Research Article Open Access
Isolation of Vibrio cholerae in Homogenized Tissues of Liver, Gall Bladder and Bile in Rabbit Model
Atif AB1*, Uday Y1, Aamenah M2, Nordin S1, Khattak MN3, Adzim MK1, Shamim AK1 and Hasnan J41Faculty of Medicine and Health Sciences, Universiti Sultan ZainalAbidin, Malaysia
3Communicable Disease Control, Alberta Health Services, Canada
4Department of Pathology, School of Medical Sciences, University Sains, Malaysia
- *Corresponding Author:
- Dr. Atif Amin Baig
Faculty of Medicine and Health Sciences
Universiti Sultan Zainal Abidin, Malaysia
Tel: +6096275587
E-mail: atifamin@unisza.edu.my
Received date: June 12, 2014; Accepted date: June 17, 2014; Published date: June 19, 2014
Citation: Atif AB, Uday Y, Aamenah M, Nordin S, Khattak MN, et al. (2014) Isolation of Vibrio cholerae in Homogenized Tissues of Liver, Gall Bladder and Bile in Rabbit Model. Microinflammation 1:103. doi: 10.4172/2381-8727.1000103
Copyright: © 2014 Atif AB, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at International Journal of Inflammation, Cancer and Integrative Therapy
Abstract
Vibrio cholerae O139 is well known as the causative agent of cholera. It is noninvasive but many reports suggested it to cause bacteremia which brings in a little controversy with the previously reported old literature especially after reported histopathological invasion pattern of Vibrio cholerae O139 and Vibrio cholerae O1 ElTor. The rabbits were processes for ileac loops inoculation of Vibrio cholerae O139 followed by biochemical and molecular analysis of the presence of Vibrio cholerae O139 in liver, gall bladder and bile. We concluded the presence of Vibrio cholerae in liver, bile and gall bladder homogenized tissue. Retrograde spreading of bacteria to the liver via the common bile duct was excluded by complete closure of the intestinal lumen distant to the duct in the intestinal lumen. However, the bacteria might traffic, probably via macrophages, to distant area in the body including the liver. Another study in future is a need to identify the lesions of Vibrio cholerae invasions within liver and gall bladder by immunohistochemistry and using GFP visibility for Vibrio cholerae passage.
Introduction
Vibrio cholerae currently includes more than 180 serogroups [1]. Vibrio cholerae of serogroup O1 is well known as the causative agent of cholera. It has become increasingly apparent that in recent years Vibrio cholerae of other O groups (non O1 Vibrio cholerae) can also cause human disease. Non O1 Vibrio cholerae has been isolated from patients with diarrhea throughout the world [2]. Recently, Vibrio choleraee O139 Bengal has emerged as the second etiological agent of cholera. It has caused epidemic in the Indian subcontinent and spread to several neighboring countries of the region which included many developed countries. It is now believed that this organism is the causative agent of the eighth cholera pandemic [3].
There are many physiological, biochemical, phenotypic and genotypic similarities between Vibrio choleraee O139 and O1 biotype ElTor. The most notable difference is the possession of a capsule by Vibrio choleraee O139 like other non O1 Vibrios, which is absent in O1 Vibrio cholerae [4]. This possession of capsule by non-O1 Vibrios confers extra virulence characteristics such as resistance to serum killing and ability to invade the bloodstream especially in patients with debilitating conditions or immunosuppression [5]. Laboratory diagnosis reflected that Vibrio choleraee O139 cause bacteremia in mice upon intradermal inoculation, whereas this ability was absent in O1 Vibrios [4]. Recent finding by Amin and colleagues [6] showed clearly the invasion pattern of Vibrio cholerae in rabbit ilium. The first case of the septicemia due to Vibrio choleraee O139 was reported in an adult patient in South India with chronic liver disease [7]. All these observations suggested that Vibrio cholerae O139 has the potential to cause bacteremia might be by invading through the intestinal distortion. We have reported the isolation of Vibrio choleraee O139 from liver, gall bladder and bile in infected adult rabbit models with bacteremia, based on hypothesis; that, the Vibrio cholerae, can invade via hepatic portal system but because of very low CFU, it has not been reported to cause bacteremia. Since, Vibrio cholerae has been reported to be a non-invasive organism, once the exact pathogenesis of Vibrio cholerae is understood with special focus on its invading capability, it will bring a new horizons to the molecular pathogenesis of Vibrio cholerae beside the association of Vibrio cholerae to elucidate the MALT and GALT responses towards development of cholera vaccines which is all focused till date towards non-invasive nature of Vibrio cholerae and Vibrio cholerae toxins.
Materials and Methods
Bacterial strain and culture conditions
Wild type strain of Vibrio cholerae O139 was used in this study. The strain was maintained in the lyophilized state and fresh ampoules were used for this study. Bacteria were grown statically and aerobically in LB broth overnight at 37°C. For intestinal invasion bacteria were harvested by centrifugation and resuspended at a concentration of 108 bacteria/ml of normal saline for injection into the ileal loops by further dilution into 107and 106CFU/ml of Vibrio cholerae.
Rabbit ileal loop procedure
The Rabbit ileal loop assays were performed as previously described [8] with minor modifications. A total of four rabbits were used for each of the three groups, (group 1 with 1x108 CFU inoculated, group 1 with 1x107 CFU inoculated, group 1 with 1 x 106 CFU inoculated) with a total of four negative non inoculated controls. The abdomens were cleaned and a midline incision was made along line alba of the abdomen and the small intestine was brought out for each rabbit. The small intestine was ligated 10 cm from the ileocecal junction and was then divided into 5 loops, by ligature using 3-O cat gut, of 5 cm each separated by 1 cm segments. Care was taken that no major blood vessel was ligated. The loops were injected with 1x102-1x107 V. cholerae in 0.5 ml LB medium with a 27G 1¼” needle. The small intestine was returned to the bowel and it was closed by catgut and silk sutures. Sterile dressing was applied on the wound and animals were returned to their cages. Limited water (but no food) was given to the animal. After 18 hours, animals were euthanized as described above. An autopsy was performed and ligated loops were recovered. Total fluid that was accumulated in each loop and the length of the loop was recorded.
