Mini Review Open Access
Is the Combination/Multi-target Therapy a New Promise for Alzheimer's Disease?
Xuekai Zhang, Jing Shi and Jinzhou Tian*
The 3rd Department of Neurology, Dongzhimen Hospital, Beijing University of Chinese Medicine, Beijing, China
- Corresponding Author:
- Jinzhou Tian
The 3rd Department of Neurology
Dongzhimen Hospital
Beijing University of Chinese Medicine, Beijing, China
Tel: 861084013380
E-mail: jztian@hotmail.com
Received date: February 18, 2016; Accepted date: February 29, 2016; Published date: March 07, 2016
Citation: Zhang X, Shi J, Tian J (2016) Is the Combination/Multi-target Therapy a New Promise for Alzheimer’s Disease? J Alzheimers Dis Parkinsonism 6:216. doi:10.4172/2161-0460.1000216
Copyright: © 2016 Zhang X, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Alzheimers Disease & Parkinsonism
Abbreviations
AD: Alzheimer’s Disease; Aβ: Beta Amyloid; P-Tau: Phosphorylated Tau; AChEi: Acetylcholinesterase (AChE) Inhibitor; CDK5: Cyclin-dependent Kinase 5; PP2A: Protein Phosphatase 2A; CaMKII: Ca2+/Calmodulin (CaM)-dependent Protein Kinase II; CaN: Calcineurin; IDE: Insulin Degrading Enzyme; syp: Synaptophysin
Since the worldwide demographic ageing, the prevalence of Alzheimer’s disease (AD) will increase dramatically. It is a well known complex and progressive disorder, which not only owing to its complex clinical symptoms including cognitive, non-cognitive (such as hallucinations, delusions, anxiety, marked agitation and associated aggressive behaviour) symptoms [1], but also because it’s complicated pathogenesis, like multiple genetic factors, oxidative stress, neuroinflammation, and vascular harms, mitochondrial dysfunction, increased amyloid and tau deposition, decreased neurotrophic factors and loss of synapses as well as neurons [2].
Although the detailed mechanism of AD has been investigated for decades, most of those laboratory results still have a long way to transform into clinical use. Currently approved pharmacotherapy only include two categories, the cholinesterase inhibitors and N-methyl D-aspartate receptor (NMDAR) antagonist [3]. They are widely used in clinic, but have been proved to be of limited efficacy and are generally considered to improve or stabilize symptoms rather than affect the underlying disease processes [4]. Thus, many pharmaceutical companies began to seek disease-modifying treatments for AD [2]. Since the important roles of Aβ and Tau accumulation in AD pathogenesis, many potential treatments targeting the accumulation of either amyloid-beta (Aβ) or tau proteins are still under ongoing investigations [5].
Humanized Anti-Aβ antibodies with high and accurate binding properties to Aβ, like the bapineuzumab and solanezumab, was a hot and promising therapeutic strategy in reducing brain Aβ via increasing its clearance [6-11]. In both preclinical studies with transgenic mice that overproduced Aβ and phase 2 clinical studies with mild-to-moderate AD patients, they showed good results in slowing Aβ deposition or reducing the amount of Aβ in the brain. However, negative results as assessed by primary outcomes in both two medicines’ phase 3 trials in treating mild-to-moderate AD were released [12,13]. The failed primary end points may in certain way indicate that targeting Aβ alone is not a reliable therapeutic strategy for the treatment of such complex and complicated disease in late stage. Combination/multi-target therapy targeting the accumulation of both amyloid and tau proteins and other facets of neuro degeneration may therefore become a reasonable strategy.
