Ischaemic Optic Neuropathy Non Arteritic/NA-AION
Received: 02-Jun-2016 / Accepted Date: 11-Jun-2016 / Published Date: 14-Jun-2016 DOI: 10.4172/2476-2075.1000119
6794Introduction
Ischaemic optic neuropathy (ION) is the commonest adult optic nerve disorder encountered worldwide and can be expected to increase in incidence in our ageing population. In a recent review of 121 cases [1] the mean age was 61 years. The condition has been classified as a) anterior (AION) affecting the optic nerve head and b) posterior (PION) involving that portion of the optic nerve behind its immediate retrolaminar portion. Furthermore there are two pathological varieties of the disease c) Arteritic (AAION) almost exclusively associated with Giant Cell Arteritis (GCA) and d) Non-arteritic (NA-AION or less correctly NAION) usually associated with diabetes, hypertension and hypercholesterolaemia. A recent treatise on the subject [2] runs to more than 600 pages.
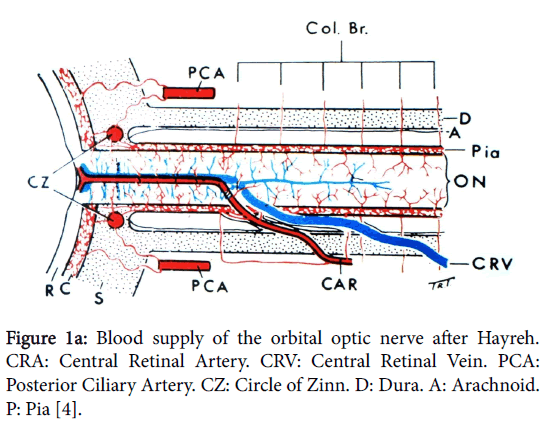
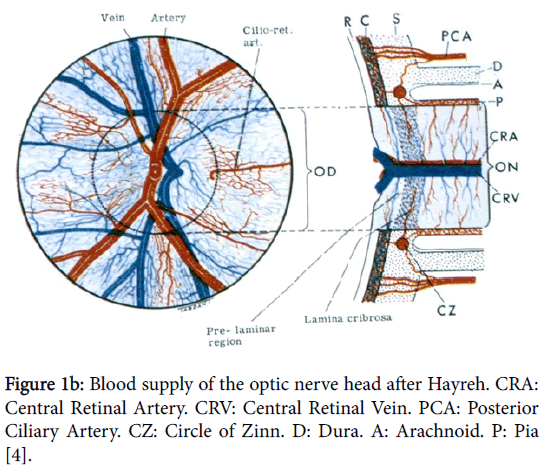
In order to understand the pathology of ION knowledge of the complex vascular anatomy of the optic nerve head (ONH) and its more posterior part is required. This was not clarified until the mid1960’s by the work again of Hayreh [3] when he showed that the ONH is supplied in the main by the ciliary vascular system and not by the ophthalmoscopically visible central retinal artery; furthermore the more posterior part of the nerve is supplied from its surrounding pial plexus fed from adjacent orbital branches of the ophthalmic artery (Figure 1a). The ophthalmic artery is the first intracranial branch of the internal carotid when it emerges from the cavernous sinus. The central retinal artery, also a branch of the ophthalmic, only supplies the surface/retinal layer of the ONH (Figure 1b) before it proceeds to supply the inner layers of the retina.
Figure 1a: Blood supply of the orbital optic nerve after Hayreh. CRA: Central Retinal Artery. CRV: Central Retinal Vein. PCA: Posterior Ciliary Artery. CZ: Circle of Zinn. D: Dura. A: Arachnoid. P: Pia [4].
Figure 1b: Blood supply of the optic nerve head after Hayreh. CRA: Central Retinal Artery. CRV: Central Retinal Vein. PCA: Posterior Ciliary Artery. CZ: Circle of Zinn. D: Dura. A: Arachnoid. P: Pia [4].