Fluid accumulation ratio
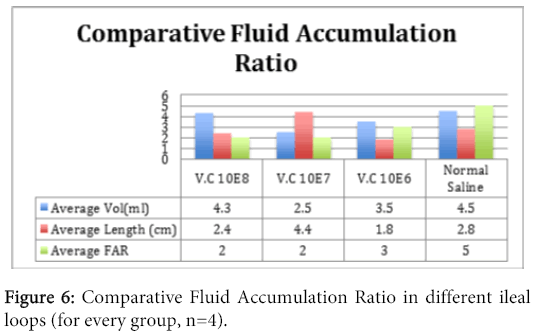
The fluid accumulation ratio (FAR=Volume of fluid/length of loop) was determined by measuring the fluid (ml) in the loops and dividing by the length (cm) of the loop. For this purpose; for each loop, the average lengths of loops were measured and its contents were emptied by gravity into a graduated cylinder to determine volume. The results were expressed as volume/length ratio to correct for variability in average loop length compared to the FAR of inoculated ileum loop with normal saline.
Isolation of Vibrio cholerae from liver
After recovering the ileums of infected rabbits, the livers of the rabbits were located and were removed from the bodies and were placed in normal saline for 5 minutes. Sections of liver were cut from all rabbits and were homogenized in LB broth; 200 µl of which was platted onto TCBS (Thiosulphate Citrate Bile Salt) agar and the plates were incubated for 16 hours at 37°C. Next day the yellowish-green Vibrio cholerae colonies were counted. Another section of liver was inoculated in APW (alkaline peptone water), an enrichment medium for Vibrio cholerae and was incubated at 37°C for 8-10 hours. A total of 200 µl of this culture was platted on TCBS agar. Next day the number of yellowish-green colonies was counted. A few of the colonies were picked and were checked for Gram staining, oxidase production and immobilization test. The colonies which gave a negative Gram reaction, positive oxidase reaction and a positive immobilization test were selected to be confirmed as Vibrio cholerae by PCR (polymerase chain reaction).
Isolation of Vibrio cholerae from gall bladder and bile
The gall bladders of the rabbits were located and were removed from the liver surface carefully. The bile was aspirated from the gall bladder and inoculated into APW. A whole gall bladder was inoculated in APW and was incubated at 37°C for 8-10 hours. Exactly 200 µl of this culture was platted on TCBS agar. Next day the number of yellowish-green colonies was enumerated. A few of the colonies were picked and were checked for gram staining, oxidase production and immobilization test. The colonies which gave a negative Gram reaction, positive oxidase reaction and a positive immobilization test were selected for confirmation to be identified as Vibrio cholerae by PCR.
Molecular confirmation of Vibrio cholerae
The PCR was carried out by the method described previously [5] For the PCR confirmation of Vibrio cholerae, two reported primers (VHMF: 5’ TGG GAG CAG CGT CCA TTG TG 3 and VHA-AS5: 5’ CAA TCA CAC CAA GTC ACT C 3’) specific for Vibrio cholerae were used. The PCR conformation was done using 1.2% agarose gel electrophoresis in TBE buffer.
Tissue processing and staining
Tissue section from infected intestine and liver were fixed in formaldehyde and processed [9] followed by micro to my and staining with hematoxylin-eosin (H and E). Few slides were counter stained with Gram staining by the methods described previously [9].
Results
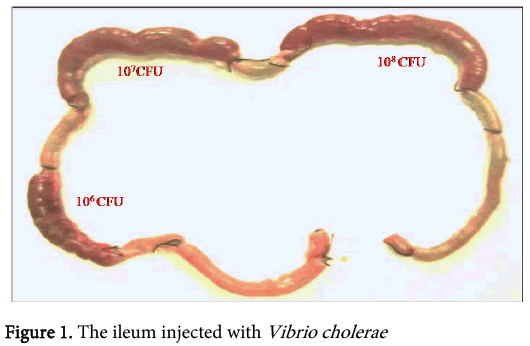
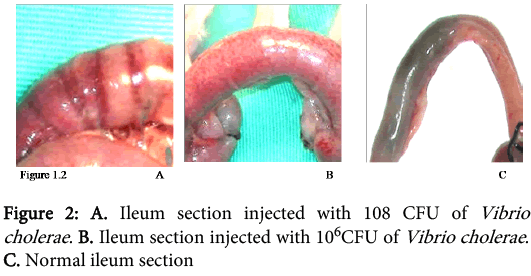
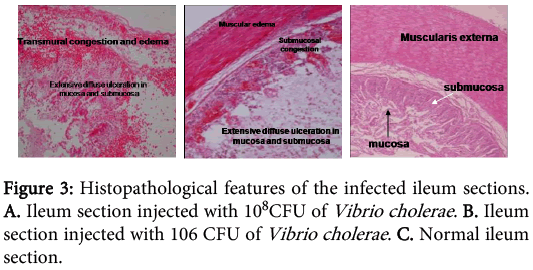
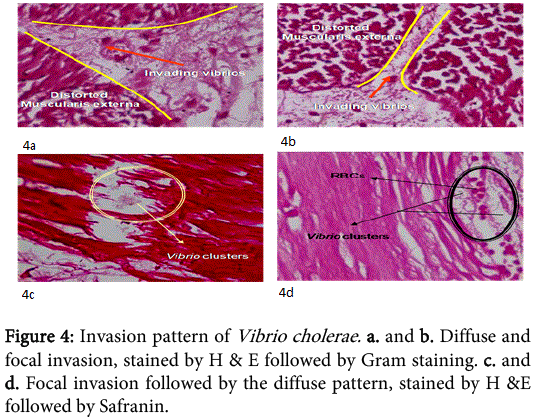
The concentrations of Vibrio cholerae inoculated into different loops of ileum were 1x106, 1x107 and 1x108.The gross pathological examination revealed serosal congestion and hemorrhage which increased with increasing concentration of inoculated Vibrio cholerae (Figure 1 and 2). The fluid accumulated in each loop was also proportional to the concentration of inoculated Vibrios and was reported in the form of FAR. The result reflects that the fluid accumulated was directly proportional to the concentration of bacteria inoculated. The histopathological sections (Figure 3) of infected ileum stained with H & E showed that the blood vessels were congested, and inflammatory cells were present in the mucosa and sub mucosa. High-powered photomicrograph showed extensive haemorrhage extending till Muscular is propria. Transmural edema and prominent congestion were seen. Muscular is propria was distorted. Later, sections from the same biopsies were stained with H and E followed by Gram staining (Figure 4). Interestingly, it was noted that in those sections, the Vibrio cholerae were found cleaving propria and creating a passage for their invasion. The area in the muscular is propria where they faced some resistance, bacteria started colonizing and then because of accumulation of lot of toxins and distortion of the adjacent fibres they started their cleavage-invasion passage pattern once again, which causes the whole ileum dysfunction.
