Research Article Open Access
Isolation and Characterization of Gasoline-Degrading Yeasts from Refined Oil-Contaminated Residues
| Goulart GG1, Coutinho JOPA1, Monteiro AS2, Siqueira EP3 and Santos VL1* | ||
| 1 Department of Microbiology, Institute of Biological Science, Universidade Federal de Minas Gerais, Belo Horizonte – MG, C.P. 486, 31270-901, Brazil | ||
| 2 Laboratório de Pesquisa em Microbiologia – Faculdade de Ciências da Saúde, Universidade Vale do Rio Doce, Governador Valadares, MG, Brazil | ||
| 3 Laboratório de Química de Produtos Naturais, Centro de Pesquisas René Rachou Fundação Oswaldo Cruz, C.P. 30190- 002, Belo Horizonte, MG, Brazil | ||
| Corresponding Author : | Vera Lúcia dos Santos Department of Microbiology ICB-UFMG, C.P. 486, 31270-901 Belo Horizonte, Brazil Tel: 5534092501 Fax: 55(31)34092730 E-mail: vlsantos@icb.ufmg.br |
|
| Received November 04, 2013; Accepted February 03, 2014; Published February 08, 2014 | ||
| Citation: Goulart GG, Coutinho JOPA, Monteiro AS, Siqueira EP, Santos VL (2014) Isolation and Characterization of Gasoline-Degrading Yeasts from Refined Oil-Contaminated Residues. J Bioremed Biodeg 5:214. doi:10.4172/2155-6199.1000214 | ||
| Copyright: © 2014 Goulart GG, et al. This is an open-a ccess article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | ||
Related article at Pubmed Pubmed  Scholar Google Scholar Google |
||
Visit for more related articles at Journal of Bioremediation & Biodegradation
Abstract
Hydrocarbon-degrading yeasts were isolated from three sites contaminated with products of refined petroleum originating from an oily residue treatment Brazilian company. A selective enrichment technique was used by supplementing medium with gasoline, which resulted in eleven isolates; two identified as Rhodotorula mucilaginosa, one as Rhodosporidium diobovatum, four as Meyerozyma (Pichia) guilliermondii, two as Wickerhamia sp., and two as Meyerozyma sp. The strains were evaluated for their growth capacity in medium containing 1% (v/v) gasoline, kerosene or lubricating oil as the only carbon source; the largest values for cellular biomass and growth rates (μ) were observed with gasoline supplementation. The strains were tolerant to aromatic (toluene and xylene) and aliphatic (hexene and n-heptane) compounds, which are part of the composition of gasoline, at concentrations up to 30 mM toluene (0.3% v/v), 20 mM xylene (0.25% v/v), 80 mM n-heptane (1.17% v/v) and 100 mM hexane (1.33% v/v). The R. mucilaginosa S47 and Meyerozyma sp. SP1 strains showed the greatest degradation percentages of gasoline, and have the potential to be used in the bioremediation of gasoline-contaminated environments.
| Keywords |
| Yeasts; Gasoline; Bioremediation; Tolerance to hydrocarbons |
| Introduction |
| Crude oil and its derivatives have been used by humans since 5,000 years B.C. In the early 19th century, the derivatives of petroleum started to be used as fuel for vehicles, components of explosives (glycerin and toluene), and as synthetic materials for clothes, solvents, and medicines, among other uses. With the increasing use of these products, there is also an increased risk of environmental contamination. The release of gasoline into the environment is a common occurrence, arising principally from leaking storage tanks; this can result in the contamination of drinking sources by mobile gasoline components, which can migrate through the soil matrix [1]. |
| Strategies for controlling environmental contamination with petroleum and its derivatives have been the subject of various studies over the past three decades. There is a great diversity of microorganisms able to utilize hydrocarbons as a sole carbon source, including bacteria, yeasts and molds. Bacteria are among the best-described hydrocarbon utilizing microorganisms [2-5]. In addition, some yeast strains display an excellent capacity to degrade oil-related compounds [6-9]. |
| The biodegradability of the most water-soluble components of gasoline, such as benzene, toluene, ethyl benzene and xylene isomers, compounds usually termed BTEX, has been clearly established using pure strains or mixed cultures [4,9,10]. However, little is known about the degradability of other gasoline components, except for some polyalkylated benzenes and some linear and branched alkanes [4,11]. In addition, the biodegradability of gasoline is not easy to assess since it consists of more than 200 identifiable components; interactions may occur between individual components and may affect, in particular, the degradation kinetics [12]. |