Thus combining medicines with different and/or complementary mechanisms of effects or using multi-target therapy targeting several steps in the neurodegenerative process of AD is a hot topic now. There are many evidences support the combination use of cholinesterase inhibitors and NMDAR antagonist. First, initial evidence from pharmacokinetic and pharmacodynamic data in healthy volunteers showed that memantine and donepezil may be safely used in combination [14]; Second, although there are controversies, some evidences from either clinical trial or systemic review indicate the combination use offer additional benefits to the patient [15-18]. Compare to moderate to severe AD patients receiving mono stable donepezil, similar patients receiving memantine (10 mg twice daily) and same dosage donepezil showed significant benefits in all four main symptom domains of AD, namely, cognition, function, behaviour, and global status [15]. Combined treatment with memantine and AChEIs was effective in patients with AD, particularly in slowing cognitive impairment and preventing the onset of agitation and aggression in elderly AD patients [19]. This may be explained by the fact of preclinical data showed these two drugs act via two different, but interconnected, pathological pathways, and that their complementary activity may produce greater effects than either drug individually [20]. However, there was also trial proved no significant benefits of the combination of donepezil and memantine over donepezil alone [21].
With the combination of behavior symptoms occur in different stages of AD patient, cognitive treatment also commonly used in the combination of non-cognitive treatments, like antipsychotic (AP) or antidepressant (AD) treatments. Evidences indicate that ADs may be reasonable pharmacological alternatives to APs in clinical management of such behavioral symptoms in AD [22]. However, there are no more detailed evidences of such combination therapy. And misuse of sedative medicines in AD patients may cause rapid deterioration. On the contrary, A preliminary open-label trial conducted in Japan suggested that the discontinuation of donepezil treatment in AD patients with behavior symptoms may produce superior efficacy and may make it possible to not increase the dosage of other psychotropic drugs [23]. And there were also evidences suggest that antipsychotic treatment may increase diabetes risk in AD patients [24].
So there are still many blanks for us to explore in the use of combination therapy. Beside the combination of two mono-target medicine, multi-target medicine or complex-component medicine may also provide some interesting clues in the treatment of AD patients. Herbal medicine or Chinese herbal formula with complex-component character may just fit into such strategy. However, few recognition has been paid to herbs in treating AD, except galanthamine and huperzine A [25,26]. Recognitions are even less in the concern of Chinese herbal formula, a combination of several herbs according to Chinese medicine theory. While, there are some very intriguing evidences about AD symptom-improving, or even disease-modifying effects of single herbs and herbal formulas in preclinical experiments and clinical trials. The following are some clinical or experimental evidences of herbal medicine in treating AD.
“Ba Wei Di Huang Wan”, a very famous Chinese herbal formula, has been reported in significantly improve Mini-Mental State Examination (MMSE) and Barthel Index of patients with AD and cerebrovascular disease [27]. “Yi-Gan San”, another traditional herbal medicine, may also significantly improve Neuropsychiatric Inventory (NPI) and Barthel Index in dementia patients [28]. Another Chinese herbal formula named GAPT, also called as GEPT in our previous papers, also showed promising effects in both preclinical and clinical studies on treating AD.