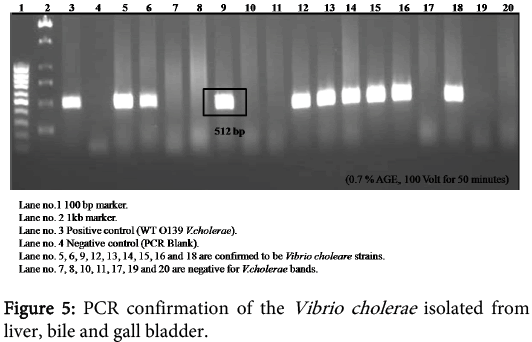
Liver biopsy which was homogenized and plated on TCBS agar were incubated at 37°C for 16 hours to count the number of Vibrio cholerae. It showed that liver had an average of 130 CFU. At the same time, the tissues from biopsies of liver and gall bladder were inoculated in different bottles of APW media for 8-10 hours followed by platting on TCBS agar. The yellowish-green colonies were picked and platted on LB broth with polymyxin. These colonies were checked for Gram staining, oxidase production and immobility testing. The Vibrio cholerae is Gram negative, oxidase positive and positive for immobility testing. The colonies which were Gram negative, oxidase positive and positive for immobility testing were confirmed at molecular level by PCR to be Vibrio cholerae (Figure 5). The bile which was aspirated from gall bladder was also inoculated in APW medium followed by platting on TCBS agar, which was followed by Gram staining, oxidase production and immobility testing. The colonies which were Gram negative, oxidase positive and positive for immobility testing were confirmed as Vibrio cholerae by PCR.
Discussion
Ingested vibrios from contaminated water or food must pass through the acid stomach before they are able to colonize the upper small intestine. Colonization is aided by way of fimbria, filamentous protein structures called toxin co regulated pilus (TCP) extended from the cell wall, that attach to the receptors on the mucosa and by the bacterium’s motility, which is helpful to penetrate the mucosa [10]. Formerly cholera was thought to cause sloughing of the intestinal mucosa by an inflammatory process. However, the intestinal mucosa was known to remain intact and without inflammatory changes [11]. The previous findings of mucosal sloughing were shown to be artifacts, based on autolytic postmortem changes. Koch first postulated in 1884 that the bacteria produced a toxin and this stimulated the massive outpouring of fluid from the intestine. De and Dutta were the first to demonstrate this toxin (now called cholera toxin) by use of culture filtrates in rabbits [12,13]. The toxin was later purified and sequenced [14] to have a molecular mass of 84000 kDa and consists of five binding (B) subunits and one active (A) subunit [15].
As we now understand the mechanism of action, the B subunits are physiologically inactive but bind the holotoxin to the GM1 ganglioside receptors in the small intestinal mucosa, and the A subunit is transported into the cell where it activates adenylate cyclase [16]. This activation leads to an increase in cyclic AMP, followed by an increase in chloride secretion in the crypt cells, and inhibition of neutral sodium chloride absorption in the villus cells, which in turn cause massive outpouring of fluid into the small intestine [17]. The volume secreted exceeds the normal absorptive capacity of the bowel and results in watery diarrhea. Most of the secretions come from the small intestine, although the toxin also inhibits water absorption by the colon [18]. The diarrheal fluid contains large amounts of sodium, chloride, bicarbonate, and potassium, but little protein or blood cells [19]. The loss of electrolyte-rich isotonic fluid leads to blood volume depletion with low blood pressure and shock and then loss of bicarbonate and potassium leads to metabolic acidosis and potassium deficiency (hypokalemia). The stools of cholera patientscontain high concentrations of cholera vibrios (up to 108 bacteria/g), and they are highly infectious. When passed into the environment, they can contaminate water sources, food and may seed an environmental reservoir [19].