| Knowledge regarding the biodegradability properties of all components of gasoline followed by an evaluation of the specific degradative capacities of the autochthonous microflora present at contaminated sites will allow an assessment of the prospects for natural attenuation and the potential use of bioremediation technologies. Bioremediation using selected microorganisms provides a good opportunity because it is environmentally friendly and cost effective. Some microbial strains can degrade hydrocarbons and utilize the resulting carbon compounds as food and energy sources for growth and reproduction. Simultaneously, the hydrocarbons are hydrolyzed from toxic into non-toxic compounds and simple inorganic compounds, such as CO2 and H2O, along with microbial biomass accumulation, through oxidation under aerobic and anaerobic conditions. To degrade organic pollutants, microorganisms must have metabolic processes to optimize the contact between microbial cells and organic pollutants, such as the production of biosurfactants, intracellular pathways to initiate the attack on organic pollutants, usually mediated by the activation and incorporation of oxygen by oxygenases and peroxidases, as well as peripheral degradation pathways to convert organic pollutants step by step into the intermediates of central intermediary metabolism [2,13]. |
| The objective of this study was to isolate, identify and investigate the potential of yeasts to degrade gasoline for use in the bioremediation of contaminated areas. Furthermore, yeast cells were characterized for changes in cell hydrophobicity after culturing in medium supplemented with different carbon sources. Additionally, the tolerance of these yeast strains to aromatic hydrocarbons (toluene and xylene) and aliphatic compounds (n-heptane and hexene) present in different fractions of gasoline was assessed, as was the potential for degradation of gasoline compounds using assays in liquid medium. |
| Materials and Methods |
| Sampling site and isolation of yeasts potentially able to degrade petroleum hydrocarbons |
| The yeast strains used in this study were isolated from samples collected from four sites contaminated with refined petroleum products. The samples were obtained from a company that treats oily waste in the metropolitan region of Belo Horizonte, Brazil. These oily wastes include gasoline, diesel oil, lubricating oil and kerosene. Samples were collected from sites that receive mixtures of oily waste (MOW), oily sludge and leachate from these sites (OL), soil from garages for trucks and heavy vehicles (S), and storage tanks for wastewater containing emulsified lubricant oils (EO). The samples were preserved on ice and transported to the laboratory for the isolation of microorganisms. |
| Aliquots of 100 μL from serial dilutions (10-1 to 10-7) were plated on Sabouraud Dextrose Agar (SDA; Difco, USA), modified with (per liter): 10.0 g casein peptone, 20 g glucose, 5 g of yeast extract and 20 g agar, and in mineral medium for fungi (MMF) containing (per liter): 3.4 g K2HPO4, 4.3 g KH2PO4, 0.3 g MgCl2.2H2O, 1.0 g (NH4) 2SO4, 0.05 g yeast extract, 20 g agar and 5 mL of a solution of trace elements (in mgL-1: MnCl.4H2O, 1; FeSO4.7H2O, 0.6; CaCl2.H2O, 2.6; Na2MoO4.2H2O, 6). Both types of medium were supplemented with chloramphenicol at a concentration of 200 mg/L and 1% gasoline. The gasoline was added to the media after they were autoclaved and homogenized in a sterile blender before being distributed onto the plates. The plates were incubated at 28°C for up to 10 days. Individual colonies on the plates representative of each morphotype were purified by streaking at least three times on SDA medium. The strains were maintained in GYMP broth (w/v- 2% glucose, 0.5% yeast extract, 1% malt extract, 0.2% NaH2PO4) with 20% (v/v) glycerol at -80°C. |
| Identification of yeasts |
| The yeasts were characterized considering morphological and biochemical properties by standard methods [14] and identification followed the keys of Kurtzman and Fell [15]. The identity was confirmed by sequencing the D1/D2 variable domains of the large subunit rDNA. For genomic DNA extraction, cells from 24-48 h old pure cultures in SDA were removed, resuspended in 2 mL saline solution, and centrifuged at 13,000 rpm for 5 min. The supernatant was discarded, the pellet was resuspended in 400 μL of extraction buffer (2% Triton X-100, 1% sodium dodecyl sulfate, 100 mM NaCl, 10 mM Tris–HCl, pH 8.0, 10 mM EDTA) and transferred to a 1.5 mL conical tube for further cell disruption for at least 20 s using approximately 80 mg of acidwashed glass beads (Sigma, 150–212 l) in combination with a strong vortex. The suspension of disrupted cells was incubated for 10 min at 65°C in order to complete cell rupture and purified by adding equal volumes of phenol–chloroform–isoamyl alcohol (25:24:1), followed by isopropanol precipitation. Subsequently, the pellet was washed in 70% ethanol and dissolved in 20–50 μL TE buffer (10mM Tris-HCl, 1.0mM EDTA, pH 8.0). |