The optic nerve blood supply is from the internal carotid system via branches of the ophthalmic artery in particular the ciliary vessels which supply the ONH, and the visual defect in NA-AION is due to a loss of perfusion in these small vessels.
Non-Arteritic ION (NA-AION)
This more common variety of ION is usually of the anterior type i.e. NA-AION and is the commonest adult optic neuropathy encountered in many parts of the world [5] associated with underlying vascular disease especially diabetes mellitus and hypertension.
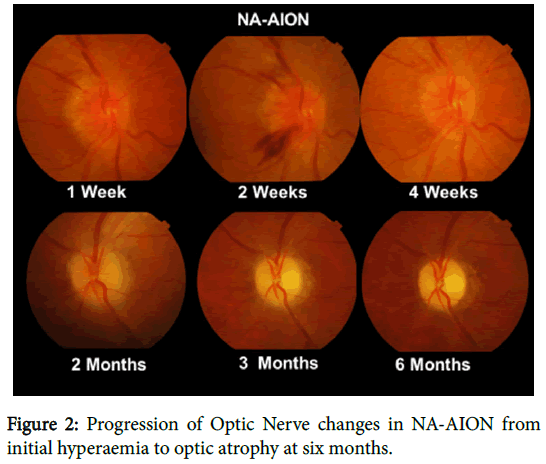
The clinical presentation of NA-AION is unmistakable when a middle aged patient presents with acute but incomplete painless visual loss usually on waking in the morning and who may have noticed loss of vision in the lower visual field. In some cases periocular discomfort not worsened on eye movement is present. Visual acuity may be normal but there is always a relative afferent pupillary defect (RAPD) and initially the optic disc is hyperaemic and swollen only becoming pale in 4-6 weeks (Figure 2). This pallor often has a yellowish appearance unlike the white pallor of a compressive lesion and disc cupping does not develop.
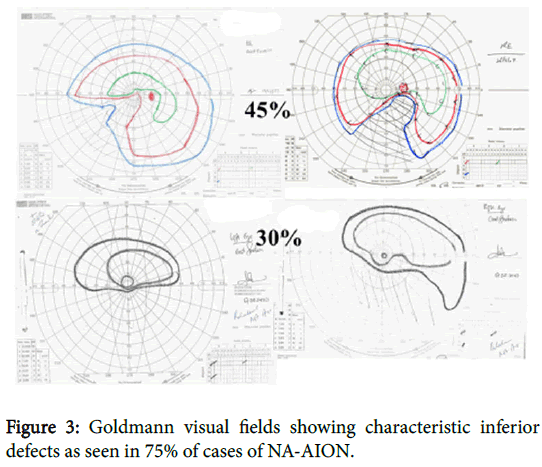
The condition usually occurs in a small so called “disc at risk” one with no significant cup. Visual field examination is essential for diagnosis and a lower altitudinal or lower nasal defect (Figure 3) will be found in 75 % of cases [1]. Where the defect is central or paracentral as in about 10 % of patients’ central vision will be affected at the outset.
Functional recovery is to be expected in only a small percentage of cases [6] and complete recovery never occurs. The condition is stable around six months with no change expected thereafter. Involvement of the fellow eye is reported in 14.5% of cases over five years [7].
There is no proven effective treatment other than control of underlying vascular disease and hypertensive patients should be told not to take treatment late in the day. A neuroprotective agent such as brimonidine tartrate eye drops may be tried and as it has an intraocular pressure lowering effect this may also be helpful, and at least in the absence of other therapy it is important to offer the patient some medication which has at least a logical basis for its use. Because the cause of the visual defect is loss of perfusion in the ONH circulation, not embolic, thrombotic or inflammatory, there is no place for anticoagulants, aspirin or corticosteroid treatment either in the acute phase of NA-AION or in its prevention.