Vibrio are said to be noninvasive until Calia et al. reported bacteremia by Vibrio parahaemolyticus suckling mice models [20]. It was followed by a report of bacteremia in Gulf Coast community with and without underlying infections. The patients with underlying infections were reported to have soft tissue infections which often progressed to fatal septicemias [21]. The same year a case of bacteremia in an infant was reported in which a 6 day old black male was reported who presented with diarrhea and biochemical evidence of severe electrolyte imbalance. Despite treatment with intravenous fluid and antibiotics, he died within 24 hours of admission. Enterotoxigenic Vibrio cholerae El Tor, serotype Inaba was isolated from blood [22]. In 1986, McClesky et al. reported a case of bacteremia by isolating non O1 Vibrio cholerae from a patient with cirrhosis after placement of LeVeen shunt [23]. Safrin and colleagues reported a case of non O1 Vibrio cholerae and prostatic abscess in a patient with idiopathic aplastic anemia and the data were compared with 23 previously reported cases of non O1 Vibrio cholerae bacteremia. The case fatality rate was reported to be 61.5% for 13 cases with majority of cases reported in immune compromised patients particularly those with hematologic malignancy or cirrhosis. Another study also reported the importance of host susceptibility to be potentially important. The Vibrio cholerae were identified in sheep blood agar as in previous findings [5]. An interesting case report came from Dhar et al in 1989 which reported the presence of non O1 Vibrio cholerae in 50 year old woman and 31 year old man with underlying liver disease presented with fever and signs of liver failure but the source of infection could not be identified in both cases with the only difference that one of the strains after being identified biochemically was non motile [24,25]. It was followed by reports from various authors describing the cases of non O1 Vibrio cholerae bacteremia in various parts of world [26-28] many of them using biochemical and microbial cultures for identifying Vibrio cholerae. Starting from January 1983 to march 1984, 26 isolates of Vibrio species were recovered from blood of patients admitted to a hospital in India in Siriraj hospital. Only 13 strains were identified as non invasiveO1 Vibrio cholerae, 3 were Vibrio vulnificus and 10 were Vibrio species. Most of the patients were adult men with cirrhosis and history of seawater exposure [29]. A similar case associated with prior gastrectomy was reported with the hypothesis that all the cases yet reported were immune deficient and this was the first case reported in healthy patient and susceptibility to such infection may have been enhanced by a prior gastrectomy for duodenal ulcer [30]. Jesudason and colleagues reported Vibrio cholerae to be the cause of gastroenteritis and extra intestinal manifestations including septicemia [31] followed by a similar report [32]. A report was published presenting a case of non O1 Vibrio cholerae septicemia with myelodysplatic syndrome in Taiwan [33].
In 1992, Jamil and colleagues reported a case of O1 Vibrio cholerae septicemia [34]. In the same year, Russel and colleagues made use of new techniques to confirm the invasion of Vibrio cholerae in rabbit ileum using different models and they found the intestinal bacterial colonization, intestinal fluid volume and onset of diarrhea caused by non O1 Vibrio cholerae. They also reported that the intestinal damage of the bacterial strains were dose dependent with necrosis of the intestinal lamina propria in RITARD rabbits [35]. A case of Vibrio cholerae septicemia in 45 year old male with a three year history of liver cirrhosis was reported and the septicemia was associated with severe underlying diseases such as leukemia and liver cirrhosis [36]. Bloodstream invasion of O139 Vibrio cholerae was reported in 1993 [7] with a similar report of non O1 meningitis in infant, in Israel and a patients with multiple myeloma respectively [37-39]. Another case of Vibrio cholerae invasion was reported in a 44 year old alcoholic man with a fever and bullous cellulites of the lower extremities with no liver cirrhosis [40]. Levine and colleagues also reported cases of septicemia followed by Vibrio infection and invasion in Gulf Coast [41]. Another case of Vibrio cholerae bacteremia was reported in patient with multiple myeloma [42]. In 1994, cases of bacteremia with non O1 Vibrio cholerae were reported associated with gastroenteritis, meningitis, liver cirrhosis and immnocompromised patients [43-46]. Boyce and colleagues reported a Biomedical Journals-14-763(R-103) Scase of bacteremia by Vibrio cholerae O139 after O139 out-break [47] with the same reports from Khan and colleagues [48] and another case of Vibrio cholerae non O1 and non O139 associated with hemorrhagic bullous skin lesions of the lower extremities [49]. A case of primary septicemia with Vibrio cholerae non O1 was reported in a burn patient [23] with a short gap with a case of nephritic syndrome with non O1 Vibrio cholerae bacteremia and peritonitis [50]. Many other cases of septicemia due to non O1 and non O139 were also reported by various researchers in different underlying disorder [51-54]. A case of Vibrio cholerae non O1 sepsis in healthy patient was reported in Spain [20] followed by a report on the infection of non O1 Vibrio cholerae in Southern Taiwan with bacteremia with concurrent spontaneous bacterial peritonitis or invasive soft tissue infection occurring solely in cirrhotic patients, self-limited acute febrile gastroenteritis that often resulted from a wound on extremities [55]. The same year another case was reported in Mexico in which a 55 year old man developed acute cholera cystitis. West et al., described the first case of septicemic acute acalculous cholecystitis caused by non O1 Vibrio cholerae is described in a healthy traveler, and bilary tract infections from V. cholerae are reviewed. Immediately after a vacation in Cancun, Mexico, a 55 year old man developed acute cholecystitis. Blood and bile cultures grew non O1 V. cholerae. At surgery, the gallbladder was acalculous, inflamed, distended and nearly ruptured. Pathogenetic factors may have included diarrhea prophylaxis with bismuth subsalicylate, distension of the gallbladder from illness induced fasting, and bacterial toxins in the gall bladder. The patients received i.v cephapirin, followed by oral cephradine for a total of 10 days, and he made a quick and complete recovery. V. cholerae should be considered in the differential diagnosis of persons from endemic areas who present with cholecystitis or acute jaundice [56]. In 1999, Albert and colleagues reported that the capsulated bacteria exhibited serum resistance and resistance to phagocytosis which resulted in disseminated infections. Vibrio cholerae O139 strains possess a thin capsule and have been found to be partially serum resistant and are partially resistant to phagocytosis [3].
