Isolated Sphenoid Sinus Disease: The MERF Experience
Received: 06-Feb-2019 / Accepted Date: 04-Mar-2019 / Published Date: 11-Mar-2019 DOI: 10.4172/2161-119X.1000364
Abstract
Introduction: Isolated disease of the sphenoid sinus is rare and represents 1 to 2% of all sinus disease. Symptoms can be vague and patients may present with headache, rhinorrhea and postnasal drip. Early identification and management is crucial to avert potential intracranial and ophthalmic complications.
Methods: A retrospective study of isolated sphenoid sinus disease was done in a tertiary ENT centre between Jan 1998 to Jan 2018 and the etiology, diagnosis and management of the various lesions was systematically evaluated.
Results: Forty seven cases of isolated sphenoid disease treated between Jan 1998 to Jan 2018 were evaluated by ENT examination, diagnostic nasal endoscopy, computed tomography of the paranasal sinuses, and in some cases with magnetic resonance imaging as needed. The most common symptom was headache (72%). The various etiologies included Allergic fungal rhino sinusitis (AFRS) (36%), acute or chronic inflammation (26%), mucocele (23%), cerebrospinal fluid leak (6%), sphenochoanal polyps (4%),coexisting mucocele with fungal sinusitis (2%) and giant cell reparative granuloma (2%). Endoscopic surgery was effective in treating all the cases of isolated sphenoid sinus disease. Conclusion: Awareness of the potential neuro-ophthalmic complications of sphenoid sinus disease is crucial. The key to successful management of isolated sphenoid sinus disease is early identification of the subclinical manifestations and timely treatment.
Keywords: Isolated sphenoid sinus disease; Endoscopic sinus surgery
Introduction
Isolated disease of the sphenoid sinus is infrequently seen in otolaryngology practice and represents 1 to 2% of all sinus disease. In most cases, the disease has an inflammatory origin, and a neoplastic cause is rare [1]. Most patients have non-specific and vague symptoms which may delay the diagnosis. The clinical presentation includes headache, nasal obstruction, rhinorrhea, postnasal drip etc. Proetz listed 13 structures adjacent to the sphenoid sinus that may be affected: cranial nerves II, III, IV, V, VI, dura mater, pituitary gland, cavernous sinus, internal carotid artery, sphenopalatine ganglion, sphenopalatine artery, pterygoid canal and the nerve [2]. Therefore, sphenoid sinus disease, if not treated early can potentially result in serious complications. Patients may then present with complications such as cranial nerve deficits, visual alteration such as visual loss or diplopia and pain or numbness [3]. The aim of the current study was to evaluate the various causes of isolated sphenoid disease, its complications and to assess the outcomes of management in a cohort who were managed in a single tertiary referral rhinology clinic in South India.
Methods
A retrospective study of all isolated sphenoid sinus disease found among adults presenting from Jan 1998 to Jan 2018 (spanning 20 years) in a tertiary care setting was done. There were twenty four men and twenty three women in the study with an age range of 19 to 70 years (mean age-46 years). The protocol for diagnosis included a comprehensive otorhinolaryngology examination, nasal endoscopy and imaging which included a Computed tomography (CT) scan of the nasal cavities and paranasal sinuses. In some patients, an Magnetic resonance imaging (MRI) scan was done as indicated to detect the presence of cerebrospinal fluid leak and when patients presented with visual changes. The etiology of sphenoid sinus disease was investigated and found to be benign or malignant pathologies (acute or chronic sphenoid sinusitis, cerebrospinalfluid leak, mucocele, sphenochoanal polyp, AFRS, giant cell granuloma). Exclusion of general medical diseases was done by blood tests and a chest X-ray. All these patients were treated by Functional endoscopic sinus surgery (FESS) by an experienced rhinosurgical team as well as with appropriate medical management. None of the patients required an open procedure or neurosurgical intervention. Absence of sinonasal symptoms and normal post-operative endoscopy was taken as the criteria for improvement after the surgery. For the statistical analysis of outcomes, the Chi-square test was used. The follow- up period in our cohort ranged from 8 months to 10 years and the mean follow-up period was 46 months.
