Isolated Splenic Metastasis in a Patient with Lung Carcinoma: Case Report and Review of the Literature
Received: 25-Aug-2015 / Accepted Date: 17-Sep-2015 / Published Date: 20-Sep-2015 DOI: 10.4172/2161-0681.1000252
Abstract
The prevalence of metastasis to the spleen is low in cancer patients. Splenic metastasis usually occurs only at the terminal stages of disease progression and is associated with multiple metastases in other sites. Only 19 cases of isolated splenic metastasis in patients with primary lung cancer have been reported in the literature. We report a case of isolated splenic metastasis in a patient with adenocarcinoma of the lung identified by CT scan and confirmed by pathologic examination and immunohistochemistry. Follow up positron emission tomography (PET) scan also identified sacral metastasis. The use of advanced imaging modalities such as PET scan may identify additional metastatic foci in patients who were previously considered to have isolated splenic metastasis.
Keywords: Lung adenocarcinoma; Isolated splenic metastasis; Positron emission tomography; Thyroid transcription factor-1
323204Abbreviations
PET: Positron Emission Tomography; CT: Computed Tomography; MRI: Magnetic Resonance Imaging; TTF-1: Thyroid Transcription Factor-1
Introduction
Lung cancer is the most common malignancy worldwide. The predominant histologic types are adenocarcinoma, squamous carcinoma, small cell carcinoma and large cell carcinoma. Among them, adenocarcinoma is the predominant type, which accounts for 40% of cases by 2006-2010 in newly published WHO Classification of Tumors of Lung, Pleura, Thymus and Heart. Lung cancers commonly spread through lymphatic or haematogenous route. Lymphatic metastases result in the involvement of ipsilateral or contralateral hilar or mediastinal lymph nodes. The liver, bone, brain and adrenal glands are frequent organs where the tumor cells metastases via blood-borne route. Primary lung cancer can also spread along the pleural surface. It is uncommon that non-hematopoietic cancers including lung cancer, metastases to the spleen. In patients with the lung cancer, splenic metastases are generally seen in the advanced stage. The cancers that have already widely spread in a body, involving multiple organs, are usually associated with a poor prognosis. Nevertheless, isolated splenic metastases are rare. A review of the literature identifies only 19 cases associated with primary lung cancer [1-3]. Here, we report a case of isolated splenic metastasis 17 months after diagnosis of a primary lung cancer.
Clinical History
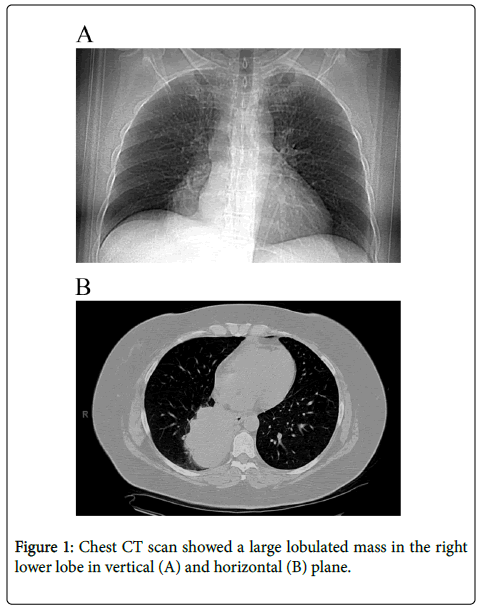
The patient is a 56 year old, female non-smoker. She had an intractable cough for 1 to 2 years, and was treated for allergies. Chest X-ray in June 2013, showed a right lower lobe density, and subsequent CT scan identified a 9.6 × 8.0 cm lobulated mass in the right lower lobe of the lung with right hilar and subcarinal adenopathy (Figure 1). Staging PET and brain MRI showed no metastases. Transbronchial biopsy demonstrated moderately differentiated adenocarcinoma, which was thyroid transcription factor-1 (TTF-1) positive by immunohistochemistry.
Before definitive resection, the patient received chemotherapy with 4 cycles of pemetrexed-cisplatin and concurrent chest 63 Gy radiation therapy with significant reduction in tumor size upon imaging. She underwent a right lower lobectomy and mediastinal lymph node dissection five months after initial presentation. Pathologic examination of the lobectomy specimen revealed a solid mass with focal necrosis measuring 5.0 × 3.8 × 3.8 cm in size. The final pathology of the residual mass showed moderately differentiated adenocarcinoma. All surgical resection margins were uninvolved by malignancy. Eighteen mediastinal and intrapulmonary lymph nodes were free of metastatic disease. The tumor was staged as ypT2a, ypN0.