The no toxigenic Vibrio cholerae O1 was also reported to cause bacteremia [57,58]. There were many similar reports with bacteremia also with non O1 Vibrio cholerae in the same year [59-62]. Santamaria and colleagues reported a case of bacteremia with O1 Vibrio cholerae in neonate with hypovolemic shock [63]. Many other reports were published in year 2003 to 2006 stating the invasion of non O1 and O139 Vibrio cholerae and bacteremia due to invasion [28,64-68].
In 2007, a case of bacteremia due to O1 Vibrio cholerae was again reported in Brazil in 70 years old patient with sepsis [69]. In 2009, Amin and colleagues reported the difference in invasion pattern of O1 and O139 Vibrio cholerae in rabbit ileum which stated the level of invasion of Vibrio choleraee O139 was more thanO1 El Tor and the pattern of invasion was different [6,70-78 ]. In this paper we also found that the O139 invaded in a diffuse pattern along with the focal pattern (Figure 3). The FAR is related to the bacterial concentration (Figure 6). Gross serosal hemorrhage (Figure 1) was also found to be similar with previous reports [6,79-83]. The histopathological sections reflected trans mural congestion and excessive ulceration in mucosa and submucosa which also support the previous report [6,84-88]. The detection of Vibrio cholerae was confirmed with all possible microbiological and molecular techniques. For the detection of Vibrio cholerae in liver, gall bladder and bile the biochemical analysis was done. The molecular analysis was done to confirm the presence of Vibrio cholerae in liver, gall bladder and bile by the method of Ravichandran et al., 2007, using specific primers for PCR.
We concluded the presence of Vibrio cholerae in liver, bile and gall bladder homogenized tissue. Retrograde spreading of bacteria to the liver via the common bile duct was excluded by complete closure of the intestinal lumen distant to the duct in the intestinal lumen. However, the bacteria might traffic, probably via macrophages, to distant areas in the body including the liver. Another study in future is a need to identify the lesions of Vibrio cholerae invasions within liver and gall bladder by immunohistochemistry and using GFP visibility for Vibrio cholerae passage.
References
- Young CC, Chuang YC, Young CD (1991) Non-O:1 Vibrio choleraee bacteremia: report of two cases. Kansenshogaku Zasshi 65: 1479-1483.
- Aldova E, Laznickova K, Stepankova E, Lietava J (1968) Isolation of non-agglutinable Virbios from an enteritis outbreak in Czechoslovakia. J Infect Dis 118: 25-31.
- Albert MJ, Qadri F, Bhuiyan NA, Ahmad SM, Ansaruzzaman M, Weintraub A, et al. (1999) "Phagocytosis of Vibrio choleraee O139 Bengal by human polymorphonuclear leukocytes." Clin Diagn Lab Immunol 6: 276-278.
- Khan AM, Albert MJ, et al. (1995) Septicemia due to Vibrio choleraee 0139 Bengal. Diagn Microbiol Infect Dis 22: 337-338.
- Sakaguchi M, Itagaki N, Funauchi M, Hasegawa H, Irimajiri K, et al. (1992) A case of Vibrio choleraee non-O1 septicemia with liver cirrhosis. KansenshogakuZasshi 66: 653-656.
- Amin A, Ali A, Kurunathan S, Cheong TG, Al-Jashamy KA, Jaafar H, ZainuddinZF, Ravichandran M, Lalitha P, at al. (2009) Comparison of histopathological features of Vibrio choleraee O1 El Tor and O139 Bengal infections in rabbit intestinal mucosa. Histol Histopathol 24: 559-565.
- Jesudason MV, Cherian AM, John TJ (1991) "Non 01 Vibrio choleraee in intestinal and extra intestinal infections in Vellore, S. India." Indian J Pathol Microbiol 34: 26-29.
- Toeg A, Berger SA, Battat A, Hoffman M, Yust I (1990) Vibrio choleraee bacteremia associated with gastrectomy. J ClinMicrobiol 28: 603-604.
- Jesudason MV, Cherian AM, John TJ (1993) "Blood stream invasion by Vibrio choleraee O139." Lancet 342: 431.
- Taylor RK, Miller mVL, Furlong DB, Mekalanos (1987) Proc. Natl Acad. Sci. USA, 84, 2833–2837.
- Sprinz H, Sribhibhadh R, Gangarosa EJ, Benyajati C, Kundel D, Halstead S (1962). Biopsy of small bowel of Thai people with special reference to recovery from Asiatic cholera and to an intestinal malabsorption syndrome. Am J Clin Pathol. 38: 43–51.
- De SN. Enterotoxigenicity of bacteria free culture filtrate of Vibrio choleraee. (1959). Nature 183: 1533.
- Dutta NK, Panse MW, Kulkrni DR. (1959). Role of cholera toxin in experimental cholera. J Bacteriol 7: 594-95.
- Finkelstein RA, LoSpalluto JJ. (1969). Pathogenesis of experimental cholera: preparation and isolation of choleragen and choleragenoid. J Exp Med 130:185-202.
- Gill, D. M. & R. S. Rappaport. (1979). Origin of the enzymatically active AI fragment of cholera toxin. J. infect. Dis, 139: 674-680.
- Holmgren J, Lonnroth I, Svennerholm L (1973). Fixation and inactivation of cholera toxin by GM1 ganglioside. Scand J Infect Dis 5:77-78.
- Field M, Fromm D, Al Awqati Q, Greenough WB III (1972). Effect of cholera toxin onion transport across isolated ileal mucosa. J Clin Invest 51:796-804.
- Speelman P, Butler T, Kabir I, Ali A, Banwell J (1986). Colonic dysfunction during cholera infection. Gastroenterology 91: 1164-1170.
