Research Article Open Access
Isolation and Characterization of Acid Soluble Collagen from the Skin of African Catfish (Clarias gariepinus), Salmon (Salmo salar) and Baltic Cod (Gadus morhua)
Robert Tylingo1*, Szymon Mania1, Anna Panek2, Rafa�?�? Pi�?�?tek3and Roman Paw�?�?owicz4
1Department of Chemistry, Technology and Biotechnology of Food, Gdansk University of Technology, Gabriela Narutowicza Street 11/12, Gdansk 80-233, Poland
2Department of Biology and Biochemistry, University of Bath, Bath BA2 7AX, United Kingdom
3Department of Molecular Biotechnology and Microbiology, Gdansk University of Technology, Gabriela Narutowicza Street 11/12, Gdansk 80-233, Poland
4Department of Chemical and Process Engineering, Gdansk University of Technology, Gabriela Narutowicza Street 11/12, Gdansk 80-233, Poland
- *Corresponding Author:
- Robert Tylingo
Department of Chemistry, Technology and Biotechnology of Food
Faculty of Chemistry, Gdansk University of Technology
ul. Gabriela Narutowicza 11/12
80-233 Gda�?�?sk, Poland
Tel: (48-58) 347-1595
Fax: (48- 58) 347-1246
E-mail: robertt@pg.gda.pl
Received date June 07, 2016; Accepted date June 21, 2016; Published date June 28, 2016
Citation: Tylingo R, Mania S, Panek A, Pi�?�?tek R, Paw�?�?owicz R (2016) Isolation and Characterization of Acid Soluble Collagen from the Skin of African Catfish (Clarias gariepinus), Salmon (Salmo salar) and Baltic Cod (Gadus morhua). J Biotechnol Biomater 6:234. doi:10.4172/2155-952X.1000234
Copyright: © 2016 Tylingo R, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Biotechnology & Biomaterials
Abstract
Acid-soluble collagen (ASC) from the fish skin of African catfish (Clarias gariepinus), Salmon (Salmo salar) and Baltic cod (Gadus morhua) was extracted and characterized. The ASC extraction yield was 75%, 73% and 68%, respectively. The denaturation and melting temperatures of African catfish ASC (29.3°C and 100.0°C) were significantly higher than ASC of Salmon and Baltic cod (20.6°C and 90.5°C; 15.2°C and 86.7°C, respectively), assessed by differential scanning calorimetry. The SDS-PAGE profile showed that each of tested ASC was the type I collagen and consisted of two different α chains, α1 and α2, as well as a β component. The FTIR spectra of all collagens indicate that the overall their chemical compositions are quite similar. The fish skin collagen is easy to prepare and represents a possible resource for use on industrial scale.
Keywords
African catfish; Salmon, Baltic cod; Collagen; Denaturation temperature
Introduction
Collagen is the most abundant protein representing nearly 30% of total proteins in the animal body [1]. It is an element of so-called tropocollagen, a right-handed triple superhelical rod consisting of three polypeptide chains, which occurs in the connective tissues, including tendons, bones and skins [2]. This protein was applied in pharmaceutical but also in biomedical materials, cosmetics and food industries, due to its features such as biocompatibility, non-toxic and well documented physical and chemical properties [3,4]. The in vitro experimental studies highlighted strong potential of matrikines (angiogenesis inhibitors derived from the domains of collagen chains) for the anticancer agents design and development [5]. Another application can be usage of some types of the collagen as a factor enhancing healing processes and proliferation of the cells culturing for transplantation [6]. The main source of industrial use of collagen in the world is bovine waste materials from meat and leather industry: Achilles tendons, leather splits, bones. Due to the almost complete utilization of these raw materials in the above-mentioned industries it is recommended to use an alternative collagen sources for the manufacture of gelatin and other products. In Europe, the consumption of gelatin exceeds its production and increases of 2-3% annually, which requires importation of this component. In addition, the occurrence of the bovine spongiform encephalopathy and reported Creutzfeldt-Jakob disease, in people aged less than 42 years, caused anxiety related with the health safety among consumers eating meat, offal and gelatin of bovine origin. In some cases collagen extracted from pigs can not be used because of the religious reasons [7]. A rich source of previously untapped collagen is skins and so-called hard wastes: heads and backbones of fish. The collagen content in the fish skin is from 14 to 25%, which is similar to the collagen content in the skins of warm-blooded animals. Desirable characteristic of collagen is thermalstability, i.e. a high denaturation and shrinkage temperature (25-36°C and 50-70°C respectively), while for gelatin the short time and high temperature of gelation (gelling temperature and melting point in the range of 20-30°C). Hardness of gel should be in the range from 80 to 300 expressed in Bloom degrees. Collagen derived from fish has a number of advantages compared with bovine and porcine collagen. It is significantly less cross-linked than the two previously mentioned collagens. Therefore, the fish collagen solubility is much higher than bovine and porcine collagen [8]. It allows to obtain the native form of collagen in the final product by using non invasive methods, while getting collagen from bovine and pig skins requires aggressive chemical and/or enzymatic treatment that causes its degradation [9-12]. The shrinkage temperature of the skin collagen isolated from cold-water fish that contains from 5.6 to 8.0% of hydroxyproline is about 40°C. The fish skin collagen coming from warm waters contains from 9.7 to 11% of hydroxyproline and exhibits shrinkage temperature in the range from 50 to 56°C. In contrast, the same temperature in the case of mammals is from 60 to 70°C. Therefore, in order to use the fish waste, the essential characteristic of the source of the raw materials is necessary to perform. It allows to specify the qualitative parameters of the resulting products. For this reason, in this work we report a comparison of fish collagen properties obtained from the skins of African catfish (Clarias gariepinus), Baltic cod (Gadus morhua) and Salmon (Salmo salar), constituting the waste of fish processing.
