Ischemia-Reperfusion Injury: A Mechanistic Concept
Received: 02-Jul-2021 / Accepted Date: 16-Jul-2021 / Published Date: 23-Jul-2021 DOI: 10.4172/asoa.1000153
About the Study
Ischemia-reperfusion injury (heart, liver, pancreas, kidney, intestine) allies with numerous clinical manifestations including acute heart failure, cerebral dysfunction, myocardial hibernation, systemic inflammatory response syndrome, multiple organ dysfunction syndrome and gastrointestinal dysfunction. These injuries are a group of complications and a complex medical ailment that poses a therapeutic ultimatum for researchers. Blood deprivation during ischemia is an undesirable event of hepatic ischemia-reperfusion injury, that is monitored by the recurrence of flow all through reperfusion, comprises a multifaceted series of events, including adenosine-5'- triphosphate depletion, mitochondrial de-energization, alterations of electrolyte homeostasis, upregulation of pro-inflammatory cytokine signaling, oxidative stress changes, and Kupffer cell activation [1].
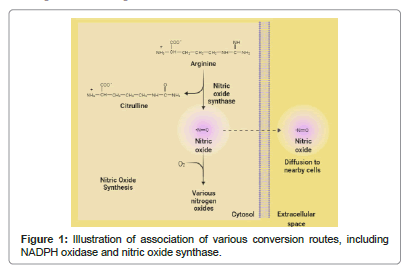
Involved pathways and numerous mediators participate altogether when Ischemia-reperfusion injury transpired and these elaborated features of the underlying mechanisms of these injuries can be a good choice to do a potential therapeutic intervention and can play a key role in the development of antioxidant therapy too [2]. Although, exposing the pathophysiology of ischemia-reperfusion injury is taught task, and especially to investigate the role of reactive oxygen species and to detect the routes of cell death pathways is a challenging aspect [3]. But, analysis of association of various conversion routes, including NADPH oxidase, xanthine oxidase system, and nitric oxide synthase is can expose the therapeutic targets also labeled (Figure 1).
Once prolonged ischemia ended, the blood supply must be reestablished, otherwise, an escalation will occurred in the rate of ROS production and local inflammation, and these unnatural disorders initiate secondary injury. These undesirable events triggered by prolonged ischemia-reperfusion injury and that induced cell damage and further initiate apoptosis, necrosis, necroptosis, and autophagy. Authors explain the mechanistic insights into reperfusion-injury by elaborating all the concerned details of the routes of cell death that transpired via these aforementioned means. These approaches will expose and elucidate the interlinked signaling pathways of cell death marked routes will be new therapeutic targets, and these types of approaches will enhance the possibilities that definitely cure ischemia-reperfusion injuries [4]. Moreover, a better understanding of the pathophysiology of ischemia-reperfusion injury will enhance the chances of successful innovation of novel treatment interventions. For example, mitochondria produce reactive oxygen species that are a key component of the mechanisms that initiate IR injury [5]. Besides it, stimulation of mitochondrial permeability transition or oxidative damage of intra-mitochondrial structures, inflammatory signaling, and extracellular remodeling, pro-apoptotic signaling, and molecules participated are other components that lead to ischemia- reperfusion injury. The impact of mitochondrial ROS on the post- infarction remodeling is also an important aspect of the mechanism underlying as per the mechanistic concept. Platelets play a key role in hemostasis and initiate the formation of thrombi in pathological surroundings. Scientific evidence suggests that platelets participate in the inflammatory progressions and initiation of acute ischemic stroke. In the end, the author believes that his view on the mechanistic concept of ischemia-reperfusion injury will offer important aspects of reactive oxygen species and will inspire the upcoming generation of researchers to find out the way to detect the routes of cell death pathways that occurred during these injuries [6].
Acknowledgment
Author (Rajiv Kumar) gratefully acknowledges his younger brother Bitto for motivation. The author acknowledges bio render for providing the facility to illustrate the diagrams (Figure 1) and again acknowledges the same.
Availability of Data And Materials
Wherever necessary, relevant citations are included in the reference section.
Competing Interests
The author has declared that no competing interest exists.
References
- Kumar R, Gulia K (2021) The convergence of nanotechnology-stem cell, nanotopography-mechanobiology and biotic-abiotic interfaces: Nanoscale tools for tackling the top killer, arteriosclerosis, strokes, and heart attacks. Nano Sel 2: 655-687.
- Li J, Li RJ, Lv GY, Liu HQ (2015) The mechanisms and strategies to protect from hepatic ischemia-reperfusion injury. Eur Rev Med Pharmacol Sci 19: 2036-2047.
- Kumar R, Chhikara BS, Gulia K, Chhillar M (2021) Cleaning the molecular machinery of cellsviaproteostasis, proteolysis and endocytosis selectively, effectively, and precisely: Intracellular self-defense and cellular perturbations. Mol Omi 17: 11-28.
- Dong Y, Chen H, Gao J, Liu Y, Li J, et al. (2019) Molecular machinery and interplay of apoptosis and autophagy in coronary heart disease. J Mol Cell Cardiol 136: 27-41.
- Kumar N, Kumar R (2013) Nanotechnology and nanomaterials in the treatment of life-threatening diseases (1st Ed.) 408.
- Naryzhnaya NV, Maslov LN, Oeltgen PR (2019) Pharmacology of mitochondrial permeability transition pore inhibitors. Drug Dev Res 80: 1013-1030.
Citation: Kumar R (2021) Ischemia-Reperfusion Injury: A Mechanistic Concept. Atheroscler Open Access. 6: 153. DOI: 10.4172/asoa.1000153
Copyright: © 2021 Kumar R. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Open Access Journals
Article Tools
Article Usage
- Total views: 1686
- [From(publication date): 0-2021 - Mar 31, 2025]
- Breakdown by view type
- HTML page views: 1079
- PDF downloads: 607