Editorial Open Access
Is There A Role for Radiotherapy In Experimental Therapeutic Drug Development?
Charles A Kunos1* and Bhadrasain Vikram21Investigational Drug Branch, Cancer Therapy Evaluation Program, USA
2Radiation Research Program, National Cancer Institute, Bethesda, Maryland, USA
- *Corresponding Author:
- Charles Kunos
Investigational Drug Branch
Cancer Therapy Evaluation Program
Division of Cancer Treatment and Diagnosis
National Cancer Institute
National Institutes of Health
9609 Medical Center Drive
Room 5W-446, Rockville
MD 20892-9760, USA
Tel: 240 276 6939
Fax: 240 276 7894
E-mail: charles.kunos@nih.gov
Received date: June 28, 2016; Accepted date: June 29, 2016; Published date: July 04, 2016
Citation: Kunos CA, Vikram B (2016) Is There A Role for Radiotherapy In Experimental Therapeutic Drug Development? . OMICS J Radiol 5:e140. doi:10.4172/2167-7964.1000e140
Copyright: © 2016 Kunos CA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Radiology
Keywords
Radiotherapy; Radiochemotherapy; Experimental chemotherapy; Precision medicine; Clinical cancer research; Cancer discovery; Clinical trials
To the Editor
Randomized clinical trials are accepted sources of evidence for evaluating and approving drug or biologic therapies on the basis of their medical safety and clinical efficacy [1]. Despite positive clinical trials with drug and biologic therapies in combination with radiation therapy, drug indications and usage specifically with radiation therapy are infrequent. Over the last decade, cetuximab [2] was the only drug product that was granted an indication and usage label from the United States Food and Drug Administration (FDA) for the use in combination with radiation therapy.
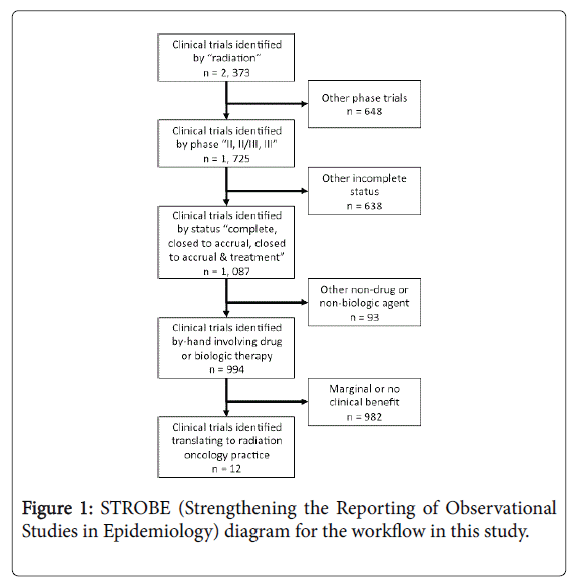
Radiation therapy, an important inducer of free hydroxyl radicals with a capacity to damage cancer DNA, has an established therapeutic role in the definitive management of brain, head and neck, lung, gastrointestinal, gynecological, and sarcoma malignancies. Better knowledge of malignant cell radiobiology has invigorated the earlier study of radiation therapy and drug or biologic therapy combinations in the clinical development plans of portfolio agents within the United States National Cancer Institute Cancer Therapy Evaluation Program (NCI CTEP). To investigate whether radiochemotherapy clinical trials positive for clinical benefit have integrated into good clinical practice, we obtained approval from NCI CTEP (Bethesda, MD) to conduct a retrospective review of anonymized phase II or phase III clinical trials registered within its databases between January 1977 and June 2016 (Figure 1).
For this study, an inquiry of the NCI CTEP Integrated Platform for Agents and Diseases (IPAD) database (version 6.1.0, Rockville, MD) was performed in June 2016 to determine the number of radiation therapy and drug or biologic therapy combination trials translating into actual radiation oncology clinical practice. Clinical trials were sorted using search terms of “radiation,” phase “II, II/III, or III,” and “complete, closed to accrual, or closed to accrual & treatment.” The workflow appears in Figure 1. A single reviewer scored by-hand clinical trials positive for radiochemotherapy clinical benefit if a radiation therapy and drug or biologic therapy combination showed any form of benefit as compared to a direct (e.g., randomized trial) or an indirect (e.g., single-arm trial versus historical control) comparator. A second reviewer independently provided quality-check. No descriptive or statistical analyses were done.
A total of 994 radiochemotherapy phase II, phase II/III, or phase III clinical trials were identified in the time period of interest (1977-2016). Twelve (1.2%) clinical trials showed clinical benefit to a radiation and drug or biologic therapy combination. The 12 clinical trials involved the radiosensitizers 5-fluorouracil, cetuximab, cisplatin, gemcitabine, temozolomide, and triapine or the radioprotector amifostine. Despite these results, only three products were granted an indication for the use of their product in combination with radiation therapy. The approved agents are cetuximab, temozolomide, and amifostine [3].
This analysis offers a contemporary review of NCI CTEP-sponsored radiation therapy and drug or biologic therapy combination clinical trials; however, this analysis does not capture all radiation oncology practice-changing clinical trials as sponsors vary around the world. Three factors likely contribute to the low number of clinically beneficial radiation-agent clinical trials. First, preclinical radiobiology describing radiation-agent dose, schedule, exposure, and effect often informs investigators late in an agent’s overall clinical development timeframe. Second, oncology market pressure often selects both monotherapy trial and regulatory strategy, leaving combination trials, such as radiation agent trials, for late-stage clinical development. And finally, these results suggest that most pharmaceutical companies do not consider radiation-drug combinations as a pathway to registration or a means of expanding the indication of an agent. Future radiation therapy and drug or biologic therapy combination clinical trials might build upon more structured preclinical radiobiology with wider support from Industry to forecast clinical success [4].
References
- US Department of Health and Human Services, U.S. Food and Drug Administration (2007) Guidance for industry:clinical trial endpoints for the approval of cancer drugs and biologics.
- US Department of Health and Human Services, U.S. Food and Drug Administration (2012) Erbitux® (cetuximab).
- US Department of Health and Human Services, U.S. Food and Drug Administration (2006)Temodar®(temozolonmide).
- Coleman CN, Higgins GS, Brown JM, Baumann M, Kirsch DG, et al. (2016) Improving the predictive value of preclinical studies in support of radiotherapy clinical trials. Clin Cancer Res.
Relevant Topics
- Abdominal Radiology
- AI in Radiology
- Breast Imaging
- Cardiovascular Radiology
- Chest Radiology
- Clinical Radiology
- CT Imaging
- Diagnostic Radiology
- Emergency Radiology
- Fluoroscopy Radiology
- General Radiology
- Genitourinary Radiology
- Interventional Radiology Techniques
- Mammography
- Minimal Invasive surgery
- Musculoskeletal Radiology
- Neuroradiology
- Neuroradiology Advances
- Oral and Maxillofacial Radiology
- Radiography
- Radiology Imaging
- Surgical Radiology
- Tele Radiology
- Therapeutic Radiology
Recommended Journals
Article Tools
Article Usage
- Total views: 10888
- [From(publication date):
August-2016 - Jul 09, 2025] - Breakdown by view type
- HTML page views : 10005
- PDF downloads : 883