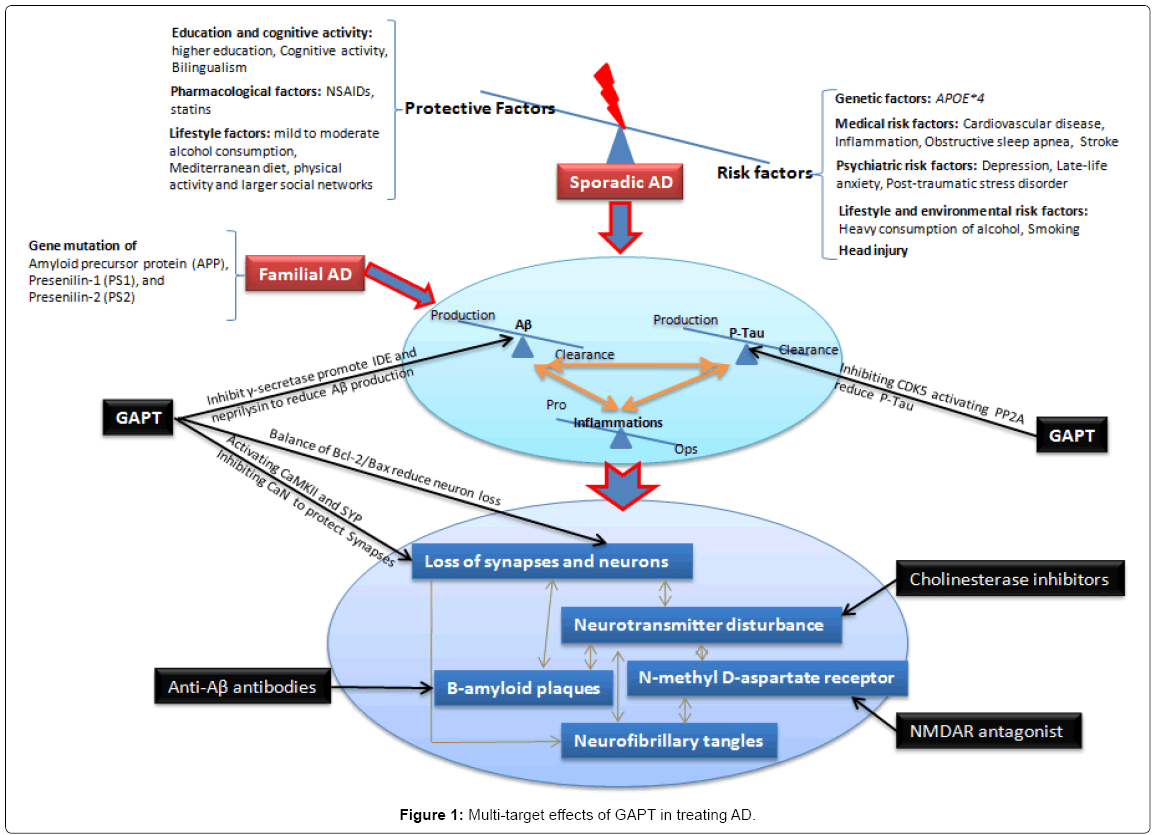
GAPT, a combination of herbal extracts, consists of eight active components pro rata of ginsenoside from ginseng, volatile oil and asarone from acorus tatarinowii schott, flavonoid glycoside from epimedium, tenuifolin from polygala, curcumine from tuber curcuma and others. Preliminary clinical studies of GAPT in treating amnestic mild cognitive impairment showed that it can significantly improved the cognitive function of patients with early-stage AD, amnestic mild cognitive impairment (MCI) [29,30]. In animal studies, GAPT extract can markedly enhance learning and memory of AD rat models induced by hippocampal injection of Aβ1-42 peptide or intravenous injection of Aβ1-40 peptide or STZ, as well as APPV717I transgenic mice and APPswe/PS1dE9 transgenic mice [31-34]. Evidences showed that GAPT may inhibit γ-secretase (presenilin-1) and promote insulin degrading enzyme and neprilysin, thus reduce Aβ levels in the brain of APPV717I transgenic mice. It may also attenuate the abnormal hyperphosphorylation of Tau protein in hippocampal neurons of APPV717I transgenic mice via inhibiting the expression of Cyclindependent kinase 5 (CDK5) and activating the expression of protein phosphatase 2A (PP2A). GAPT also showed significant effectiveness in synaptic protection either before or after the formation of amyloid plaques inAPPV717I mice, which is exerted partially through activating the expression of Ca2+/calmodulin (CaM)-dependent protein kinase II (CaMKII) and synaptophysin, as well as inhibiting the expression of Ca2+/Calmodulin-dependent protein phosphatase 2B (calcineurin, CaN). CaMKII and CaN have been found to play important roles in memory processes and neuronal degeneration. It also showed significant preventive and therapeutic effects in repairing neurons structure, increasing synapses number, as well as restoring balance of Bcl-2/Bax in the hippocampus of APPswe/PS1dE9 transgenic mice. Bcl- 2/Bax ratio has well proved as important balance in apoptosis signaling pathway. Above all, GAPT showed significant promise in restoring the dynamic balance state of Aβ and hyperphosphorylated Tau metabolism via targeting multiple-targets (Figure 1).