Material and Methods
Raw materials
To research the skins of three species of fish living in different temperature ranges were used. All fish used in the study come from artificial culture. African catfish (Clarias gariepinus) obtained from Nemex Sp. z o.o. (Warsaw, Poland) with the average size and weight 0.7 m and 1.0 kg, respectively. Optimal temperature for growth of African catfish was 28-30°C (Teugels, 1986). Salmon (Salmo salar) collected from Morpol S.A. (Ustka, Poland) with the average size and weight 0.8 m and 2.5 kg, respectively. Its optimal temperature for growth was 16-18°C. Baltic cod (Gadus morhua) with 5-7°C temperature of habitat collected form Szkuner sp. z o. o. (Wladyslawowo, Poland) with the average size 0.5 m and weight 1.0 kg. Cod is a cold-water species which prefers water temperatures around 10°C. The skins of fresh fish were mechanically separated. The residues of adhering tissues were removed manually. After thorough mixing of the skins, the samples (approximately 500 g) were prepared and stored in polyethylene bags at -20°C until used.
Extraction of acid soluble collagen (ASC)
Pretreatment: In first step the residue lipids from the skin were removed by rinsig with distilled water at 4°C (1:5, w:v), then centrifugation the samples at 2400×g for 5 min at 4°C and then physical separation the upper oil layer. The skin was separated from water and frozen.
Extraction: In the first step the frozen samples were minced in a meat grinder using mesh diameter=3 mm. The fragmented skins were mixed with 0.1 M NaOH solution (1:6, w/v) and kept at 4°C to remove non-collagenous proteins. The process lasted 48 h. After that, treated skins were washed with cold water (4°C) to remove NaOH until the wash water reached neutral pH. The fish skins were bleached with 3% H2O2 solution for 24 h at 4°C for the removal pigments more effectively, and washed with cold water (4°C) again. All procedures were conducted on ice. The extraction of collagen was performed with 0.5 M acetic acid for 72 h with continuous stirring at 4°C. The extract was centrifuged at 2400×g for 30 min at the same temperature. The collagen solution obtained after extraction with acetic acid was placed in dialysis bags (Spectrapor 2 Dialysis Tubing Standard RC12000 D-14000 D) and immersed in double-distilled water until getting 6.5 pH values. The dialysis was carried out at 6oC with constant stirring. The dialysis solution was changed every 24 h. After 7 days of dialysis, process has been completed. Such prepared samples were dried by freeze-drying method (CHRIST Alpha 2-4 LSC, Osterode am Harz, Germany). Obtained collagen was stored in the airtight container at 4°C. All used reagents were of analytical grade.
The composition of skin
The dry weight, fat, ash contents of fish skins were determined according to the method of AOAC [13].
Yield
The yield of ASCs was calculated based on the dry weight of starting material after pretreatment:
Yield (g/100 g)=(Weight of lyophilized collagen)/(Weight of initial dry fish by-product after pretreatment) × 100
Hydroxyproline content
The hydroxyproline content was determined using the colorimetric method recommended by the ISO 3496:1994 [14] after the material was hydrolyzed in 6 M hydrochloric acid for 6 h at 105ºC. The hydrolyzed samples (50 mL) were mixed with a buffered chloramines-T regent (450 mL, pH 6.5), and the oxidation was allowed to proceed for 25 min at room temperature. Ehrilich’s aldehyde reagent (500 mL) was added to each sample, mixed gently and the chromophore was developed by incubating the samples at 65ºC for 20 min. The absorbance of reddish purple complex was measured at 550 nm using Alpha Helios Spectrometer.
Amino acid composition
The dry collagen samples were hydrolyzed under reduced pressure in 6 M HCl at 110°C for 24 h. The hydrolysates were conducted by the Pico Tag method using Pico-tag HPLC system (Pico-tag, Napa, USA). The chromatographic column Waters Pico Tag®, 60Å, 4 μm, 3.9 mm × 300 mm was used. A gradient elution was used with the weak eluent (A) being 0.14 M sodium acetate containing 0.5 ml/L TEA adjusted to pH 6.35 with glacial acetic acid, and the strong eluent (B) was 60% ACN in water. The program was convex cure from 10 to 51% B in 10 min, followed by a washing step at 100% B before returning to initial conditions (10% B). The total flow rate of the column was 1.0 mL/min, injection volume of 8 μL. The detection wavelength was 254 nm. The separation process was monitored by Waters Millenium32 Chromatography Software v. 4.0 Software..