| D1/D2 divergent domains were amplified by PCR as described by Lachance et al. [16] using the primers NL-1 (5';-GCATATCAATAAGCGGAGGAAAAG-3') and NL-4 (5';-GGTCCGTGTTTCAAGACGG-3'). The amplified DNA was concentrated, cleaned (Kit Wizard Plus SV Minipreps DNA Purification System; Promega, USA), and sequenced in a Mega BACETM1000 automated sequencing system (Amersham Biosciences, USA). The sequence data were aligned with the Electropherogram Quality Analysis program (http://asparagin.cenargen.embrapa.br). The yeasts were identified by searching databases using the BLAST sequence analysis tool (http://.ncbi.nlm.nih.gov/BLAST/). |
| Assays of yeast growth in mineral medium containing gasoline, kerosene and lubricating oil as carbon sources |
| The yeasts were inoculated at a final concentration of 0.02 optical density unit at 600 nm in 60 mL flasks containing 10 mL of MMF plus 1% (v/v) of lubricating oil (Daido Dairoll PA-5A), commercial gasoline (Petrobras, Brazil) or kerosene (Ica, Brazil) as the sole source of carbon. |
| To standardize the inoculum, stock cultures of yeast cells grown on DAS for 48 h at 25°C and washed in saline solution (NaCl 0.85% w/v) were transferred to MMF. The optical density (O.D.) at 600 nm was read and the absorbance values were used to calculate the inoculum’s size to obtain flasks with a cell density of 0.02 O.D. (corresponding to 3.32 log10 CFU mL-1). All bottles were sealed with rubber stoppers and sealed with plastic film to prevent the evaporative loss of hydrocarbons. The vials were incubated for 120 h at 28°C and 180 rpm and the growth (CFU mL-1) was determined by yeast colony counts of appropriate decimal dilutions on Sabouraud agar. The cellular density data were converted to mgL-1 using a calibration curve of biomass (mg L-1) against density expressed as CFU mL-1.The specific growth rate μ, was calculated from equation, μ=ΔlnX/Δt, where X is biomass (mgL-1) and t is time (h).All tests were performed in triplicate. In control experiments, yeast cells were inoculated into MMF without the addition of the evaluated carbon sources, and were subjected to the same conditions of incubation as the test trials. |
| Assays of tolerance to organic compounds present in gasoline |
| The yeasts were inoculated into MMF (0.02 O.D. 600 nm) supplemented with 2% peptone and toluene, xylene, hexane or n-heptane at various concentrations (5-100 mM). The flasks were incubated at 28°C and 180 rpm for 48 h. After this period, cell growth, indicative of tolerance to organic solvents, was evaluated by measuring the optical density of samples of cultures at 600 nm. In control experiments, cells were grown similarly in MMF, without the addition of organic compounds. All tests were performed in triplicate. |
| Detection of biosurfactant/bioemulsifier producti |
| For the determination of surface-active compound production during growth in liquid medium supplemented with gasoline, the emulsifying and surfactant activity of culture supernatants were evaluated. The yeasts were cultivated in Erlenmeyer flasks containing 50 mL of MMF supplemented with 0.05% (w/v) peptone and 1% (v/v) gasoline under agitation at 180 rpm at 28°C. After incubation for up to 9 days, the yeast cells were separated from the growth medium by centrifugation at 5,000 rpm for 20 min at 4°C. The supernatant was filtered through a 0.45 μm Millipore membrane filter and preserved by refrigeration at 4°C until detection the of emulsifying activity (E24) was performed using the method of Cameron et al. [17]. |
| In these assays, 4 mL aliquots of the cell free filtrate were mixed with 6 mL of toluene or kerosene in a test tube and vortexed vigorously for 2 min. After 24 h, the proportion of emulsified toluene was compared with the total volume of toluene added. The emulsification index (E24) was estimated as the height of the emulsion layer divided by the total height and multiplied by 100. Uninoculated culture medium was used as the negative control for the experiment. In addition, the surface tension of the cell-free culture broth was determined by the Ring method using a KRUSS tensiometer (K10T Hamburgo). |
| Microbial adhesion to hydrocarbons |
| Changes in cell surface hydrophobicity during growth in liquid medium supplemented with glucose or gasoline was assessed by the microbial adhesion to hydrocarbon method (MATH) described by Rosenberg et al. [18], with modifications. Yeast cells were grown in 60 mL flasks containing 10 mL of MMF supplemented with 1% (w/v) yeast extract and 1% (v/v) gasoline or 1% (w/v) glucose, under the same conditions as the biodegradation experiments. After growth, the cells were washed twice with a PUM buffer (g L-1): K2HPO4, 19.7; KH2PO4, 7.26; H2NCONH2, 1.8 and MgSO4.7H2O, 0.2, suspended in the buffer to an optical density of 0.7 O.D. at 600 nm (A0). Next, 500 μL of hydrocarbons were added to 2 mL of the microbial suspension, vortexed for 2 min and equilibrated for 30 min. The bottom aqueous phase was carefully removed with a Pasteur pipette and the O.D. at 600 nm was measured (A1). The degree of hydrophobicity was calculated as [(A0 - A1)/A0] X 100%. |
| Gasoline degradation assays |