There are also many interesting clues in the field of single herbs and extracts or derivative from Chinese herbs. For example, curcumin, Ginkgo biloba and Ginsenosides. All extracts exert a broad range of bioactivity and neuroprotective effects. Curcumin is a well-known food additive in Indian cuisine and is used widely in both Ayurvedic medicine and Chinese medicine. In a 4 weeks randomized, double-blind, placebo-controlled human trial, curcumin (400 mg) has been shown to significantly improve working memory and mood after treatment [35]. In animal studies of curcumin in APPswe/PS1dE9 double transgenic AD mice also showed improved spatial learning and memory ability [36,37]. Such effects may conducted through reducing Aβ40, Aβ42, and aggregation of Aβ-derived diffusible ligands in the mouse hippocampal CA1 area, increase the expression of synapse-related proteins PSD95 and Shank1 and improve structure and plasticity of synapse. Ginkgo biloba is among the most widely used complementary alternative medicines for preservation of cognitive health in aging [38]. The effect in AD treatment include preventing free radical-induced damage, increasing intracellular levels of antioxidant enzymes and restoring calcium homeostasis, as well as attenuating neuron apoptosis, inhibiting membrane lipid peroxidation, anti-inflammatory and direct inhibiting Aβ aggregation [39]. Although previous perspective clinical trial indicate that Ginkgo biloba 120 mg twice a day was not effective in reducing the incidence of AD or all-cause dementia in subjects without dementia at baseline [40], a recent systematic review and meta-analysis of randomized placebo-controlled trials on Ginkgo biloba extract EGb 761® evidenced the clinical efficacy, safety, and tolerability of Ginkgo biloba extract EGb 761 at daily doses of 240 mg in the treatment of patients with dementia [41]. Panax ginsengis another commonly used herbal medicine for the treatment of weakness and fatigue in Asia for thousands of years. Its main active components are ginsenosides, which also have a variety of benefits, including anti-inflammatory, antioxidant, and neuroprotective effect [42]. Ginsenosides have showed effectiveness on AD in vitro and in vivo models [43-45]. An open-label investigation about Panax ginseng in AD patients indicate that 12 weeks ginseng administrition improved the cognitive performance scales according to mini-mental state examination (MMSE) and Alzheimer disease assessment scale (ADAS), and the improved function disappeared after the ginseng withdrawal [46].
Conclusion
Above all, there are evidences of both single herbs and herbal formulas in improving AD symptoms, even in modifying disease effects in clinical trials or preclinical experiments. And many preclinical experiments showed such effects are exerted through a multi-target way. Since the pathogenesis of AD is a complex process involving so many signaling pathways, and development of effective mono-target disease modifying drugs has been proven to be a difficult task. Therefore, these encouraging preclinical and clinical results may well suggest that multi target effect of herbal extracts is a new promise for Alzheimer’s disease. And the multi-component and multi-target characteristics of Chinese herbs may well be considered as a new promise for treating the complex AD in different stages.
Acknowledgements
This work was supported by the 111 Project (No.B08006), the Technological Platform of Clinical Evaluation and Research for New Herbal Medicinal Products (No.2011ZX09302-006-01), the Study of Secondary Prevention with Chinese Herbal Medicine for Chronic Diseases (No.Z111107056811043) and National Natural Science Foundation of China Project (No. 81473518, 81573824 and 81503625).
References
- Dillon C, Serrano CM, Castro D, Leguizamón PP, Heisecke SL, et al. (2013) Behavioral symptoms related to cognitive impairment. Neuropsychiatr Dis Treat 9: 1443-1455.
- Venigalla M, Gyengesi E, Sharman MJ, Munch G (2015) Novel promising therapeutics against chronic neuroinflammation and neurodegeneration in Alzheimer's disease. Neurochem Int: 30061-30069.