- Molla AM, Rahman M, Sarker SA, Sack DA, Molla (1981). AStool electrolyte content and purging rates in diarrhea caused by rotavirus, enterotoxigenic E. coli, and V cholerae in children. J Pediatr 98: 835–38
- Catalá Barceló MT, Núñez Sánchez JC, BalaguerMartínez JV, Borrás Salvador R (1998) Vibrio choleraee non 01 sepsis in a healthy patient: review of reported cases in Spain. Rev ClinEsp 198: 850-851.
- Boyce TG, Mintz ED, Greene KD, Wells JG, Hockin JC, Morgan D, Tauxe RV, et al. (1995) "Vibrio choleraee O139 Bengal infections among tourists to Southeast Asia: an intercontinental foodborne outbreak." J Infect Dis 172: 1401-1404.
- Crump JA, Bopp CA, Greene KD, Kubota KA, Middendorf RL, Wells JG, Mintz ED, et al. (2003) "Toxigenic Vibrio choleraeesero group O141-associated cholera-like diarrhea and bloodstream infection in the United States." J Infect Dis 187: 866-868.
- McCleskey FK, Hastings JR, Winn RE, Adams ED Jr (1986) "Non-01 Vibrio choleraee bacteremia--complication of a Le Veen shunt." Am J Clin Pathol 85: 644-646.
- El-Hiday AH, Khan FY, Al Maslamani M, El Shafie S (2006) "Bacteremia and spontaneous bacterial peritonitis due to Vibrio choleraee (non-O1 non-O139) in liver cirrhosis." Indian J Gastro enterol 25: 107.
- Eltahawy AT, Jiman-Fatani AA, Al-Alawi MM (2004) "A fatal non-01 Vibrio choleraee septicemia in a patient with liver cirrhosis." Saudi Med J 25: 1730-1731.
- Fernandez-Natal, I. and M. Alcoba-Leza (1996) "Non-O1 Vibrio choleraee bacteraemia without diarrhoea." Lancet 348: 67.
- Regules JA, Horvath LL, Chung KK (2008) "Invasive Vibrio choleraee infection following burn injury." J Burn Care Res 29: 551-554.
- Folgueira MD, López MM, García J, Peña P (1991) "[Bacteremia caused by Vibrio choleraee non-01]." Enferm Infecc Microbiol Clin 9(4): 254-255.
- Thungapathra M, Sharma C, Gupta N, Ghosh RK, Mukhopadhyay A, et al. (1999). Construction of a recombinant live oral vaccine from a non-toxigenic strain of Vibrio choleraee O1 serotype inaba biotype E1 Tor and assessment of its reactogenicity and immunogenicity in the rabbit model. Immunol.Lett. 68: 219-227.
- West BC, Silberman R, Otterson WN (1998) Acalculouscholecystitis and septicemia caused by non-O1 Vibrio choleraee: first reported case and review of biliary infections with Vibrio choleraee. Diagn Microbiol Infect Dis 30: 187-191.
- Johnson JA, Salles CA, Panigrahi P, Albert MJ, Wright AC, Johnson RJ, Morris JG., Jr, et al. (1994) Vibrio choleraee O139 synonym bengal is closely related to Vibrio choleraee El Tor but has important differences. Infect Immun62: 2108–2110.
- Poulos JE, Cancio M, Conrad P, Nord HJ, Altus P (1994) Non 0-1 Vibrio choleraee septicemia and culture negative neutrocyticascites in a patient with chronic liver disease. J Fla Med Assoc 81: 676-678.
- Wistrom, J (1989) "A case of non-O:1 Vibrio choleraee bacteremia from northern Europe." J Infect Dis 160: 732.
- Jhon D. Bancroft., Alan Stevens (1990) Theory and Practice of Histological Techniques. Churchill Livingstone.
- Safrin S, Morris JG Jr, Adams M, Pons V, Jacobs R, Conte JE Jr, et al. (1988) Non-O:1 Vibrio choleraee bacteremia: case report and review. Rev Infect Dis 10: 1012-1017.
- Santamaría Muñoz R, Ramírez Aguilera P, Pansza R, Acevedo E, Hernández Estradae , et al. (2002) Vibrio choleraee sepsis in the neonate. An Esp Pediatr 57: 361-363.
- Namdari H, Klaips CR, Hughes JL (2000) A cytotoxin-producing strain of Vibrio choleraee non-O1, non-O139 as a cause of cholera and bacteremia after consumption of raw clams. J Clin Microbiol 38: 3518-3519.
- Rudensky B, Marcus EL, Isaacson M, Lefler E, Stamler B, Sechter I, et al. (1993) "Non-O group 1 Vibrio choleraee septicemia in Israel." Isr J Med Sci 29: 54-55.
- Russell RG, Tall BD, Morris JG Jr (1992) "Non-O1 Vibrio choleraee intestinal pathology and invasion in the removable intestinal tie adult rabbit diarrhea model." Infect Immun 60: 435-442.
- Lee YL, Hung PP, Tsai CA, Lin YH, Liu CE, Shi ZY (2007) "Clinical characteristics of non-O1/non-O139 Vibrio choleraee isolates and polymerase chain reaction analysis of their virulence factors." J Microbiol Immunol Infect 40: 474-480.
- Lin CJ, Chiu CT, Lin DY, Sheen IS, Lien JM, et al. (1996) Non-O1 Vibrio choleraee bacteremia in patients with cirrhosis: 5-yr experience from a single medical center. Am J Gastroenterol 91: 336-340.
- Stanfield JT, Mc Cardell BA, Madden JM (1987) "Campylobacter diarrhea in an adult mouse model." Microb Pathog 3: 155-165.