Denaturation temperature (Td) by DSC measurement
Differential scanning calorimetry (DSC) experiments were performed using CSC 6300 Nano-DSC III differential scanning microcalorimeter (Calorimetry Sciences Corp., Lindon, UT) with capillary cell volume of 0.299 mL in the temperatures range: 2 to 50°C, 1 to 60°C and 5 to 60°C for collagen isolated from: Baltic cod, Salmon and African catfish, respectively. The analysis was performed with a scanning rate of 1°Câ�?�?min-1 for all collagens. The experimental data were recorded using DSCRun software (Calorimetry Sciences Corp., Lindon, UT). In the experiments were used collagen samples in 0.1 M acetic acid at concentration of: 0.60 mg × mL−1, 0.50 mg × mL−1 and 0.55 mg × mL−1 for isolates from Baltic cod, Salmon and African Catfish skins, respectively. Before each measurement, the samples were degassed with stirring in an evacuated chamber for 10 min at ice and then carefully loaded into the cells. Calorimetric cells were kept under excess of 0.3 MPa to prevent degassing during the scan. The reversibility of the thermal transition was verified by checking the reproducibility of the calorimetric trace in second heating of the sample after cooling from the first scan and 10 min equilibration at low temperature. A 0.1 M acetic acid dialysis solution obtained for each analyzed collagen was used in both cells to determine baselines that were subtracted from the sample runs to generate the DSC thermograms that were analyzed. To obtain the Cp ex (heat capacity of the collagen solutions) the Nano Analyze software (TA Instruments) was used for a baseline subtraction and determination of the melting temperature.
The melting temperature of collagen fibers
The melting temperature of collagen fibers was determined by Differential Scanning Calorimetry, using TA 3000 calorimeter purchased from Mettler (Texas, USA). To measure, the collagen was weighed into aluminum crucibles in amount of 1-2 mg. The measurement was performed in the temperature range from 25 to 140°C, at a heating rate of 5°C/min.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE)
The SDS-PAGE was performed using 8% separation and 5% stacking gel. Preparations were made at a concentration of 10 mgâ�?�?cm-3 in sample buffer (Tris-HCl, pH 6.8 containing 2-mercaptoethanol, 2% SDS). Before the application on gel, the samples were heated at 50°C for 10 min. A 10 μL sample was loaded per path. As a standard to estimate the molecular weights of proteins High-molecular weight markers (Sigma Chemical Co., St. Louis, Mo, USA) were used. Separation of the proteins was carried out at a flow 70 V for thickening gel and 150 V for the separation gel. Protein bands were stained with Coomassie Brilliant Blue R250 dissolved in water, methanol, and acetic acid (4:5:1, v/v/v) and destained using a solution containing methanol, water and acetic acid (1:8:1, v/v/v).
FTIR measurement
The FTIR spectra of dry collagen preparation were measured using the FTIR spectrometer (Nicolet 8700; Thermo Electron Corp., Waltham, MA) equipped with the Golden Gate (Specac Corp., Oprington, UK) ATR accessory with a single reflection diamond crystal. The temperature of the crystal was maintained at 25.0 ± 0.1°C by using an automatic temperature controller (Specac Corp.) coupled with the ATR accessory. In each measurement, 64 scans were collected with a resolution of 4 cm−1 and the range of 4000-750 cm−1. The spectrum of collagen of the plate was measured and later substracted from every measured spectrum as the background. After measuring all FTIR spectra corresponding to a selected strain and backgroung substraction, the average spectrum was calculated. The spectrometer was purged with dry nitrogen to diminish the negative influence of water vapor.
Statistical analysis
All methods of the collagen extraction and analysis were replicated three times. The mean values with standard deviations (SD) were reported.
Results and Discussion
Isolation of fish skin collagen
The material for the study were skins of Baltic cod, Salmon and African catfish obtained by their mechanically separation from fish. The following table provides basic composition, for enabling the determination the efficiency of collagen protein extraction (Table 1). Pre-treatment of raw materials helped reduce the amount of noncollagenous proteins in preparations and fat, mainly in preparations derived from the skins of Salmon and African catfish. The main component of crude skin protein is collagen. In case of Cod, Salmon and African catfish skin constitutes approximately 80% of the total protein. The collagen content is most often determined based on the hydroxyproline content using an appropriate conversion factor. This makes it possible to determine the efficiency of the fish skin collagen extraction process by determining the content of hydroxyproline in skins and in the resulting mass of the preparation. Determination the exact conversion factor made using two techniques: by calculating from amino acid composition and dialysis carrying out (Table 2). Dialysis was performed as described in section 2.2 [14]. Purified and filtered on the filter paper preparations were frozen and freeze-dried [1]. Knowledge of its value enabled determination of the collagen content in the raw material and efficiency of extraction. The extraction yield was determined comparing collagen content in the raw material, the dialysis and freeze-drying preparations, using a P-Dia conversion factor (Table 2). Losses in the range of 25-30% may be caused by the fact that cross-linked collagens may have limited solubility in dilute organic acid, as well as its some minor fragments in non-helical form were removed during dialysis.