- Schmidt R, Hofer E, Bouwman FH, Buerger K, Cordonnier C, et al. (2015) EFNS-ENS/EAN Guideline on concomitant use of cholinesterase inhibitors and memantine in moderate to severe Alzheimer's disease. Eur J Neurol 22: 889-898.
- Cummings JL, Isaacson RS, Schmitt FA, Velting DM (2015) A practical algorithm for managing Alzheimer's disease: what, when, and why? Ann Clin Transl Neurol 2: 307-323.
- Hyman BT, Phelps CH, Beach TG, Bigio EH, Cairns NJ, et al. (2012) National Institute on Aging–Alzheimer's Association guidelines for the neuropathologic assessment of Alzheimer's disease. Alzheimer's Dement 8: 1-13.
- Shankar GM, Li S, Mehta TH, Garcia-Munoz A, Shepardson NE, et al.(2008) Amyloid-[beta] protein dimers isolated directly from Alzheimer's brains impair synaptic plasticity and memory. Nature Medicine 14: 837-842.
- Zago W, Buttini M, Comery TA, Nishioka C, Gardai SJ, et al. (2012) Neutralization of soluble, synaptotoxic amyloid β species by antibodies is epitope specific. J Neurosci 32: 2696-2702.
- Buttini M, Masliah E, Barbour R, Grajeda H, Motter R, et al. (2005) Beta-amyloid immunotherapy prevents synaptic degeneration in a mouse model of Alzheimer's disease. The J Neurosci 25: 9096-9101.
- Farlow M, Arnold SE, van Dyck CH, Aisen PS, Snider BJ, et al. (2012) Safety and biomarker effects of solanezumab in patients with Alzheimer's disease. Alzheimers Dement 8: 261-271.
- Dodart JC, Bales KR, Gannon KS, Greene SJ, DeMattos RB, et al. (2002) Immunization reverses memory deficits without reducing brain Abeta burden in Alzheimer's disease model. Nat Neurosci 5: 452-457.
- Blennow K, Zetterberg H, Rinne JO, Salloway S, Wei J, et al. (2012) Effect of immunotherapy with bapineuzumab on cerebrospinal fluid biomarker levels in patients with mild to moderate Alzheimer disease. Arch Neurol 69: 1002-1010.
- Doody RS, Thomas RG, Farlow M, Iwatsubo T, Vellas B, et al. (2014) Phase 3 trials of solanezumab for mild-to-moderate Alzheimer's disease. N Engl J Med 370: 311-321.
- Salloway S, Sperling R, Fox NC, Blennow K, Klunk W, et al. (2014) Two phase 3 trials of bapineuzumab in mild-to-moderate Alzheimer's disease. N Engl J Med 370: 322-333.
- Periclou AP, Ventura D, Sherman T, Rao N, Abramowitz WT (2004) Lack of pharmacokinetic or pharmacodynamic interaction between memantine and donepezil. Ann Pharmacother 38: 1389-1394.
- Gauthier S, Molinuevo JL (2013) Benefits of combined cholinesterase inhibitor and memantine treatment in moderate-severe Alzheimer's disease. Alzheimers Dement9: 326-331.
- Tariot PN, Farlow MR, Grossberg GT, Graham SM, McDonald S, et al . (2004) Memantine treatment in patients with moderate to severe Alzheimer disease already receiving donepezil: a randomized controlled trial JAMA 291: 317-324.
- Porsteinsson AP, Grossberg GT, Mintzer J, Olin JT, Memantine MEMMDSG (2008) Memantine treatment in patients with mild to moderate Alzheimer's disease already receiving a cholinesterase inhibitor: a randomized, double-blind, placebo-controlled trial. Curr Alzheimer Res 5: 83-89.
- Farrimond LE, Roberts E, McShane R (2012) Memantine and cholinesterase inhibitor combination therapy for Alzheimer's disease: a systematic review. BMJ Open 2.