Results
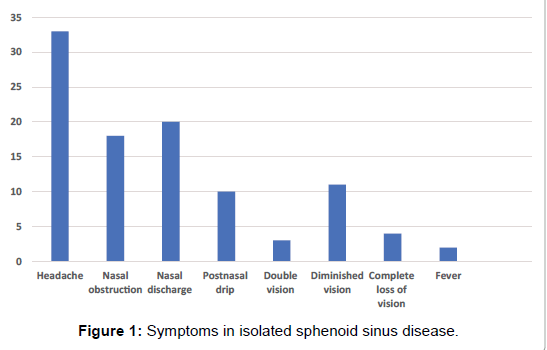
A retrospective study of forty seven patients with isolated sphenoid sinus disease was done over a twenty-year period in a tertiary care hospital. The age range of the patients was 19 to 70 years (mean age- 46 years) and there were twenty four men and twenty three women in the study. Inclusion criteria included patients with disease confined to the sphenoid sinus. Patients who had disease in the other paranasal sinuses were excluded from the study. The most common symptom was headache which was mainly retro-orbital (33 patients) followed by nasal discharge (20 patients), nasal obstruction (18 patients), postnasal drip (10 patients), fever (2 patients), double vision (3 patients), diminished vision (11 patients), complete loss of vision (4 patients) (Figure 1).