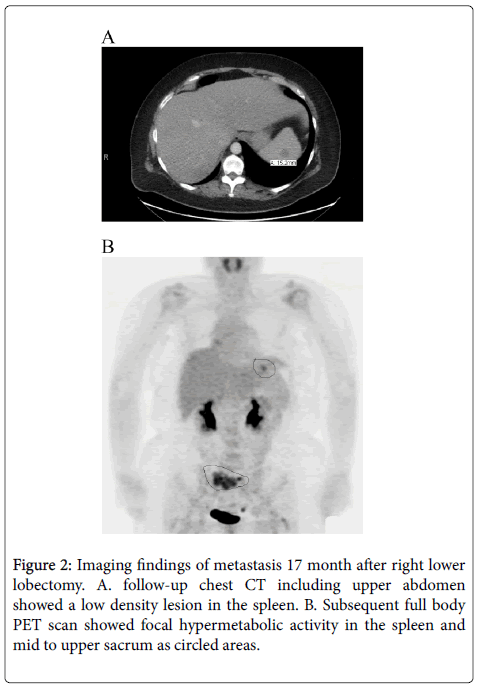
After surgery, the patient was disease free for seventeen months when surveillance CT scan identified a 1cm solitary low-density lesion in the spleen. Subsequent PET demonstrated hypermetabolic activity in the spleen as well as in the sacrum (Figure 2).
Brain MRI showed no metastases. The patient received chemotherapy followed by radiotherapy to the sacral lesion. Follow-up PET showed that hypermetabolic activity in the spleen was resolved and there was interval improvement in the sacrum. Total splenectomy was performed to remove any residual tumor.
Pathologic Findings
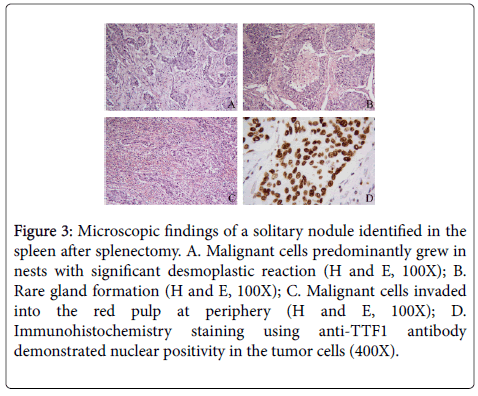
One 1.2 cm red-brown nodule was identified in the splenectomy specimen. Sectioning revealed the nodule to have a yellow center and red-brown periphery. Microscopic examination showed replacement of splenic tissue by tumor cells and surrounding prominent desmoplasia. The tumor cells predominantly grew in nests or cords, although focal rare glandular structures were present. At the edge of the nodule, the tumor cells invaded into the red pulp. Tumor cells showed strong nuclear positivity for TTF-1. Morphologic and immunochemical results were consistent with metastatic adenocarcinoma from the lung primary (Figure 3).
Figure 3: Microscopic findings of a solitary nodule identified in the spleen after splenectomy. A. Malignant cells predominantly grew in nests with significant desmoplastic reaction (H and E, 100X); B. Rare gland formation (H and E, 100X); C. Malignant cells invaded into the red pulp at periphery (H and E, 100X); D. Immunohistochemistry staining using anti-TTF1 antibody demonstrated nuclear positivity in the tumor cells (400X).
Clinical Follow-Up
The patient was discharged on the second postoperative day. A PET scan one month after splenectomy showed benign postoperative changes in the splenic bed and significant improvement of the sacral metastasis.
Discussion
Compared to other organs, the spleen is relatively well-protected from metastasis from non-hematopoietic malignancy due to its anatomy and physiology. Anatomically, the spleen's lack of afferent lymphatic channels reduces its likelihood of receiving metastatic cancer cells from the lymphatic system. Physiologically, the spleen has a high concentration of angiogenesis inhibition factor, resulting in an environment that does not favour growth of metastatic cancer cells [4,5]. Additionally, the high density of splenic lymphoid cells plays a role in immune surveillance, helping to remove any lodging cancer cells before they are able to proliferate. The incidence of splenic metastasis in non-hematopoietic malignancy is therefore very low: 4.4% and 3.0% in large autopsy studies [5,6]. Lung and breast cancer, as well as melanoma, are the most common tumors which metastasize to the spleen [5-7].
The reported incidence of splenic metastasis from primary lung cancer is 1.2 to 5.6% [8,9]. In this setting, splenic metastasis is frequently seen in the terminal stage as part of a diffuse metastatic process, where an average of 3 to 6 other organs are typically involved [5,6]. In two independent autopsy studies performed in 1995 and in 2001, all patients with splenic metastasis associated with lung cancer had disseminated abdominal disease [8,9]. The interval between the diagnosis of the primary lung tumor and splenic lesion varies from 0 to 8 months [10].