Structure of skin collagen
The amino acid composition: The amino acid composition of collagen from the compared fish skins was shown in the Table 3. Glycine was the major amino acid of each tested collagen, accounted for about 32.2% to 37.1% of total amino acids, depends on the species. Tested collagen samples were mainly composed of alanine, proline, glutamic acid and hydroxyproline, what is similar to collagens from the other fish species [15]. There was no cysteine and tryptophan in each collagen. In addition, in collagen from the skin of African catfish did not have hydroxylysine. Methionine content (from 14 to 19 residues/1000 residues) was the similar value of the other studies [16]. The highest amount of amino acid residues was observed for Salmon skin collagen (193/1000 residues), which was slightly lower than the brown banded bamboo shark skin (204/1000 residues) [17] and striped catfish skin (206/1000 residues) [18]. Amounts of amino acid residues in collagen from African catfish (161/1000 residues) and Baltic cod (156/1000 residues) were lower than compared literature values. The amino acid content in each case was lower than 22% of porcine skin collagen [19]. The highest degree of hydroxylation of proline residues was achieved for African catfish skin collagen (43.5%), similar to the 43% of channel catfish [20]. Results for Salmon and cod collagen were 33.7% and 34.6%, similar to the Malaysian catfish – 29.7% [21] and somewhat less than walleye polloc – 37.5% [22].
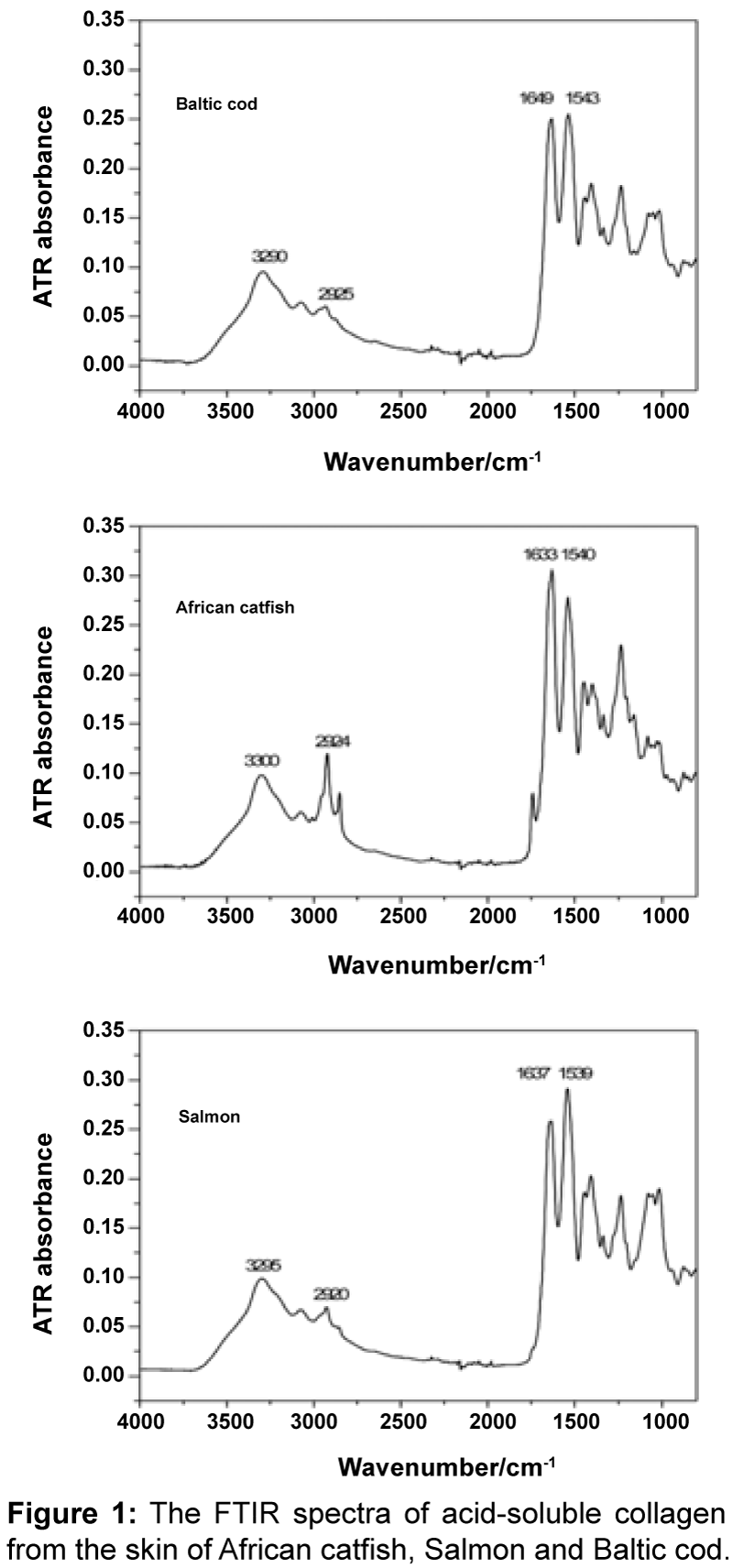
FTIR spectroscopy: The FTIR spectra of Baltic cod, African catfish and Salmon collagens are presented in Figure 1. The IR spectra of ASCs are similar and have characteristic absorbency peaks corresponding to amide I, II, A, and B bands. Absorption bands corresponding to N-H stretching vibrations (amide A) occur in the wavenumber range of 3400-3440 cm-1. Amide A bands of African catfish, Salmon and Baltic cod ASCs were found at 3300, 3295, 3290 cm-1, respectively. A shift of the amide A in this group may indicate that N-H bonds are involved in a slightly different hydrogen bond network [23]. Peaks related to the asymmetrical stretch of alkyl groups, were found at 2924 cm-1 (African catfish), 2920 cm-1 (Salmon) and 2925 cm-1 (Baltic cod). The amide I band (1600–1700 cm-1) corresponds to the carbonyl group stretching vibrations and can be used to analyze the secondary structure of protein [23]. The maxima of these bands for African catfish, Salmon and Baltic cod collagens spectra were found at 1633, 1637 and 1649 cm-1, respectively. This means that secondary structures of these collagens are significantly different. The amide II band was found at ca. 1550 cm-1 and can be attributed to N-H in-plane bend and CN stretching vibrations [23]. The band position does not varies a lot (1540 cm-1 - African catfish, 1539 cm-1 – Salmon, and 1543 cm-1 - Baltic cod), although some variations in intensity may be observed (in relation to the amide I band intensity). The IR spectra of all collagens indicate that the overall their chemical compositions are quite similar. However, the differences in the shape of the amide I and II suggest that secondary structure differs slightly. These structural differences may translate into different properties of samples or be an effect of different sample preparation.