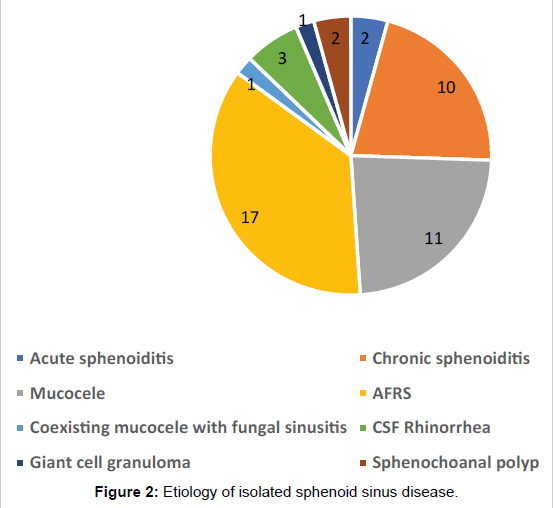
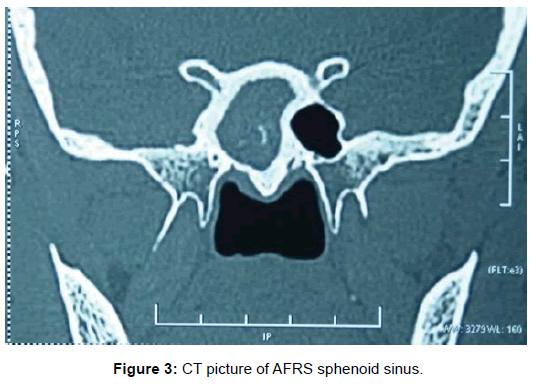
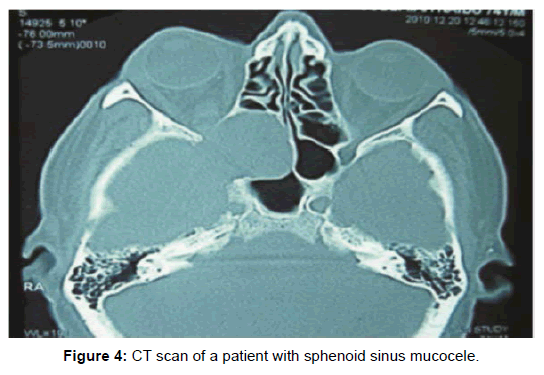
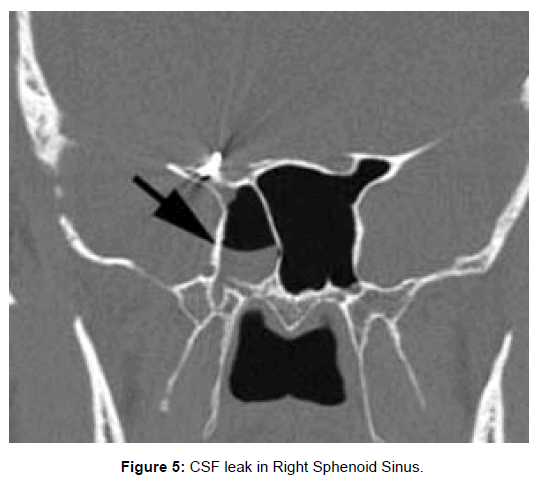
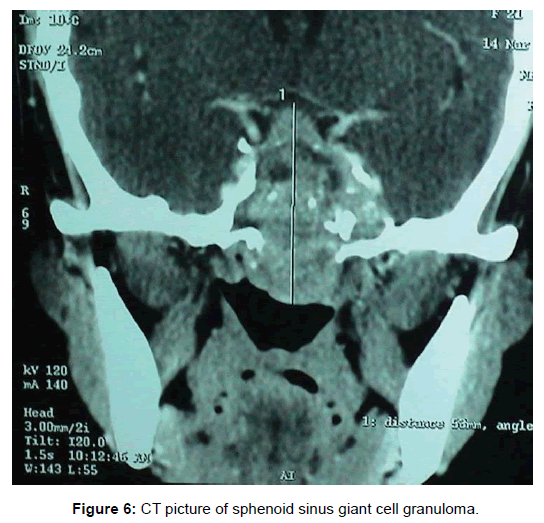
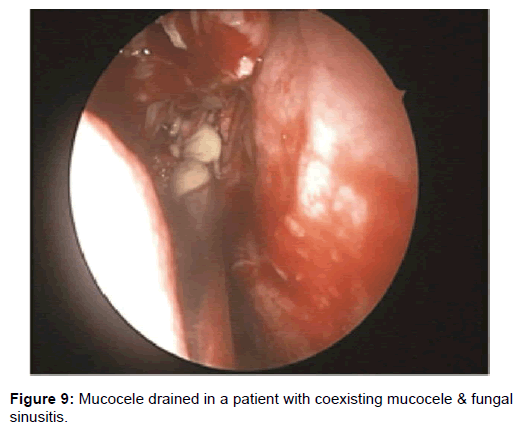
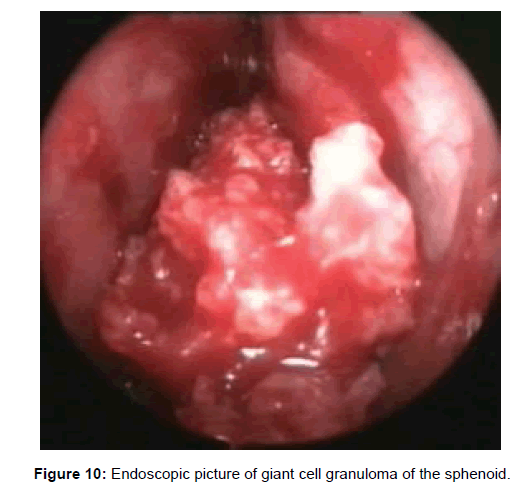
All 47 patients underwent Computed tomographic (CT) imaging, while 23 patients underwent both CT and Magnetic resonance (MR) imaging. The etiology of isolated sphenoid sinus disease was acute sphenoiditis (2 patients), chronic sphenoid inflammation due to allergy (10 patients), mucocele (11 patients), sphenochoanal polyp (2 patients), allergic fungal sinusitis (17 patients), coexisting mucocele with fungal sinusitis (1 patient), Cerebrospinal fluid (CSF) leak (3 patients), giant cell granuloma (1 patient) (Figure 2). The etiology was evident on imaging (Figures 3-6).