| The yeasts were cultured for 7 days in 60 mL flasks containing 10 mL of MMF plus 1% (v/v) gasoline at 28°C, with agitation at 180 rpm. All bottles were sealed with rubber stoppers and sealed with plastic film to prevent the evaporative loss of hydrocarbons. After this period, the residual gasoline was extracted from the medium for analysis. All tests were performed in triplicate. The control for these experiments consisted of MMF containing gasoline without an inoculum, and the flasks were subjected to the same incubation conditions of the trial test. |
| Extraction of gasoline constituents by the Solid Phase Micro- Extraction (SPME) method |
| To extract the gasoline constituents and quantify the aromatic hydrocarbons during the biodegradation process, an SPME fiber, coated with a 100 μm polydimethylsiloxane layer (Supelco) was pierced through the Teflon-silicone septum of the flasks with samples and pushed down into the middle of the headspace created by heating the flasks at 90°C in a heater block (Reacti-Therm III, Pierce). The fiber was exposed to the gaseous phase for 5 min. The fiber was then retracted and immediately inserted into the injector of the gas chromatograph for thermic desorption of compounds and analysis. |
| Chromatographic and spectrometric analyses were performed by GC-MS (Shimadzu™, Model 17A/QP5050A) using a PTE™-5 (Supelco) chromatographic column with a size of 30 m x 0.25 mm x 0.25 μm. The temperature was programmed to vary linearly from 40°C to 270°C at a rate of 7°C min-1 and maintained for 22 min. Helium was the carrier gas with a flux of 50 mL min-1, and the interface temperature was 280°C. The injection of samples and the control into the GC-MS system was carried out in triplicate. The data were obtained using CLASS 5000 software (Shimadzu) and analyzed using AMDIS software (Automated Mass Deconvolution and Identification System, version 2.1, National Institute of Standards and Technology (NIST), USA). The individual hydrocarbons were identified using the NIST Mass Spectral Search software, based on similarities between their mass spectra and those provided by the NIST/EPA/NIH compound library, version 2.0. Control peak areas were used as a point of reference for the remaining compounds (100%) in the untreated system. Sample peak areas were reported as a percentage of the control peak area. The gasoline degradation potential of yeasts was expressed as the reduction in the total area of the chromatograms and of the peaks corresponding to gasoline compounds, in relation to the control sample. In the chromatographic analysis, we used a solution of hexadecane in ethyl acetate (1:1,600,000) as the internal standard for the normalization of data. The compound used as the internal standard, as well as its concentration, were previously standardized. For this, 1 μL of this solution was added to the samples with a 10 μL syringe (Hamilton # 701), immediately before SPME extraction. |
| Results |
| Isolation, characterization and identification of yeasts with degradation potential of oil hydrocarbons |
| In total, eleven morphotypes were isolated from three of the four sites contaminated with oil at cell densities varying from 102 to 106 CFU mL-1 or CFU g-1 of the residues. Two isolates were from sites that received a mixture of oily waste, eight from the oily sludge and leachate from these sites and the soil of a garage for trucks and heavy vehicles, and one from the top of a wastewater storage tank containing emulsified lubricant oils (Table 1). |
| The morphological and biochemical properties and rDNA sequence analysis of the 11 selected strains of yeast indicated that six strains belong to the genus Meyerozyma, two to the genus Rhodotorula, two to the genus Wickerhamia and one to the genus Rhodosporidium. The 26S rRNA gene sequences have been deposited in the NCBI gene bank and the accession numbers are shown in Table 1. In general, strains of the same species differed from 0 to 3 nucleotides within the sequence of the D1/D2 domain, i.e. from 0% to 0.5% different. The strains that had six or more nucleotide substitutions (1%) non-continuous were considered different species [19]. Based on this criterion, yeasts S47, S48, S49, S53, ESC1, SJ1 and SV1 could be identified to the species level (similarity>99%), while isolates S51 and SP1 were identified to the genus level since the similarity varied between 97 and 98%. It is possible that isolates MR58 and MR73 are new species within the genus Wickerhamia sp. |
| Growth characteristics of yeasts in mineral medium containing gasoline, kerosene and lubricating oil |