| Skin | Dry weight [%] | *Total nitrogen [%] | Fat content [%] | Ash [%] |
|---|---|---|---|---|
| Cod | 30.0 ± 0.71 | 4.5 ± 0.008 | 1.03 ± 0.18 | 2.2 ± 0.03 |
| Salmon | 31.0 ± 0.68 | 4.3 ± 0.009 | 0.91 ± 0.23 | 3.1 ± 0.04 |
| African catfish | 29.2 ± 0.64 | 4.7 ± 0.009 | 1.01 ± 0.15 | 2.3 ± 0.01 |
*The amount of protein is the total nitrogen multiplied by universal conversion factor equal: 6.25.
Table 1: The composition of used raw materials.
| Collagen | Hydroxyproline content in raw material [%] | Hydroxyproline content isolated colagen preparation [%] | Extraction efficiency [%] | P-Dia* | P-AAC** |
|---|---|---|---|---|---|
| Cod | 1.411 ± 0.0321 | 6.50 ± 0.142 | 68 | 15.38 | 15.41 |
| Salmon | 1.714 ± 0.0317 | 8.03 ± 0.151 | 73 | 12.45 | 12.66 |
| African catfish | 1.615 ± 0.0413 | 8.25 ± 0.173 | 75 | 12.12 | 12.01 |
*P-Dia – Conversion factor determined by dialysis carrying out **P-AAC – Conversion factor determined based on amino acid composition (Table 3)
Table 2: Extraction efficiency and conversion factor used for collagen content calculating.
| Amino acid | Arfican catfish | Salmon | Baltic cod |
|---|---|---|---|
| Hydroxyproline | 70.5 ± 0.5 | 65.0 ± 0.4 | 54.5 ± 0.5 |
| Aspartic acid | 47.2 ± 0.2 | 56.3 ± 0.3 | 50.5 ± 0.6 |
| Threonine | 29.3 ± 0.3 | 20.4 ± 0.2 | 23.3 ± 0.2 |
| Serine | 33.7 ± 0.2 | 51.0 ± 0.2 | 66.4 ± 0.4 |
| Glutamic acid | 78.1 ± 0.1 | 72.5 ± 0.5 | 72.0 ± 0.5 |
| Glycine | 322.6 ± 0.2 | 371.3 ± 1.0 | 347.9 ± 0.2 |
| Alanine | 110.0 ± 0.4 | 102.3 ± 0.9 | 101.4 ± 0.1 |
| Cysteine | - | - | - |
| Valine | 22.1 ± 0.3 | 14.5 ± 0.5 | 15.2 ± 0.1 |
| Methionine | 14.6 ± 0.1 | 17.8 ± 0.6 | 19.2 ± 0.2 |
| Isoleucine | 12.9 ± 0.3 | 10.9 ± 0.4 | 10.5 ± 0.1 |
| Leucine | 28.1 ± 0.4 | 21.0 ± 0.1 | 24.6 ± 0.5 |
| Tyrosine | 6.1 ± 0.1 | 2.0 ± 0.1 | 5.0 ± 0.2 |
| Phenylalanine | 19.2 ± 0.2 | 12.2 ± 0.4 | 19.3 ± 0.3 |
| Lysine | 31.7 ± 0.6 | 20.2 ± 0.1 | 27.3 ± 0.3 |
| Histidine | 7.5 ± 0.1 | 12.5 ± 0.6 | 6.4 ± 0.1 |
| Arginine | 49.3 ± 0.3 | 56.8 ± 0.2 | 53.2 ± 0.7 |
| Proline | 123.0 ± 0.2 | 96.5 ± 0.1 | 102.8 ± 0.8 |
| Hydroxylysine | - | 3.1± 0.1 | 7.1 ± 0.1 |
| Tryptophan | - | - | - |
| Molar mass [kDa] | 110146 ± 13 | 107809 ± 15 | 109027 ± 11 |
-amino acid not present in the sample Values are mean ± SD (n=3, P<0.05)
Table 3: Amino acid composition of fish skin collagen isolated from African catfish, Salmon and Baltic cod (residues per 1000 total amino acid residues).