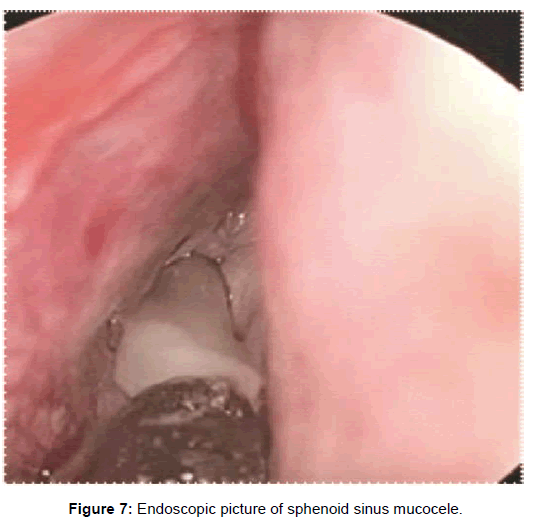
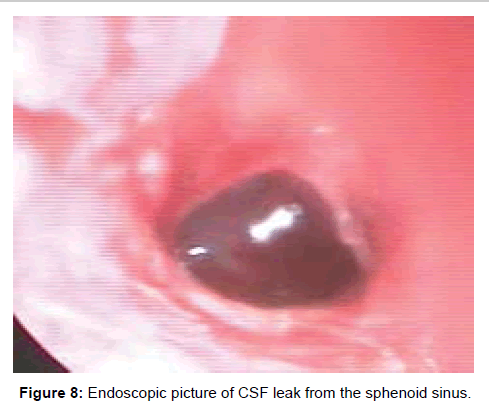
Two patients with acute sphenoid sinusitis were successfully managed medically with antibiotics and ten patients with chronic sphenoid sinusitis were managed with topical corticosteroids and anti-histamines. The remaining thirty five patients were managed by functional endoscopic sinus surgery (Figures 7-10).
All patients had successful management of their sphenoid disease. There were no intra-operative or post-op complications in any of the patients. Diplopia due to oculomotor palsy improved in two patients after the surgery. Vision recovered completely in 7 of the 11 patients who complained of diminished vision. These patients underwent surgery within a week after onset of diminished vision. Patients who had diminished vision for a prolonged period before the procedure did not regain vision after surgery and patients with complete pre-operative loss of vision did not regain their vision after the procedure (Table 1).
| Ophthalmic symptoms | Mucocele | AFRS | Chi-square test | ||
|---|---|---|---|---|---|
| Pre-operative | Post-operative | Pre-operative | Post-operative | ||
| Double vision | 3 | 1 | - | - | 0.27 |
| Diminished vision | 3 | 1 | 8 | 3 | 0.005 |
| Complete loss of vision | 1 | 1 | 3 | 3 | Not applicable |
Table 1: Pre-operative and post-operative ophthalmic symptoms in sphenoid sinus disease.
In patients with CSF rhinorrhea, CSF was noted coming from the sphenoid sinus ostium during diagnostic nasal endoscopy. The CT scans in these patients demonstrated bony dehiscence of the sphenoid sinus walls as well as partial or complete opacification of the sinus. The MR findings included hyperintense signal on T2-weighted images within the sinus consistent with fluid. These patients underwent transnasal endoscopic water tight repair of CSF leak with fat, fascia, muscle, surgicel and fibrin glue.
Overall there were no recurrences of isolated sphenoidal disease in our cohort of medical and surgically managed patients over their follow up period which ranged from 8 months to 10 years with the mean follow-up period being 46 months. This highlights the consistent success of our management protocol studied over the two decades.