| The yeast strains utilized the evaluated compounds as the sole source of carbon and energy, which was evident by an increase in cell density after incubation. The growth profile varied depending on the yeast strains and substrate type (p <0.05) (Table 2). In general, gasoline was shown to be the least supportive carbon source, followed by kerosene; the lowest values were observed for oil (p<0.05). In the medium with gasoline, strains R. mucilaginosa S47, Meyerozyma sp. SP1 and S49 and S53 of M. guilliermondii showed the best growth; in the medium with kerosene, strains S49 and SJ1 of M. guilliermondii, Meyerozyma sp. S51, and R. diobovatum S48 showed the best growth; and in the presence of oil, S53, S49, SP1 and S48 showed the best results. These results clearly indicate that different oils were degraded and utilized by the strains in various proportions, probably depending on the complexity and aliphatic and aromatic nature of the oily substrate. |
| In general, we observed a positive correlation between the growth rate (μ) and biomass production from the growth medium supplemented with gasoline (r=0.97, p=0.00), kerosene (r=0.8, p=0.0019) and oil (r=0.89, p=0.0001) (Table 2). |
| The highest growth rates (μ) also occurred in medium supplemented with gasoline, and in most isolates, high values were observed at 48 h of incubation (p <0.05). In the medium containing lubricating oil, Wickerhamia sp. MR58 showed significant growth after 24 h of incubation, R. mucilaginosa ESC1 after 48 h, and the others after 120 h. In the medium with kerosene, R. diobovatum S48 grew after 24 h of incubation, Wickerhamia MR73 and M. guilliermondii SV1 strains were not able to grow, and others grew up after 120 h of incubation. In the control experiments, there was no growth of yeasts. Independent of the growth substrate, the highest growth rates were observed for the strains S49, SP1, S53, ESC1, S48 and S47. |
| Organic solvent tolerance assays |
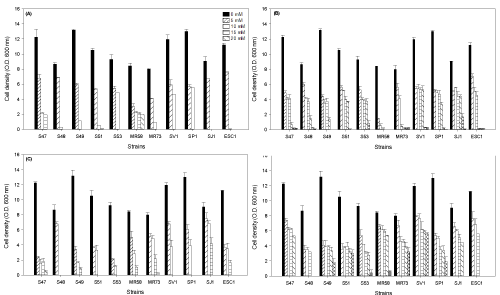
| The yeasts showed different profiles of tolerance to aromatic (toluene and xylene) and aliphatic (n-hexane and heptane) compounds that are part of the composition of gasoline, as well to the different concentrations of the compounds evaluated. The strains S53, SJ1 and SV1 of M. guilliermondii were tolerant up to 30 mM toluene (0.3% v/v), Wickerhamia sp. MR58 up to 20 mM xylene (0.25% v/v), R. mucilaginosa S47 up to 80 mM n-heptane (1.17% v/v) and S49, S51, S53, MR58, MR73, SV1 and SP1 were tolerant up to 100 mM hexene (1.33% v/v) (Figure 1). The growth of microorganisms was also different for the evaluated compounds, and some yeasts reached a cell density greater than 7 units of optical density at 600 nm at some concentrations. In control experiments without organic compounds, the optical density of the isolates ranged from 6.5 to 13 units. |
| Detection of biosurfactant/bioemulsifier production |
| Samples of the yeast culture supernatants were not able to form a stable emulsion using kerosene and toluene as the organic phase and did not present surfactant activity, expressed by a reduction in the surface tension of the growth medium (data not shown). This, however, does not exclude the possibility of biosurfactant production by these microorganisms, which should be evaluated by additional testing. |
| Determination of hydrophobicity of cells grown in MMF supplemented with gasoline or glucose |
| To evaluate the effect of different substrates on cell hydrophobicity, changes in hydrophobicity after growth in mineral medium supplemented with gasoline (1% v/v) or glucose (1% w/v) were evaluated (Figure 2). Hydrophobicity was expressed as the percentage of cells that adhered to hexadecane. Isolates were considered hydrophobic when more than 50% of the cells migrated from the PUM buffer toward hexadecane and hydrophilic when more than 50% of cells remained in the buffer [20]. |
| Cells of strains S47 and ESC1 of R. mucilaginosa, R. diobovatum S48, M. guilliermondii S53, strains MR58, MR73 of Wickerhamia sp. and Meyerozyma sp. SP1 became hydrophobic after growth in mineral medium augmented with gasoline. However, all yeasts showed values of adhesion to hexadecane lower than 40% after growth in medium containing glucose and were considered hydrophili |
| Assays of gasoline degradation |
| The hydrocarbon profile in the growth media after 7 days of growth was analyzed by GC-MS and compared with the hydrocarbon profile from a control flask where the FMM medium and gasoline were kept together under identical conditions. The potential of yeasts to degrade gasoline (1% v/v) was expressed as a reduction in the total area of the chromatograms and of the peaks corresponding to the constituents of gasoline compared to the control sample (Table 3). All hydrocarbon classes evaluated, i.e. aromatic compounds, linear, branched and cyclic alkanes as well as alkenes, were substantially degraded after 7d. Hydrocarbons belonging to the same class were found to be consumed at various rates by different isolates. R. mucilaginosa S47 and Meyerozyma sp. SP1 degraded gasoline more effectively than any of the other strains; S47 reduced the area under the primary peaks by >60% and 99.5% of the total area of the chromatogram relative to the uninoculated control. For R. mucilaginosa SP1, the reduction in the area of the chromatogram was 97.1% and >95% for peak areas of the individual compounds analyzed. R. mucilaginosa ESC1, as well as strains S53, S49 and SV1 of M. guilliermondii showed a reduction in the total area of 93.31, 87.4, 47.9 and 69.5%, respectively. The other strains showed lower reductions both in the total area of the chromatogram (values ranging from 2.1 to 28.3%) and the areas of the individual peaks. |
