I, Tendera M (2005) Efficacy of ivabradine, a new selective If inhibitor, comnpared with atenolol in patients with chronic stable angina. European Heart Journal 26: 2529-2536.
- Tardif JC, Ponikowski P, KahanT(2009) Efficacy of the If current inhibitor ivabradine in patients with chronic stable angina receiving beta-blocker therapy: a 4-month, randomized, placebo-controlled trial. European Heart Journal 30: 540-548.
- Fox K, Ford I, Steg PG (2008) (Ivabradine for patients with stable coronary artery disease and left-ventricular systolic dysfunction (BEAUTIFUL): a randomised, double-blind, placebo-controlled trial. Lancet 372: 807-816.
- Montalescot G, SechtemU (2013)Achenbach S, et al. ESC Guidelines on the management of stable coronary artery disease. The Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J 34: 2949-3003.
- Drouin A, Gendron ME, Thorin E (2008) Chronicheart rate reduction by ivabradine prevents endothelial dysfunction in dyslipidaemic mice. Br J Pharmacol154:749-757.
- Custodis F, Baumhäkel M, Schlimmer N (2008) Heart rate reduction by ivabradine reduces oxidative stress, improves endothelial function, and prevents atherosclerosis in apolipoprotein E-deficient mice. Circulation 117: 2377-2387.
- Schirmer SH, Baumhakel M, Custodis F (2012) Heart- rate reduction by If-channel inhibition with ivabradine restores collateral artery growth in hypercholesterolemic atherosclerosis. Eur Heart J 33:1223-1231.
- Aljaroudi W, Alraies MC, Halley C (2012)Impact of progression of diastolic dysfunction on mortality in patients with normal ejection fraction. Circulation 125: 782-788.
- Senni M, Tribouilloy CM, Rodeheffer RJ (1998) Congestive heart failure in the community: a study of all incident cases in Olmsted County, Minnesota, in 1991. Circulation 98: 2282-2289.
- AngejaBG, Grossman W (2013) Evaluation and management of diastolic heart failure.Circulation 107: 659-663.
- Dominguez-Rodriguez A, Consuegra-Sanchez L, Blanco-Palacios G (2012) Anti- inflammatory effects of ivabradine in patients with acute coronary syndrome: a pilot study. Int J Cardiol158: 160-162.
- MottramPM, MarwickTH (2005) Assessment of diastolic function: what the general cardiologist needs to know. Heart 91: 681-695.
- Okura H, Takada Y, Kubo T (2006) Tissue Doppler-derived index of left ventricular filling pressure, E/E´, predicts survival of patients with non-valvular atrial fibrillation. Heart 92: 1248-1252.
- Dedkov EI, Zheng W, Christensen LP (2007) Preservation of coronary reserve by ivabradine-induced reduction in heart rate in infarcted rats is associated with decrease in perivascular colagen. American Journal of Physiology- Heart and Circulatory Physiology 293: H590-H598.
- Milliez P, Messaoudi S, Nehme J (2009) Beneficial effects of delayedivabradine treatment on cardiac anatomical and electrical remodeling in rat severe chronic heart failure. American Journal of Physiology- Heart and Circulatory Physiology 296: H435-H441.
- Heusch G (2008) Pleiotropic action(s) of the bradycardic agent ivabradine: cardiovascular protection beyond heart rate reduction. British journal of Pharmacology 155:970-971.
- Heusch G, Skyschally A, Schulz R (2011) Cardioprotection by IvabradineThrough HeartRate Reduction and Beyond. J CardiovascPharmacolTher 16: 281-284.
- Canet E, Lerebours G, Vilaine JP (2011) Innovation in coronary artery disease and heart failure: clinical benefits of pure heart rate reduction with ivabradine. Ann N Y AcadSci 1222: 90-99.
- Ferrari R, Campo G, Gardini E (2005) Specific and selective If inhibition: expected clinical benefits from pure heart rate reduction in coronary patients. Eur Heart J Suppl7: H16-H21.
Relevant Topics
Recommended Journals
- Journal of Lung Cancer Diagnosis & Treatment
- Advances in Cancer Prevention
- Breast Cancer: Current Research
- Cancer Surgery
- Immunology: Current Research
- Current Trend in Gynecologic Oncology
- Journal of Cancer Diagnosis
- Journal of Gastrointestinal Cancer and Stromal Tumors
- Cervical Cancer: Open Access
- Journal of Mucosal Immunology Research
- Journal of Oncology Research and Treatment
- Journal of Orthopedic Oncology
- Journal of Prostate Cancer
- Research and Reviews on Pathogens
Article Tools
Article Usage
- Total views: 14689
- [From(publication date):
December-2014 - Nov 21, 2024] - Breakdown by view type
- HTML page views : 10285
- PDF downloads : 4404