Discussion
Isolated disease of the sphenoid is a rare entity and represents 1 to 2% of all sinus disease. Most patients have inflammatory pathology, and a neoplasm is rare [1]. Akin to our experience over two decades, similar literature of the diverse pathologies encountered by other authors is discussed here, to emphasize the existence of this unique entity aptly termed as Isolated sphenoid sinus disease (ISSD).
Neoplastic lesions account for 15–16% of isolated sphenoid sinus disease cases [4]. The symptoms of sphenoid sinus disease can be nonspecific and delayed presentations are very often seen. Most patients have headache as the presenting symptom and its prevalence ranges from 28% in tumors to 98% ininflammatory lesions [3]. Author, reported headache as the primary presenting complaint in 72.5% of patients [5]. Hence, sphenoid sinus disease should be considered in patients presenting with subacute or chronic headache [6]. Patients may present with rhinorrhea, postnasal drip, nasal obstruction [1].
Sphenoid sinus disease may at times be detected radiographically as an incidental finding while evaluating some other abnormality [5]. If sinonasal symptoms are ignored and the diagnosis missed, delayed diagnosis and treatment can lead to serious complications [1]. Intra-cranial structures, orbital contents, or both may be affected by complications. This is due to the close anatomical proximity of the sphenoid sinus with the brain, meninges, pituitary gland, optic nerve, internal carotid artery, the sphenopalatine artery and ganglion and the pterygoid canal and its nerve, cavernous sinus, and cranial nerves (oculomotor, trochlear, ophthalmic, and maxillary branches of the trigeminal nerve and abducent nerve) [1,7]. Thus, sphenoid sinusitis can lead to orbital cellulitis and abscess, orbital apex syndrome, blindness, sepsis, meningitis, epidural and subdural abscess, cerebral infection, pituitary abscess, cavernous sinus thrombosis, sepsis and internal carotid artery thrombosis [2].
Trigeminal nerve symptoms have been reported in 10% to 30% of inflammatory diseases, and cranial nerve findings in 12% to 70% of cases [8]. The abducent nerve is the most common cranial nerve to be involved resulting in diplopia. This occurs in 6% of inflammatory cases and in 50% of neoplastic disease. In 1979, it was reported that in 78% of the cadavers, the bony wall between the optic nerve and sphenoid sinus was found to be thinner than 0.5 mm and in 8% of cadavers there was no bony structure between the sphenoid sinus and internal carotid artery [7].
Contralateral abducens nerve palsy has been reported as a rare complication of unilateral isolated sphenoid sinusitis [9]. Retrobulbar optic neuropathy secondary to isolated sphenoid sinus disease has been reported. Possible mechanisms of optic nerve involvement include compressive optic neuropathy secondary to mucoceles and/ or pyoceles; direct extension of sinus infection to the optic nerve from suppurative sinusitis with invasion of the nerve by local bacteria and secondary inflammatory occlusive vasculitis causing optic neuritis. A rare hypothesis that has been proposed includes demyelination caused by stimulation of the immune system by an infection in the sphenoid sinus [10]. Occlusion of the carotid and basilar arteries secondary to sphenoid sinus disease has been reported [11].
Sphenoid sinus disease presents several management challenges. The initial signs and symptoms of sphenoid sinus disease are often similar regardless of the etiology [5]. Diagnosis is based on a thorough history taking, ENT examination, nasal endoscopy and a CT scan of the paranasal sinuses. Diagnostic nasal endoscopy may be normal or may reveal mucoid, purulent discharge or the presence of polyps. Patients may have acute bacterial sphenoid sinusitis which is usually caused by Streptococcus pneumoniae and Haemophilus influenzae. Acute isolated sphenoid sinusitis is a rare condition, especially in young children. Approximately 80% of pediatric patients with intracranial complications of sinusitis have sphenoid involvement [2].