| Discussion |
| Gasoline, kerosene and oil degrading yeasts were isolated from three different sites contaminated with oily wastes. Many authors have reported that microorganisms isolated from polluted sites have a greater capacity to degrade pollutants in degradation assays in reactors at the laboratory or pilot scale, probably because they are already adapted to grow in environments with these compounds [21], given the selective pressure that these environments have on the local microbiota. As example, microorganisms isolated from soil contaminated with diesel oil were able to degrade 80% of the diesel fuel added to the growth medium (400 mg L-1) in 4 weeks, whereas the microflora from uncontaminated soil degraded 67% of the diesel oil in the same period [22]. |
| The best growth of yeast in medium containing gasoline, as compared with oil and kerosene, probably occurred because gasoline was used as the model hydrocarbon in culture media for the selection of yeasts. These data suggest that the methodology used in the isolation was adequate to obtain microorganisms with the potential to degrade gasoline. The growth observed in this assay was an indication that the yeasts were utilizing some components of gasoline, which was the sole source of carbon available in the culture medium. |
| The pattern of differential growth of yeasts in media containing gasoline, kerosene and lubricating oil may have resulted from different degrees of tolerance to the various constituents of these substrates. While gasoline has aromatic hydrocarbons with 7-9 carbons as the major constituent and acyclic aliphatic hydrocarbons with 5 and 6 carbons in a small fraction, kerosene is rich in C8-C18 hydrocarbons, which are mainly n-alkanes. In turn, lubricating oil is rich in longer chain hydrocarbons (C20-C35) and n-alkanes with 11 to 20 carbons in small amounts. This diversity of constituents leads to different levels of toxicity of these fuels. This pattern was observed by Wongs et al. [23] that evaluated of growth of Pseudomonas aeruginosa WATG isolated from water samples from an old kerosene tank in medium containing gasoline, kerosene, diesel oil or lubricating oil (10 g L-1). The bacteria presented the highest growth rates in diesel fuel, followed by kerosene, lubricating oil and gasoline. |
| Although yeasts have the capacity to degrade hydrocarbons, there are only a few citations on these yeasts capable of degrading compounds derived from refined petroleum. Previous studies have been conducted on naturally existing oil-degrading yeasts of the genera isolated in this study. Lahav et al. [24] isolated Meyerozyma (Pichia) guilliermondii and R. mucilaginosa in evaporation ponds contaminated with industrial wastes, which were able to utilize diverse hydrocarbons as carbon sources, including anthracene and phenanthrene. Pan et al. [25] isolated a strain of P. anomala 2.2540 from petroleum contaminated soil, which had the ability to degrade polyaromatic hydrocarbons such as naphthalene, dibenzothiophene, phenanthrene and chrysene in different concentrations, alone or combined. Margesin et al. [26] isolated 61 strains of bacteria and 28 strains of yeast from diverse uncontaminated cold environments. These strains used representative fractions of oil hydrocarbons for their growth, such as n-alkanes and monoaromatic and polyaromatic hydrocarbons, at low temperatures. Of the bacterial strains, 6% used n-hexadecane for growth and 13% used phenol, phenanthrene and anthracene, while 79% of the yeast strains used n-hexadecane and 21-32% used phenol, anthracene or phenanthrene. Three strains identified as Rhodotorula and one as Candida were able to grow in all hydrocarbons tested, which did not occur with any bacterial strain. Y. lipolytica AF 335977, C. viswanathii CVU 45752, C. palmioleophila CPU 45758 and Meyerozyma (Pichia) guilliermondii AF 257270 [27], isolated from soil contaminated with oil, have also been described as degraders of different hydrocarbons. |