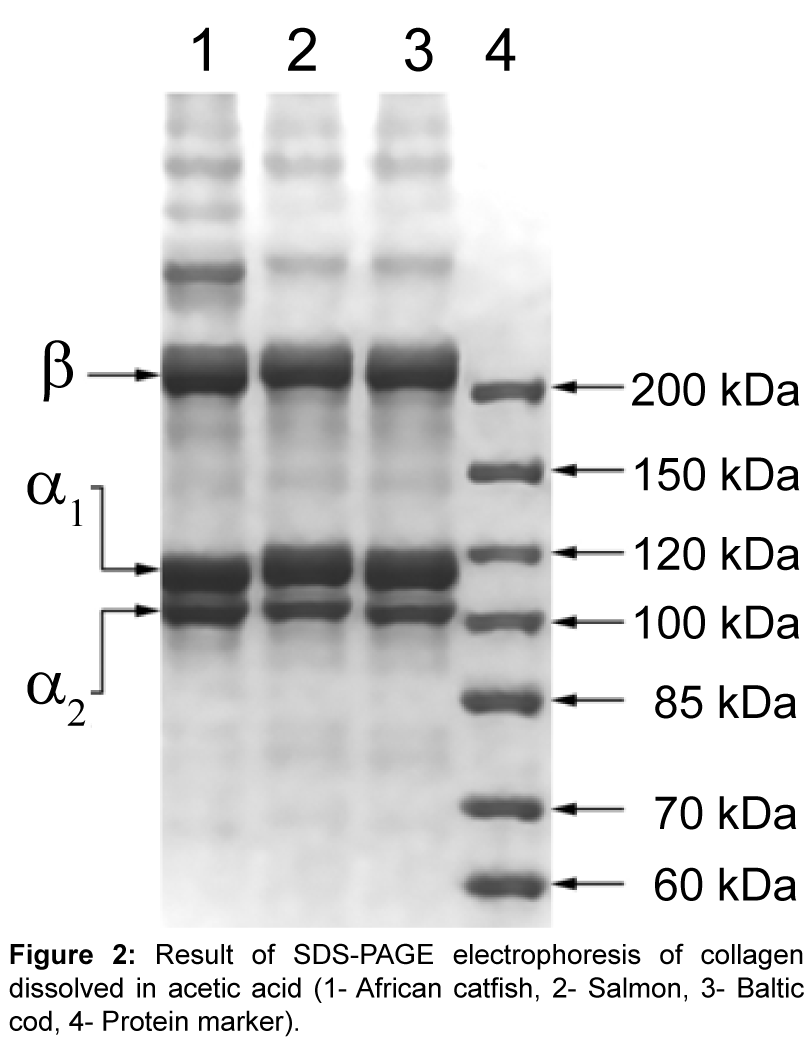
SDS-PAGE electrophoresis: Samples of African catfish, Salmon and Baltic cod collagen, dissolved in acetic acid, were subjected to electrophoresis in 8% separating gel in denaturing conditions (Figure 2). All tested samples of collagen showed the same distribution of the individual components of which protein was made. Subunits migrated at the same rate. The collagens consisted of chains: α (α1 and α2 chains - two distinct types of varying the rate of migration) and β dimer (β chain), but γ elements were not visible. The existence of at least two different subunits showed that the main of collagen from the researched fish skins could be collagen type I. Based on the electrophoretic patterns of collagen, it was impossible to accurately determine the presence of the α3 chain (the identical migration rate like the α1 chain) [24]. The sample was not completely hydrolyzed because on gel could not be seen additional bands indicative of the presence of other proteins or contamination of the sample.
Thermal stability of ASC
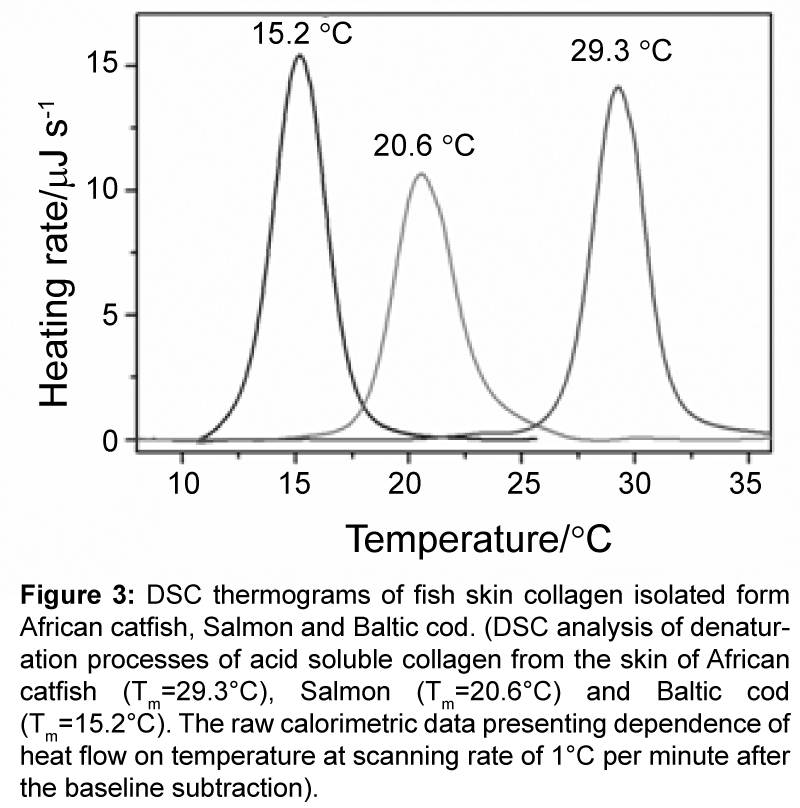
To investigate the denaturation temperatures of the ASC, a series of DSC experiments, with scanning rate of 1.0°C �?�? min-1, have been conducted. The DSC thermograms presenting experimental dependencies of excess heat capacity (Cpex) on temperature for analyzed collagens are represented on the Figure 3.