CT scans in sphenoid inflammation reveal mucosal thickening, and partial or total opacification of the sinus. Bony wall sclerosis may be present in some cases with a chronic inflammatory process [4]. Fungal sinusitis represents 15 to 20% of all cases. Decreased aeration of the sphenoid sinus constitutes a risk factor for fungal sinusitis. Diabetes mellitus and other causes of immunosuppression are risk factors for development of sphenoid sinus infections. In fungal sinusitis, opacification of the sphenoid, often with associated hyperdense foci, bony wall thickening or expansion may be noted [4]. Isolated sphenoid sinusitis complicated by meningitis and multiple cerebral infarctions in a renal transplant recipient has been reported [11].
Mucoceles are seen in the sphenoid in 2% of cases. The common presenting symptoms mentioned are progressively worsening headache, visual loss, decreased visual acuity, visual field defect, diplopia, rhinorrhoea, and nasal congestion [12]. CT scan features of mucocele show a homogenous, non-enhancing, expansile sinus mass completely filling the potential sinus cavity expanding or remodeling surrounding bone margins. Mucoceles generally do not enhance with contrast, but acutely inflamed mucopyoceles may show enhancement. The content of the sinus is variable, depending on the degree of hydration, ranging from near water attenuation to hyper-attenuating as secretions become increasingly thick and dehydrated. On MRI, the signal intensity of mucoceles varies in accordance with fluid content, presence of a proteinaceous component or hemorrhage. They usually have low signal intensity on T1 weighted images and a signal void on T2 weighted image sequence due to inspissated debris [13].
Fibrous dysplasia of the sphenoid presents as a ground-glass pattern on CT and typically shows a heterogeneous pattern hypointense in T1W MRI and intermediate intensity in T2W MRI. The sphenoid is an extremely rare site for malignancy. Squamous cell carcinoma is uncommon in the sphenoid sinus. Metastatic disease has been reported to involve the sphenoid sinus, with prostate, renal, and lung cancers predominating [5]. Non-Hodgkin’s lymphoma of the sphenoid sinus has been rarely reported and may present with oculomotor palsy. In patients with malignancies, the finding usually seen is an expansile mass leading to bone destruction with irregular shape and poorlydefined margins.
In CSF leak, air-fluid level can be observed and small bony dehiscences are found in the posterior and lateral walls of the sphenoid [4]. CSF rhinorrhea may be associated with sphenoid encephaloceles. Magnetic resonance imaging in cases of encephalocele demonstrates herniated brain parenchyma, meninges, and CSF within the sinus [5]. Sphenoid sinus pathologies such as pituitary adenoma, Langerhans cell histiocytosis, solitary plasmacytoma, and chordoma have been reported [14]. Angiographic studies and intervention may be required if vascular lesions are suggested on CT or MR imaging studies [5]. Surgical intervention is the primary treatment for most patients with isolated sphenoid sinus disease. Advanced imaging techniques have allowed safe management of sphenoid sinus lesions [5]. Endoscopic endonasal approach is the preferred treatment in these patients [15]. In patients with optic nerve involvement, recovery of vision depends on the duration of visual loss before surgery, the severity of the visual disturbance, and the severity of sphenoid sinus disease. Visual disturbances lasting more than 6 months may be irreversible and indicate a poorprognosis [16].
Conclusion
A high index of suspicion is essential to diagnose sphenoid sinus disease, as sinonasal symptoms may be non-specific. CT scan of the paranasal sinuses is the gold standard investigation, although an MRI may be required in the presence of complicated or advanced disease. The sinus has a close relationship to vital neuro-vascular structures. Hence, timely intervention is essential to avert neuro-ophthalmic complications of isolated sphenoid disease.
References
- Marcolini TR, Safraider MC, Socher JA, Lucena GO (2015) Differential diagnosis and treatment of isolated pathologies of the sphenoid sinus: retrospective study of 46 cases. Int Arch Otorhinolaryngol 19: 124-129.