| Tolerance of yeasts to the organic solvents correlated to the hydrophobicity of the solvent used, with higher values of tolerance observed for more hydrophobic solvents (n-heptane and hexane). Qun et al. [28] correlated the hydrophobicity of the solvent and its toxicity to microbial cells using the logarithm of the octanol/water partition coefficient (LogP). They observed that less hydrophobic solvents, with LogP values lower than 2, were more toxic to microbial cells, solvents with LogP between 2 and 4 were somewhat less toxic and solvents with a LogP value greater than 4, i.e. more hydrophobic, were well-tolerated by microorganisms. It has been suggested that less hydrophobic solvents (such as toluene and xylene) can better penetrate the cell, leading to the denaturation of enzymes and a reduction in metabolic activity. These authors tested the tolerance of Saccharomyces cerevisiae type II (Sigma) to ethanol, sec-butyl alcohol, butyl acetate, n-hexane, n-heptane, n-octane, n-decane and dodecane, and found lower tolerance of microorganisms to ethanol and sec-butyl alcohol, and greater tolerance to dodecane, which was the most hydrophobic of the tested compounds. In our study, yeasts were less tolerant to toluene and xylene, with LogP values of 3.0 and 2.5, respectively, and more tolerant to hexene and n-heptane, with LogP values of 3.4 and 4.0, respectively. |
| Hughes et al. [29] evaluated the tolerance of filamentous fungi and bacteria isolated from Antarctica to 10 hydrocarbons by measuring cell growth, and observed that the fungi were more tolerant than bacteria. The authors observed that aromatic hydrocarbons inhibited growth more than aliphatic hydrocarbons, which was also observed in the present study. Yeasts and bacteria have shown tolerance values depending on the organism studied and the site of isolation. Zahir et al. [30] tested the toluene tolerance of bacteria isolated from soil contaminated with hydrocarbons and from rhizospheric soil. Growth was observed in the presence of toluene concentrations ranging from 20 to 100 mM, which corresponded to concentrations from 0.21 to 1% (v/v); greater tolerance was observed for the microorganisms isolated from contaminated soils. These isolates showed greater tolerance to toluene than the yeasts evaluated in the present study, which tolerated up to 30 mM toluene (0.3% v/v). Segura et al. [9] isolated five marine bacteria that were tolerant to 0.1% v/v toluene and benzene, but sensitive to 0.1% ethyl benzene or xylene. In our study, yeasts tolerated up to 0.3% toluene and 0.25% xylene. |
| In the assays to assess the hydrophobicity of cells grown in MMF supplemented with gasoline or glucose, higher values were observed for cells grown in gasoline, suggesting that hydrophobicity may have been induced by the hydrocarbons found in this fuel. These results are consistent with those obtained by Chrzanowski et al. [8], who evaluated the influence of phenol (hydrophilic) or n-alkane (hydrophobic) on the cell surface hydrophobicity of Candida maltosa, Meyerozyma (Pichia) guilliermondii and Y. lipolytica; the authors observed that, in cells grown in phenol, hydrophobicity varied between 25 and 40%, whereas for cells grown in the presence of alkanes, hydrophobicity reached 90%. A strain of Y. lipolytica degrader of hydrocarbons showed cell surface hydrophobicity greater than 90% after growth in the presence or absence of hydrocarbons [31]. |
| In addition to the production of biosurfactants, cell surface hydrophobicity can be considered an important factor in controlling the assimilation of hydrocarbons. It has been suggested that cells with greater hydrophobicity are more likely to adhere to hydrophobic compounds than those with lower hydrophobicity, and are better at assimilating hydrocarbons [13,31,32]. Under the growth conditions used here, the yeasts did not produce biosurfactants. Several studies have reported the influence of medium composition on the yield of biosurfactants, mainly concerning sources of carbon and nitrogen. Batista et al. [33] reported that bacteria grown in medium containing glucose presented higher kerosene emulsifying activity as compared to culture with fructose, sucrose (2% w/v) or kerosene (0.5% w/v). In another study, Kim et al. [31] observed that Y. lipolytica cultures from medium with various petroleum hydrocarbons showed lower surface tension values than cultures grown in medium with hydrophilic compounds such as glucose and peptone. In our study, the yeasts were grown only in medium containing gasoline as the carbon source, which may not be suitable for the production of biosurfactants. Another alternative would be to study the emulsifying capacity of the whole culture, not only the supernatant, as has been reported in some studies. Menezes Bento et al. [34] observed the emulsification of diesel oil by total cultures of various bacterial strains, but the activity was not observed for culture supernatants, indicating that an extracellular emulsifier was not produced. Some microbial cells present high surface hydrophobicity and tensoactive activity. Others produce extracellular vesicles that play an important role in the uptake of hydrophobic compounds by cells with high surfactant activity. The vesicles and microbial cells with surfactant activity are classified as particulate biosurfactants [32]. |