- Abbott SL, Serve H, Janda JM (1994) "Case of Aeromonas veronii (DNA group 10) bacteremia." J Clin Microbiol 32: 3091-3092.
- Chang-Chien, C. H (2006) "Bacteraemic necrotizing fasciitis with compartment syndrome caused by non-O1 Vibrio choleraee." J Plast Reconstr Aesthet Surg 59: 1381-1384.
- Rabadan, P. M. and E. Vilalta (1989) Non-O:1 Vibrio choleraee bacteremia. Rev Infect Dis 11: 667.
- Thamlikitkul, V Thomas M, Cherian T, Raghupathy P (1990) Vibrio bacteremia in Siriraj Hospital thai Non-O:1 Vibrio cholerae bacteremia and peritonitis in a patient with nephrotic syndrome. Pediatr Infect Dis J 15: 276-277.
- Calduch Broseta JV, Segarra Soria MM, ColominaAvilés J, LlorcaFerrandiz C, Pascual Pérez R, et al. (2003) Septicemia caused by Vibrio choleraee non-01 in immuno compromised patient. An Med Interna 20: 630-632.
- Ko WC, Chuang YC, Huang GC, Hsu SY (1998) Infections due to non-O1 Vibrio choleraee in southern Taiwan: predominance in cirrhotic patients.Clin Infect Dis 27: 774-780.
- Lee MH, Leu HS, Huang SH (1993) Bacteremic cellulitis caused by non-O1 Vibrio choleraee: report of a case. J Formos Med Assoc 92: 472-474.
- Wang K, Chao CH, Liu IM, Liu CY (1991) Non-0:1 Vibrio choleraee bacteremia: a case report and literature review. Zhonghua Yi XueZaZhi (Taipei) 48: 232-236.
- Coovadia YM, Bhamjee A, Isaäcson M (1983) "Vibrio choleraee bacteraemia in a newborn infant. A case report." S Afr Med J 64: 405-406.
- Florman AL, Cushing AH, Byers T, Popejoy S (1990) Vibrio choleraee bacteremia in a 22-month-old New Mexican child. Pediatr Infect Dis J 9: 63-65.
- Heath CH, Garrow SC, Golledge CL (2001) Non-O1 Vibrio choleraee: a fatal cause of sepsis in northern Australia. Med J Aust 174: 480-481.
- Ninin E, Caroff N, El Kouri D, Espaze E, Richet H, Quilici ML, Fournier JM, et al. (2000) Nontoxigenic Vibrio choleraee O1 bacteremia: case report and review. Eur J Clin Microbiol Infect Dis 19: 489-491.
- Kontoyiannis DP, Calia KE, Basgoz N, Calderwood SB (1995) Primary septicemia caused by Vibrio choleraee non-O1 acquired on Cape Cod, Massachusetts. Clin Infect Dis 21: 1330-1333.
- Yamai S, Okitsu T, Shimada T, Katsube Y. Distribution of serogroups of Vibrio choleraee non-O1 non-O139 with specific reference to their ability to produce cholera toxin, and addition of novel serogroups. Kansenshogaku Zasshi. 1997: 71:1037–1045.
- Gordon MA, Walsh AL, Rogerson SR, Magomero KC, Machili CE, Corkill JE, Hart CA, et al. (2001) "Three cases of bacteremia caused by Vibrio choleraee O1 in Blantyre, Malawi." Emerg Infect Dis 7: 1059-1061.
- Phetsouvanh R, Nakatsu M, Arakawa E, Davong V, Vongsouvath M, Lattana O, Moore CE, Nakamura S, Newton PN, et al. (2008) Fatal bacteremia due to immotile Vibrio choleraeesero group O21 in Vientiane, Laos - a case report. Ann Clin Microbiol Antimicrob 7: 10.
- Ismail EA, Shafik MH, Al-Mutairi G (2001) A case of non-O:1 Vibrio choleraee septicemia with meningitis, cerebral abscess and unilateral hydrocephalus in a preterm baby.Eur J Clin Microbiol Infect Dis 20: 598-600.
- Jamil B, Ahmed A, Sturm AW (1992) Vibrio choleraee O1 septicaemia. Lancet 340: 910-911.
- Magnusson, M. R, S. P. Pegg (1996) Vibrio choleraee non-O1 primary septicaemia following a large thermal burn. Burns 22: 44-7.
- Tan KK, Sin KS, Ng AJ, Yahya H, Kaur P (1994) Non-O1 Vibrio choleraeesepticaemia: a case report. Singapore Med J 35: 648-649.
- Shelton CH 3rd, Martino RL, Ramsey KM (1993) "Recurrent non-0:1 Vibrio choleraee bacteremia in a patient with multiple myeloma." Cancer 72: 105-107.
- Berghmans T, Crokaert F, Sculier JP (2002) "Vibrio choleraee bacteremia in a neutropenic patient with non-small-cell lung carcinoma." Eur J Clin Microbiol Infect Dis 21: 676-678.
- Calia, F. M. and D. E. Johnson (1975) "Bacteremia in suckling rabbits after oral challenge with Vibrio parahaemolyticus." Infect Immun 11: 1222-1225.
- Dhar R, Ghafoor MA, Nasralah AY (1989) "Unusual non-serogroup O1 Vibrio choleraee bacteremia associated with liver disease." J Clin Microbiol 27: 2853-2855.
- Royo G, Martín C, Fuentes E, Elía M, Fernández J, Cuesta A, et al. (1993) Bacteremia caused by Vibrio choleraee 0:1.Enferm Infecc Microbiol Clin 11: 228.
- Suankratay C, Phantumchinda K, Tachawiboonsak W, Wilde H (2001) Non-serogroup O:1 Vibrio choleraee bacteremia and cerebritis. Clin Infect Dis 32: E117-9.