- Monti A, Katsanoulas C, Farini M (2008) Acute isolated sphenoid sinusitis in a 4-year-old child: a rare case with an atypical presentation. Signa Vitae 3: 51-54.
- Fooanant S, Angkurawaranon S, Angkurawaranon C, Roongrotwattanasiri K, Chaiyasate S (2017) Sphenoid Sinus Diseases: A Review of 1,442 Patients. Int J Otolaryngol 2017: 1-7.
- Sieskiewicz A, Lyson T, Olszewska E, Chlabicz M, Buonamassa S, et al. (2011) Isolated sphenoid sinus pathologies – the problem of delayed diagnosis. Med Sci Monit 17: 179-183.
- Martin TJ, Smith TL, Smith MM, Loehrl TA (2002) Evaluation and Surgical Management of Isolated Sphenoid Sinus Disease. Arch Otolaryngol Head Neck Surg 128: 1413-1419.
- Celenk F, Gulsen S, Gonuldas B, Baysal E, Durucu C, et al. (2015) Isolated sphenoid sinus disease: An overlooked cause of headache. J Craniomaxillofac Surg 43: 1914-1917.
- Ceylan K, Kizilkaya Z, Emir H, Samim E (2006) An Unusual Presentation for 6th Nerve Palsy: Isolated Chronic Sphenoid Sinusitis and Bilateral Abducens Palsy. Internet J Otorhinolaryngol 6: 1-5.
- Ruoppi P, Seppa J, Pukkila M, Nuutinen J (2000) Isolated Sphenoid Sinus Diseases: Report of 39 Cases. Arch Otolaryngol Head Neck Surg 126: 777-781.
- Siu J, Sharma S, Sowerby L (2015) Unilateral isolated sphenoid sinusitis with contralateral abducens nerve palsy - A rare complication treated in a low-resource setting. J Otolaryngol Head Neck Surg 44: 9-11.
- Chafale VA, Lahoti SA, Pandit A, Gangopadhyay G, Biswas A (2015) Retrobulbar optic neuropathy secondary to isolated sphenoid sinus disease. J Neurosci Rural Pract 6: 238-240.
- Steadman CD, Salmon AHJ, Tomson CRV (2004) Isolated sphenoid sinusitis complicated by meningitis and multiple cerebral infarctions in a renal transplant recipient. Nephrol Dial Transplant 19: 242-244.
- Strek P, Zagolski O, Składzien J, Oles K (2007) Sphenoid sinus pyocele with intracranial extension managed under endoscopic guidance: a report of an extremely rare case. B-ENT 3: 149-151.
- Sinha BK, Adhikari P (2008) Sphenoid sinus mucocele with blindness: A rare presentation. Nepal Med Coll J 10: 204-206.
- Lin TH, Fang SY, Ho HC (2009) Isolated Sphenoid Sinus Disease: Analysis of 11 Cases. Tzu Chi Med J 2: 227-232.
- Tang IP, Brand Y, Prepageran N (2016) Evaluation and treatment of isolated sphenoid sinus diseases. Curr Opin Otolaryngol Head Neck Surg 24: 43-49.
- Lee LA, Huang CC, Lee TJ (2004) Prolonged visual disturbance secondary to isolated sphenoid sinus disease. Laryngoscope 114: 986-990.
Citation: Natarajan K, Begum T, Anto R, Panicker A, Kumar RS, et al. (2019) Isolated Sphenoid Sinus Disease: The MERF Experience. Otolaryngol (Sunnyvale) 9: 363. DOI: 10.4172/2161-119X.1000364
Copyright: ©2019 Natarajan K, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 4935
- [From(publication date): 0-2019 - Apr 16, 2025]
- Breakdown by view type
- HTML page views: 4104
- PDF downloads: 831