The ASC from Baltic cod, Salmon and African catfish denatures thermally at temperatures: 15.2°C, 20.6°C and 29.3°C, respectively. In the reheating scan no visible endothermic effects were detected, indicating that the transition process was irreversible for all analyzed ASC. The observed denaturation peaks are typical as determined so far for other collagen molecules and characterize by the very small of 0.2 J/Kg heat capacity increment of unfolding (ΔCpex). The determined denaturation temperatures of skin collagen from Baltic cod, Salmon and African catfish well correlate with data for other fish: 10°C (ice fish) [25] 18°C (halibut) [26]; 25.0°C (bullhead shark), 25.6°C (chub mackerel), 26.5°C (Japanese sea-bass) [8]; 28.6°C (bigeye snapper) [27] and 34.8°C (silver carp) [10]. These melting temperatures well correlate with temperatures of fish habitat and are higher for warmwater species (Table 4). Published so far thermodynamic data underline strong correlation between collagen melting enthalpies and the content of hydroxyproline [26]. The skin collagen from Baltic cod, Salmon and African catfish contain 54, 65 and 70 hydroxyproline per 1000 residues, respectively. This very well correlates with determined melting temperatures of these ASC.
Conclusion
The most resistant to denaturation turned out to collagen isolated from the skins of African catfish, which denaturation temperature is 29.3°C. Although it is a value lower than that of mammalian collagen, provides less restrictive conditions for the isolation and processing, whereby represents a possible resource for use on an industrial scale. Despite the each of tested collagen high share of glycine, alanine and proline in each studied collagens, their amino acid compositions were different. Although each of the tested collagens has a high content of amino acids such as proline and hydroxyproline, the total content of these residues were the highest in the case of collagen from the skin of African catfish. This confirms dependence, the higher the content of the hydroxyproline, the higher denaturation temperature of collagen preparation. The highest stability of collagen obtained from the African catfish skin was also confirmed by melting temperature determination by DSC measurement. The rationality of results also confirmed the fact that the African catfish, opposed to the Salmon and Cod, is a species living in warm waters. Moreover, electrophoresis and FTIR analysis showed no differences in the construction in all of the researched fish skin collagens. The knowledge about their properties gives possibility to introduce chemical and enzymatic modifications to get the preparations with established and desirable properties.
| Collagen | Melting temperature [°C] |
|---|---|
| African catfish | 100.0 ± 0.51 |
| Salmon | 90.5 ± 0.67 |
| Baltic cod | 86.7 ± 0.31 |
Table 4: Melting temperatures of fish skin collagens obtained by Differential Scanning Calorimetry measurement.
Figure 3: DSC thermograms of fish skin collagen isolated form African catfish, Salmon and Baltic cod. (DSC analysis of denaturation processes of acid soluble collagen from the skin of African catfish (Tm=29.3°C), Salmon (Tm=20.6°C) and Baltic cod (Tm=15.2°C). The raw calorimetric data presenting dependence of heat flow on temperature at scanning rate of 1°Cper minute after the baseline subtraction).
Acknowledgement
The authors acknowledge with thanks the financial support received under research Grant No. N312 258540 from the Ministry of Science and Higher Education. The authors express their gratitude to Ph.D. Piotr Bruzdziak for his help in preparing FTIR spectra and their analysis.
References
- Pati F,Adhikari B, Dhara S (2010) Isolation and characterization of fish scale collagen of higher thermal stability. BioresourTechnol 101: 3737-3742.
- Muyonga JH, Cole CGB, DuoduKG (2004) Characterisation of acid soluble collagen from skins of young and adult Nile perch (Latesniloticus). Food Chem 85: 81–89.
- Hoyer B, Bernhardt A, Lode A, Heinemann S, Sewing J, et al. (2014) Jellyfish collagen scaffolds for cartilage tissue engineering. ActaBiomater 10: 883-892.
- Jo I, Lee JM, Suh H, Kim H (2007) Bone tissue engineering using marrow stromal cells. Biotechnol Bioprocess Eng 12: 48-53.