Where central vision is involved (10% of cases) in the presence of a hyperaemic swollen optic disc as in NA-AION a mistaken diagnosis of optic neuritis (ON) is often made. However ON is commoner in younger patients who usually demonstrate a dense central or paracentral scotoma and recovery with or without treatment is the more usual outcome.
Posterior ischaemic optic neuropathy is a diagnosis of exclusion [8] when a compressive cause has been eliminated by neuroimaging. The patient again presents with acute visual loss but central vision is preferentially involved because the more posterior optic nerve is supplied from the surrounding pial plexus and its centre is the watershed area where the macular axons are situated.
Discussion
As the name ION indicates the condition has been accepted as of ischaemic/vascular cause until recently [9] when it has been suggested that a possible alternative aetiology may be found in the vitreous and it has even been suggested that the disease should be renamed “vitreous traction optic neuropathy”. There is some support for considering another cause because one might expect an ischaemic disc to be pale at outset as seen in the arteritic variety (AAION) but the NA-AION disc is hyperaemic at outset and only become pale later, however its regular association with underlying vascular disease and angiographic studies by Hayreh [10] clearly point to a vascular underlying cause.
Unfortunately there are no undisputed pathological reports of acute NA-AION unlike those demonstrating AAION as shown earlier [4]. In Cogans 1966 book [11] there is pathological specimen shown (fig 116) with a retro laminar infarct which could be NA-AION but there is no case report given to support the diagnosis. A more recent paper by Knox et al. [12] on the histopathology of 193 laboratory specimens with a diagnosis of ION may contain some cases of NA-AION but again there are no supporting reports in any of these non arteritic specimens illustrated.
References
- Cullen JF, Chung SH (2012) Non-arteritic Anterior Optic Neuropathy (NA- AION): outcome for visual acuity and visual field defects, the Singapore scene 2. Singapore Med J 53: 88-90.
- Hayreh SS (1969) Blood supply of the optic disc and its role in optic atrophy, glaucoma and oedema of the optic disc. Brit J Ophthalmol 53: 721-748
- Cullen JF (2016) Ischaemic Optic Neuropathy – Arteritic. Optom Open Access 1: 1-3.
- Cullen JF, Por YM (2007) Ischaemic optic neuropathy –the Singapore scene. Singapore Med J 48: 246-251.
- Hayreh SS, Zimmerman MB (2008) Non-arteritic anterior ischemic optic neuropathy: Natural history of visual outcome. Ophthalmology 115: 298-305.
- Newman NJ, Schirer RA, Langerby P, Kelman S, Feldon S, et al. (2002) The fellow eye in NAION: Report from the ION decompression trial follow up study. Am J Ophthalmol 134: 312-328.
- Hayreh SS (2004) Posterior Ischaemic Optic Neuropathy: Clinical features pathogenesis and management. Eye 18: 1188-1206.
- Parsa CF, Hoyt WF (2015) Non arteritic anterior ischemic optic neuropathy: A misnomer. Rearranging pieces of a puzzle to reveal a non-ischemicpapillopathy caused by vitreous separation. Ophthalmology 122: 439-442.
- Hayreh SS (2015) Re: Parsa et al.: Non-arteritic anterior ischemic optic Neuropathy: A misnomer. Rearranging pieces of a puzzle to reveal a non-ischemia papillopathy caused by vitreous separation.Ophthalmology 122: 439-442
- Knox DL, Kerrison JB, Green WR (2000)Histopathologic studies of Ischemic Optic Neuropathy. Trans Am OphthSoc 95: 203-220.
Citation: Cullen JF (2016) Ischaemic Optic Neuropathy – Non Arteritic/NA-AION. Optom Open Access 1: 119. DOI: 10.4172/2476-2075.1000119
Copyright: © 2016 Cullen JF. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 15988
- [From(publication date): 6-2016 - Nov 23, 2024]
- Breakdown by view type
- HTML page views: 15185
- PDF downloads: 803