Very rarely, isolated splenic metastasis, where the spleen is the only site of metastasis, may be seen in patients with lung cancer [1,11]. Most patients with isolated splenic metastasis of lung cancer were asymptomatic (10/19) [1-3] as was our patient. The metastasis was most commonly identified on routine follow up. Occasionally patients presented with abdominal pain (5/19), splenic rupture (3/19) or fever (1/19). Pulmonary adenocarcinoma was seen most often in isolated splenic metastasis (8/19), followed by squamous cell carcinoma (6/19). Large cell undifferentiated carcinoma, poorly differentiated carcinoma, and carcinoid tumor can also cause isolated metastasis to the spleen [1]. The majority of isolated splenic metastases are associated with tumors in the left lung (11/19); however, recently reports show an increase in right lung primaries as seen in our case [1-3].
Grossly, splenic metastasis presents in three patterns: macronodular, micronodular and diffuse [7,8,10]. In the macronodular pattern, the metastasis can be either solitary or multiple nodules. The micronodular pattern is characterized by the presence of scattered uniform miliary nodules. In the diffuse pattern, the splenic parenchyma is completely replaced by tumor cells [7]. On imaging, splenic metastases can appear as solid (as in our case), cystic or solidcystic lesion [1,12].
In a patient with a history of malignancy, any new indeterminate splenic lesion should be considered as a metastasis, until proven otherwise. A whole body scan should be provided to search for additional metastases. In our case, follow-up CT scan identified a new indeterminate low-density lesion in the spleen, and a subsequent PET found sacral metastasis. Although, there are no guidelines for treating isolated splenic metastasis splenectomy was performed in most reported cases. Splenectomy not only eliminates malignant deposits but also prevents spontaneous splenic rupture secondary to metastasis. In our case, the spleen was initially felt to be the sole metastatic focus. It was only by whole body PET scan that another hypermetabolic focus in the sacrum was identified. It is likely that patients who were previously considered to have isolated splenic metastasis would also have other metastatic foci if whole body PET scans were performed. The prognostic significance of isolated splenic metastasis is unclear due to the rarity of reported cases. The detection of isolated splenic metastasis can be synchronous or metachronous to the primary tumor, and may be identified between two months to eight years after diagnosis of primary lung cancer. The vast majority of reported patients died 1 to 49 months post splenectomy, but rare cases of prolonged survival up to 8 years after splenectomy have been reported [2]. With the advance of imaging techniques and improvement in follow-up care of cancer patients, reported isolated splenic metastasis has more than doubled since the year 2000. With increasing case numbers, further study may help to clarify the clinical significance of isolated splenic metastasis in primary lung cancer.
References
- Dias AR, Pinto RA, Ravanini JN, Lupinacci RM, Cecconello I, et al. (2012) Isolated splenic metastasis from lung squamous cell carcinoma. World J Surg Oncol 10: 24.
- Sardenberg RA, Pinto C, Bueno CA, Younes RN (2013) Non-small cell lung cancer stage IV long-term survival with isolated spleen metastasis. Ann Thorac Surg 95: 1432-1434.
- Eisa N, Alhafez B, Alraiyes AH, Alraies MC (2014) Abdominal pain as initial presentation of lung cancer. BMJ Case Rep 2014.
- Sánchez-Romero A, Oliver I, Costa D, Orduña A, Lacueva J, et al. (2006) Giant splenic metastasis due to lung adenocarcinoma. Clin Transl Oncol 8: 294-295.
- Berge T (1974) Splenic metastases. Frequencies and patterns. Acta Pathol Microbiol Scand A 82: 499-506.
- Schon CA, Gorg C, Ramaswamy A, Barth PJ (2006) Splenic metastases in a large unselected autopsy series. Pathol Res Pract 202: 351-356.
- Comperat E, Bardier-Dupas A, Camparo P, Capron F, Charlotte F (2007) Splenic metastases: clinicopathologic presentation, differential diagnosis, and pathogenesis. Arch Pathol Lab Med 131: 965-969.
- Satoh H, Watanabe K, Ishikawa H, Yamashita YT, Ohtsuka M, et al. (2001) Splenic metastasis of lung cancer. Oncol Rep 8: 1239-1241.
- Kinoshita A, Nakano M, Fukuda M, Kasai T, Suyama N, et al. (1995) Splenic metastasis from lung cancer. Neth J Med 47: 219-223.
- Lam KY, Tang V (2000) Metastatic tumors to the spleen: a 25-year clinicopathologic study. Arch Pathol Lab Med 124: 526-530.
- Schmidt BJ, Smith SL (2004) Isolated splenic metastasis from primary lung adenocarcinoma. South Med J 97: 298-300.
- Tang H, Huang H, Xiu Q, Shi Z (2010) Isolated splenic metastasis from lung cancer: ringleader of continuous fever. Eur Respir Rev 19: 253-256.
Citation: Cai Q, Kragel P (2015) Isolated Splenic Metastasis in a Patient with Lung Carcinoma: Case Report and Review of the Literature. J Clin Exp Pathol 5:252. DOI: 10.4172/2161-0681.1000252
Copyright: © 2015 Cai Q et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 17343
- [From(publication date): 12-2015 - Jul 02, 2025]
- Breakdown by view type
- HTML page views: 16367
- PDF downloads: 976