- Abboud CS, Ferreira CE, Barbosa VL, Araújo DA, Zandonadi EC, Pasternak J, et al. (2007) "Septicemia caused by Vibrio choleraee O1 biotype El Tor, in Sao Paulo, Brazil." Braz J Infect Dis 11: 300-301.
- Abbott SL, Seli LS, Catino M Jr, Hartley MA, Janda JM, et al. (1998) "Misidentification of unusual Aeromonas species as members of the genus Vibrio: a continuing problem." J Clin Microbio l36: 1103-1104.
- Anderson AM, Varkey JB, Petti CA, Liddle RA, Frothingham R, Woods CW, et al. (2004) Non-O1 Vibrio choleraee septicemia: case report, discussion of literature, and relevance to bioterrorism. Diagn Microbiol Infect Dis 49: 295-297.
- Bonner JR, Coker AS, Berryman CR, Pollock HM (1983) "Spectrum of Vibrio infections in a Gulf Coast community." Ann Intern Med 99(4): 464-469.
- Chan HL, Ho HC, Kuo TT (1994) "Cutaneous manifestations of non-01 Vibrio choleraee septicemia with gastroenteritis and meningitis." J Am Acad Dermatol 30: 626-628.
- Cheng NC, Tsai JL, Kuo YS, Hsueh PR (2004) "Bacteremic necrotizing fasciitis caused by Vibrio choleraee serogroup O56 in a patient with liver cirrhosis." J Formos Med Assoc 103: 935-938.
- Choi SM, Lee DG, Kim MS, Park YH, Kim YJ, Lee S, Kim HJ, Choi JH, Yoo JH, Kim DW, Min WS, Shin WS, Kim CC, et al. (2003) "Bacteremic cellulitis caused by non-O1, non-O139 Vibrio choleraee in a patient following hematopoietic stem cell transplantation." Bone Marrow Transplant 31(12): 1181-1182.
- Christenson B, Soler M, Nieves L, Souchet LM (1997) "Septicemia due to a non-0:1, non-0:139 Vibrio choleraee." BolAsoc Med P R 89: 31-32.
- Esparcia AM, Cañizares R, Roig P, Martínez A (2000) "[Bacteremia by Vibrio cholerae no 01, two cases]." Enferm Infecc Microbiol Clin 18: 49-50.
- Fernández JM, Serrano M, De Arriba JJ, Sánchez MV, Escribano E, Ferreras P, et al. (2000) Bacteremic cellulitis caused by Non-01, Non-0139 Vibrio choleraee: report of a case in a patient with hemochromatosis. Diagn Microbiol Infect Dis 37: 77-80.
- Halabi M, Haditsch M, Renner F, Brinninger G, Mittermayer H, et al. (1997) Vibrio choleraee non-O1 septicaemia in a patient with liver cirrhosis and Billroth-II-gastrectomy. J Infect 34: 83-84.
- Levine WC and PM Griffin (1993) Vibrio infections on the Gulf Coast: results of first year of regional surveillance. Gulf Coast Vibrio Working Group. J Infect Dis 167: 479-483.
- Liou CW, Lui CC, Cheng MH (2001) A case of intracerebral abscess caused by non-O1 Vibrio choleraee. Eur J ClinMicrobiol Infect Dis 20: 678-680.
- Naidu LS, Bakerman PR, Saubolle MA, Lewis K (1993) Vibrio choleraee non-0:1 meningitis in an infant. Pediatr Infect Dis J 12: 879-881.
- Piersimoni C, Morbiducci V, Scalise G (1991) Non-O1 Vibrio choleraee gastroenteritis and bacteraemia. Lancet 337: 791-2.
- Ravichandran M, Ali SA, Rasheed HW, Kurunathan S, Yean CY, et al. (2006). Construction and evaluation of a O139 Vibrio choleraee vaccine candidate based on a hemA gene mutation. Vaccine 24: 3750–3761
- Rerknimitr R, Chanyaswad J, Kongkam P, Kullavanijaya P (2008) Risk of bacteremia in bleeding and nonbleeding gastric varices after endoscopic injection of cyanoacrylate. Endoscopy 40: 644-649.
- Restrepo D, Huprikar SS, VanHorn K, Bottone EJ (2006) O1 and non-O1 Vibrio choleraee bacteremia produced by hemolytic strains.DiagnMicrobiol Infect Dis 54: 145-148
- Stypulkowska-Misiurewicz H, Pancer K, Roszkowiak A (2006) "Two unrelated cases of septicaemia due to Vibrio choleraee non-O1, non-O139 in Poland, July and August 2006." Euro Surveill 11: E061130.2.
- Su BA, Tang HJ, Wang YY, Liu YC, Ko WC, Liu CY, Chuang YC, et al. (2005) In vitro antimicrobial effect of cefazolin and cefotaxime combined with minocycline against Vibrio choleraee non-O1 non-O139. J MicrobiolImmunol Infect 38: 425-429.
Relevant Topics
Recommended Journals
- Journal of Lung Cancer Diagnosis & Treatment
- Advances in Cancer Prevention
- Breast Cancer: Current Research
- Cancer Surgery
- Immunology: Current Research
- Current Trend in Gynecologic Oncology
- Journal of Cancer Diagnosis
- Journal of Gastrointestinal Cancer and Stromal Tumors
- Cervical Cancer: Open Access
- Journal of Mucosal Immunology Research
- Journal of Oncology Research and Treatment
- Journal of Orthopedic Oncology
- Journal of Prostate Cancer
- Research and Reviews on Pathogens
Article Tools
Article Usage
- Total views: 19310
- [From(publication date):
September-2014 - Apr 04, 2025] - Breakdown by view type
- HTML page views : 14634
- PDF downloads : 4676