- Monboisse JC,Oudart JB,Ramont L,Brassart-Pasco S,Maquart FX (2014) Matrikines from basement membrane collagens: A new anti-cancer strategy. BiochimBiophysActa 1840: 2589-2598.
- Ramanauskaite G, �?½alalyte D, Kašeta V, Vaitkuviene A, Kalediene L, et al. (2014) Skin extracellular matrix components accelerate the regenerative potential of Lin-cells. Cent Eur J Biol 9: 367-373.
- Zelechowska E, Sadowska M, Turk M (2010) Isolation and some properties of collagen from the backbone of Baltic cod (Gadusmorhua). Food Hydrocolloid 24: 325-329.
- Nagai T, Suzuki N (2000) Isolation of collagen from fish waste material skin, bone and fins. Food Chem 68: 277–281.
- Fernandes RMT, CoutoNeto RG, Paschoal CWA, Rohling JH, Bezerra CWB (2008) Collagen films from swim bladders: Preparation method and properties. Colloids Surf B Biointerfaces62: 17-21.
- Rodziewicz-Motowidlo S, Sladewska A, Mulkiewicz E, Kolodziejczyk A, Aleksandrowicz A, et al. (2008) Isolation and characterisation of a thermally stable collage preparation from the outer skin of the silver carp (Hypophthalmichthysmolitrix). Aquaculture 285: 130-134.
- Sadowska M, Kolodziejska I, Niecikowska C (2003) Isolation of collage from the skin of Balic cod (Gadusmorhua). Food Chem 81: 257-262.
- Senaratne LS, Park PJ, Kim SK (2006) Isolation and characterization of collagen from brown backed toadfish (Lagocephalusgloveri) skin. BioresourTechnol 97: 191-197.
- Helrich K (1990) AOAC. In: Official methods of analysis. Virginia.
- Anonymous (1978) Meat and meat products-determination of L(-)hydroxyproline content (reference method). International Standard ISO 3496: 1994(E).
- Skopinska-Wisniewska J,Olszewski K,Bajek A,Rynkiewicz A,Sionkowska A (2014) Dialysis as a method of obtaining neutral collagen gels. Mater SciEng C Mater BiolAppl 40: 65-70.
- Minh Thuy le T, Okazaki E, Osako K (2014) Isolation and characterization of acid-soluble collagen from the scales of marine fishes from Japan and Vietnam. Food Chem 149: 264-270.
- Wang L, An X, Yang F,Xin Z, Zhao L, et al. (2008) Isolation and characterisation of collagens from the skin, scale and bone of deep-sea redfish (Sebastesmentella). Food Chem 108: 616-623.
- Kittiphattanabawon P, Benjakul S, Visessanguan W, Shahidi F (2010) Comparative study on characteristics of gelatin from the skins of brownbanded bamboo shark and blacktip shark as affected by extraction conditions.Food Hydrocolloid 24: 164-171.
- Singh P, Benjakul S, Maqsood S, Kishimura H (2011) Isolation and characterisation of collagen extracted from the skin of striped catfish (Pangasianodonhypophthalmus). Food Chem 124: 97–105.
- Ikoma T, Kobayashi H, Tanaka J, Walsh D, Mann S (2003) Physical properties of type I collagen extracted from fish scales of Pagrus major and Oreochromisniloticas. Int J BiolMacromol 32: 199-204.
- Liu HY, LiD, Guo SD (2007) Studies on collagen from the skin of channel catfish (Ictaluruspunctaus). Food Chem 101: 621–625.
- Kiew PL, Mashitah MD (2013) Isolation and characterization of collagen from the skin of Malaysian catfish (HybridClariassp.). J Korean SocAppl Bi 56: 441-450.
- Yan M, Li B, Zhao X, Ren G, Zhuang Y, et al. (2008) Characterization of acid-soluble collagen from the skin of walleye pollock (Theragrachalcogramma). Food Chem 107: 1581-1586.
- Jeong H, Venkatesan J, Kim S (2013) Isolation and characterization of collagen from Marine fish (Thunnusobesus). Biotechnol Bioprocess Eng 18: 1185-1191.
- Kimura S, Takema Y, Kubota M (1981) Octopus skin collagen. Isolation and characterization of collagen comprising two distinct alpha chains. J BiolChem 256: 13230-13234.
- Burjanadze TV (1979) Hydroxyproline content and location in relation to collagen thermal stability. Biopolymers 18: 931-938.
- Privalov PL (1982) Stability of proteins. Proteins which do not present a single cooperative system. Adv Protein Chem 35: 1-104.
Relevant Topics
- Agricultural biotechnology
- Animal biotechnology
- Applied Biotechnology
- Biocatalysis
- Biofabrication
- Biomaterial implants
- Biomaterial-Based Drug Delivery Systems
- Bioprinting of Tissue Constructs
- Biotechnology applications
- Cardiovascular biomaterials
- CRISPR-Cas9 in Biotechnology
- Nano biotechnology
- Smart Biomaterials
- White/industrial biotechnology
Recommended Journals
Article Tools
Article Usage
- Total views: 16503
- [From(publication date):
June-2016 - Mar 31, 2025] - Breakdown by view type
- HTML page views : 15012
- PDF downloads : 1491