- Gareri P, Putignano D, Castagna A, Cotroneo AM, De Palo G, et al .(2014) Retrospective study on the benefits of combined Memantine and cholinEsterase inhibitor treatMent in AGEd Patients affected with Alzheimer's Disease: the MEMAGE study. J Alzheimers Dis 41: 633-640.
- Parsons CG, Danysz W, Dekundy A, Pulte I (2013) Memantine and Cholinesterase Inhibitors: Complementary Mechanisms in the Treatment of Alzheimer’s Disease. Neurotox Res 24:358-369.
- Howard R, McShane R, LindesayJ, Ritchie C, Baldwin A, et al. (2012) Donepezil and Memantine for Moderate-to-Severe Alzheimer's Disease. New England Journal of Medicine 366:893-903.
- Huang TY, Wei YJ, Moyo P, Harris I, Lucas JA, et al. (2015)Treated Behavioral Symptoms and Mortality in Medicare Beneficiaries in Nursing Homes with Alzheimer's Disease and Related Dementias. J Am GeriatrSoc 63: 1757-1765.
- Hidenobu Suzuki, Yuichi Inoue, KatsunakaMikami, Keishi Gen (2014) The influence and changes in the dosages of concomitantly used psychotropic drugs associated with the discontinuation of donepezil in severe Alzheimer’s disease with behavioral and psychological symptoms on dementia: a preliminary open-label trial. Ther Adv Psychopharmacol 4 : 37-42.
- Chang KJ, Hong CH, Lee Y, Lee KS, Roh HW, et al. (2015) Effect of Psychotropic Drugs on Development of Diabetes Mellitus in Patients With Alzheimer's Disease. Medicine (Baltimore) 94: e919.
- Yang G, Wang Y, Tian J, Liu JP (2013) Huperzine A for Alzheimer's disease: a systematic review and meta-analysis of randomized clinical trials. PLoS One 8: e74916.
- Caramelli P, Laks J, Palmini ALF, Nitrini R, Chaves MLF (2014) Effects of galantamine and galantamine combined with nimodipine on cognitive speed and quality of life in mixed dementia: a 24-week, randomized, placebo-controlled exploratory trial (the REMIX study). Arq Neuropsiquiatr 72: 411-417.
- Iwasaki K, Kobayashi S, Chimura Y, Taguchi M, Inoue K, et al. (2004) A randomized, double-blind, placebo-controlled clinical trial of the Chinese herbal medicine "bawei di huang wan" in the treatment of dementia. J Am Geriatr Soc 52: 1518-1521.
- Iwasaki K, Satoh-Nakagawa T, Maruyama M, Monma Y, Nemoto M, et al. (2005) A randomized, observer-blind, controlled trial of the traditional Chinese medicine Yi-Gan San for improvement of behavioral and psychological symptoms and activities of daily living in dementia patients. J Clin Psychiatry 66: 248-252.
- Wei MQ, Tian JZ, Shi J, Ma FY, Miao YC (2012) Effects of Chinese medicine for promoting blood circulation and removing blood stasis in treating patients with mild to moderate vascular dementia: a randomized, double-blind and parallel-controlled trial. Zhong Xi Yi Jie He Xue Bao 10: 1240-1246.
- Miao YC, Tian JZ, Shi J, Mao M (2012) Effects of Chinese medicine for tonifying the kidney and resolving phlegm and blood stasis in treating patients with amnestic mild cognitive impairment: a randomized, double-blind and parallel-controlled trial. Zhong Xi Yi Jie He Xue Bao 10: 390-397.
- Tian J, Xu Y, Shi J, Yin J, Sheng S, et al. (2006) Effect of GETO extract on myelin sheath structure and myelin basic protein content in the brain with AD model. Alzheimer's & Dementia: The Journal of the Alzheimer's Association 2: S601.
- Tian J, Shi J, Zhang L, Yin J, Hu Q, et al. (2009) GEPT extract reduces Abeta deposition by regulating the balance between production and degradation of Abeta in APPV717I transgenic mice. Curr Alzheimer Res 6: 118-131.