| The ability of isolated yeasts to utilize gasoline as a carbon source was investigated by GC-MS analysis. All hydrocarbon classes evaluated, i.e. aromatic compounds, linear, branched and cyclic alkanes as well as alkenes, were substantially degraded after 7 days (Table 3). Hydrocarbons belonging to the same class were found to be consumed at various rates by different isolates. The aromatics were found to be readily consumed, in agreement with the results reported for some individual aromatics, such as benzene, toluene, ethyl benzene, o-, m- and p-xylene [35-37]. For other polyalkylated aromatics, the nature and relative positions of alkyl chains on the ring significantly influenced the rate of biodegradation. Concerning alkanes, cyclic alkanes were found to be degraded, although Ridgway et al. [3] noted the low occurrence of pure strains able to grow with cyclic alkanes as the sole carbon source. Moreover, the simultaneous degradation of iso- and n-alkanes provided further evidence for the high efficiency of the isolates compared with microflora of other origins, whereas the degradation of iso-alkanes took place only after total exhaustion of n-alkanes [38,39]. In general, the residual components of gasoline were mostly branched alkanes. In particular, 3-ethylhexane and trimethyl alkanes with either a quaternary carbon (2,2,5-trimethylhexane and 2,2,4-tri-methylpentane,) or alkyl chains on consecutive carbons (2,3,3-trimethylpentane and2,3,4-trimethylpentane) appeared to be the most recalcitrant compounds of gasoline. |
| In this assay, the yeasts showed good potential for the degradation of gasoline compared to the degradation experiments described in the literature. The degradation of gasoline by microbiota isolated from urban wastewater activated sludge was investigated by Solano-Serena et al. [40], who observed a degradation rate of 74% (400 mg L-1) with two days of incubation, and 94% after 23 days of incubation. Bacteria isolated by Lu et al. [5] from contaminated soil near a gas station were able to grow at up to 2 g L-1 gasoline; however, the optimal concentration for growth was 1 g L-1 and a better degradation rate was obtained for Pseudomonas sp. Q10, corresponding to 62.6%. Yerushalmi and Guiot [36] reported the complete degradation of gasoline by the microbiota of soil contaminated by this fuel, which degraded 16.1 to 600 mg L-1 of gasoline after 2.5 and 16 days, respectively. |
| Although several studies have related cell surface hydrophobicity to an increased binding capacity of cells to hydrophobic compounds, and consequently the ability to degrade these compounds [8,13,31] a direct relationship between cell surface hydrophobicity and degradation was not found in the present study. Strains S47 and ESC1 of R. mucilaginosa, Meyerozyma sp. SP1 and M. guilliermondii S53, which had highest potential to degrade gasoline, became hydrophobic after growth in this fuel, but R. diobovatum S48 and strains MR58 and MR73 of Wickerhamia sp., although hydrophobic, did not show the potential to degrade gasoline under the evaluated conditions. Strain M. guilliermondii S49 did not become hydrophobic, but was able to significantly degrade gasoline. |
| Conclusion |
| In this work, we described new yeast strains belonging to the genera Meyerozyma, Rhodotorula, Wickerhamia and Rhodosporidium capable to grow in medium containing 1% (v/v) gasoline, kerosene or lubricating oil as the only carbon source; and the highest growth rates (μ) were observed in medium supplemented with gasoline. The strains were tolerant to aromatic (toluene and xylene) and aliphatic (hexene and n-heptane) compounds, which are part of the composition of gasoline. R. mucilaginosa S47 and Meyerozyma sp. SP1 showed the highest degradation percentages for gasoline. These results highlight the potential of yeast isolates to be used in the bioremediation of sites highly contaminated with gasoline and petroleum hydrocarbons. |
| Acknowledgements |
| The authors are thankful for the financial support from the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Fundação de Amparo a Pesquisa do Estado de Minas Gerais (FAPEMIG), Pró-Reitoria de Pesquisa da UFMG, Comissão de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Petróleo Brasileiro S.A and Vale S.A. company. |
References
|
Tables and Figures at a glance
| Table 1 | Table 2 | Table 3 |
Figures at a glance
 |
 |
| Figure 1 | Figure 2 |
Relevant Topics
- Anaerobic Biodegradation
- Biodegradable Balloons
- Biodegradable Confetti
- Biodegradable Diapers
- Biodegradable Plastics
- Biodegradable Sunscreen
- Biodegradation
- Bioremediation Bacteria
- Bioremediation Oil Spills
- Bioremediation Plants
- Bioremediation Products
- Ex Situ Bioremediation
- Heavy Metal Bioremediation
- In Situ Bioremediation
- Mycoremediation
- Non Biodegradable
- Phytoremediation
- Sewage Water Treatment
- Soil Bioremediation
- Types of Upwelling
- Waste Degredation
- Xenobiotics
Recommended Journals
Article Tools
Article Usage
- Total views: 15673
- [From(publication date):
February-2014 - Jul 09, 2025] - Breakdown by view type
- HTML page views : 10944
- PDF downloads : 4729