- Shi J, Zhang X, Yin L, Wei M-q, Tian J GAPT extract prevents Aß deposition induced synaptic dysfunction in APPV717I transgenic mice may partially through adjusting the expression of CaMKII and CaN. In press.
- Shi J, Zhang X, Yin L, Wei M-q, Ni J, Li T, Tian J The preventive and therapeutic effects of GAPT extracton synapse loss in APPswe/PS1dE9 transgenic mice via adjusting BCL2/BAX balance. In press.
- Cox KH, Pipingas A, Scholey AB (2015) Investigation of the effects of solid lipid curcumin on cognition and mood in a healthy older population. J Psychopharmacol 29: 642-651.
- Ringman JM, Frautschy SA, Cole GM, Masterman DL, Cummings JL (2005) A potential role of the curry spice curcumin in Alzheimer's disease. Curr Alzheimer Res 2: 131-136.
- Wang P, Su C, Li R, Wang H, Ren Y, et al. (2014) Mechanisms and effects of curcumin on spatial learning and memory improvement in APPswe/PS1dE9 mice. J Neurosci Res 92: 218-231.
- Franke AG, Heinrich I, Lieb K, Fellgiebel A (2014) The use of Ginkgo biloba in healthy elderly. Age (Dordr) 36: 435-444.
- Tan MS, Yu JT, Tan CC, Wang HF, Meng XF, et al. (2015) Efficacy and adverse effects of ginkgo biloba for cognitive impairment and dementia: a systematic review and meta-analysis. J Alzheimers Dis 43: 589-603.
- DeKosky ST, Williamson JD, Fitzpatrick AL, Kronmal RA, Ives DG, et al. (2008) Ginkgo biloba for prevention of dementia: a randomized controlled trial. JAMA 300: 2253-2262.
- Gauthier S, Schlaefke S (2014) Efficacy and tolerability of Ginkgo biloba extract EGb 761® in dementia: a systematic review and meta-analysis of randomized placebo-controlled trials. ClinInterv Aging 9: 2065-2077.
- Hügel HM, Jackson N, May BH, Xue CC (2012) Chinese herbs for dementia diseases. Mini Rev Med Chem 12: 371-379.
- Zhao H, Li Q, Zhang Z, Pei X, Wang J, et al. (2009) Long-term ginsenoside consumption prevents memory loss in aged SAMP8 mice by decreasing oxidative stress and up-regulating the plasticity-related proteins in hippocampus. Brain Res 1256: 111-122.
- Tu LH, Ma J, Liu HP, Wang RR, Luo J (2009) Theneuroprotective effects of ginsenosides on calcineurin activity and tau phosphorylation in SY5Y cells. Cell MolNeurobiol 29:1257-1264.
- Qian YH, Han H, Hu XD, Shi LL (2009) Protective effect of ginsenoside Rb1 on beta-amyloid protein(1-42)-induced neurotoxicity in cortical neurons. Neurol Res 31: 663-667.
- Heo JH, Lee ST, Chu K, Oh MJ, Park HJ, et al. (2008) An open-label trial of Korean red ginseng as an adjuvant treatment for cognitive impairment in patients with Alzheimer's disease. Eur J Neurol 15: 865-868.
Relevant Topics
- Advanced Parkinson Treatment
- Advances in Alzheimers Therapy
- Alzheimers Medicine
- Alzheimers Products & Market Analysis
- Alzheimers Symptoms
- Degenerative Disorders
- Diagnostic Alzheimer
- Parkinson
- Parkinsonism Diagnosis
- Parkinsonism Gene Therapy
- Parkinsonism Stages and Treatment
- Stem cell Treatment Parkinson
Recommended Journals
Article Tools
Article Usage
- Total views: 11097
- [From(publication date):
March-2016 - Apr 02, 2025] - Breakdown by view type
- HTML page views : 10221
- PDF downloads : 876