Research Article Open Access
Is Fortification of Methionine Necessary in Soya Bean (Glycine Max) Based Feeds for Oreochromis andersonii (Castelnau, 1861) Raised in Semi-Concrete Ponds?
Kefi AS*, Chungu NP, Mupenda N and Mumba CNational Aquaculture Research and Development Centre, P. O. Box 22797, Kitwe, Zambia
- *Corresponding Author:
- Kefi AS
National Aquaculture Research and Development Centre
P. O. Box 22797, Kitwe, Zambia
Tel: 260 979 255 620/260 969 426 244
E-mail: askefi@yahoo.com
Received date: July 08, 2013; Accepted date: September 25, 2014; Published date: September 25, 2014
Citation: Kefi AS, Chung UP, Mupenda N, Mumba C (2014) Is Fortification of Methionine Necessary in Soya Bean (Glycine Max) Based Feeds for Oreochromis andersonii (Castelnau, 1861) Raised in Semi – Concrete Ponds?. J Marine Sci Res Dev 4:154. doi:10.4172/2155-9910.1000154
Copyright: © 2014 Kefi AS, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Marine Science: Research & Development
Abstract
An experiment was conducted to determine the optimal amount of methionine that can be incorporated in the soya bean based Oreochromis andersonii feeds. Three levels (2%, 4% and 6%) of methionine included in soya bean based feed (30% and 10% crude protein and crude lipid respectively) were tested on O. andersonii for seventy eight (78) days in hapas erected in a semi–concrete pond (250 m2 ) arranged in a complete randomized design. Specific growth rate (SGR% day-1), mean body weight gain (MBWG) (g) and apparent feed conversion efficiency (AFCE %) differed (P<0.05) among the treatments although the 4% and 6% gave similar (P>0.05) SGR (%), MBWG (g) and AFCE (%) but higher than the control and the lowest methionine level used in the experiment. Trend analysis showed a linear relationship (F=3.358, P=0.02) between Gonadosomatic Index (GSI) and methionine level with the highest methionine level producing fish with the highest GSI. Furthermore, the highest methionine level produced a significantly higher (P<0.05) GSI than in other treatments. However, females and larger sized fish appeared to mature earlier than males and smaller fish respectively. Fish body moisture and ash were similar (P>0.05) in all the treatments. It is, therefore, recommended that soya bean based feed can be fortified with 4% methionine to improve the O.andersonii growth. An economic analysis is required to ascertain the cost effectiveness of using the synthetic amino acid in soya bean fish feeds for O. andersonii.
Keywords
Methionine; Oreochromis andersonii; Soya bean; Growth and Reproduction
Introduction
The use of soya bean has now generally been accepted as the best alternative to the use of fish meal in the livestock feeds including fish. This is because it has similar balanced amino acid (AA) profile although its methionine level is lower than that of fish meal from as low as 0.55% in the full fat soya beans [1]. It is considered to meet requirements for some fish species [2,3] such as common carp, tilapia, channel catfish, salmonids, red drum, striped bass and marine shrimp [4]. It has also relatively high amounts of vitamins (thiamine, niacin, B–Complex and carotene) [5,6]. Furthermore, soya bean is readily available on the market at a relatively lower price [7]. If properly cooked, its low levels of cellulose and complex carbohydrates provide an additional advantage of using the plant in aquaculture nutrition. It has also low fibre content coupled with high digestibility [8].
Osman et al. [9] speculated low levels of both methionine and lysine in commercial fish feeds. Some authors [10] have, therefore, recommended the fortification of plant based diets with synthetic methionine in order to balance the AAs. Methionine is a sulfur containing and an essential AA required by fish as well as terrestrial vertebrates for normal growth and metabolic functions. This is because methionine is used in the protein synthesis and as a sulphur source which is needed for synthesis of other sulphur – containing biochemicals [11]. It is in the form of S–adenosyl–methionine which serves as a principal donor of methyl group, a major contributor to the whole body pool of carbon units that are required for trans–methylations, the biosynthesis of choline, thymidine, polyamines and creatine [12-14].
Oreochromis andersonii is indigenous to Zambia and has been adopted as the fish species of choice for aquaculture. It is naturally in lagoons of the Upper and Middle Zambezi River, Kafue systems [15] and Lake Kariba [16]. Adults occupy deep water while juveniles remain inshore. It feeds on detritus and zooplankton although bigger individuals also take insects and other invertebrates. In aquaculture the requirement for soya bean protein and lipid has been studied. Kefi et al. [17] found 30% crude protein to be ideal economically. Furthermore, Kefi et al. [18] observed that the minimal lipid requirement was lower than 10% although the optimal lipid requirement was observed to be 15%.
Although there are similar studies on methionine that have been conducted in other fish species, there has been no published work on O. andersonii. For instance Osman et al. [9] found 3.05% methionine of the crude protein to be optimal for O. niloticus. The experiment was conducted to determine the optimal methionine inclusion levels in soya bean based diet fed to O. andersonii juveniles.
Materials and Methods
The study to investigate the optimal methionine levels in soya bean based feed was carried out at National Aquaculture Research and Development Centre (NARDC), Kitwe for seventy eight days in hapas placed in a 250 m2 semi-concrete pond. Oreochromis andersonii was the experimental fish species used in this study with varying levels of methionine (2%, 4% and 6%) as treatments. The percentages of methionine used were based on what Osman et al. [9] reported in O. niloticus and a slight lower methionine of 2% was used as the start level. The experimental pond was prepared by draining it, setting up of hapas (1 × 0.9 × 1 m) and refilling with water to 60 cm level. The treatments were allocated to hapas in a randomized block design in triplicates. Two hundred and fifty O. andersonii (19.58 ± 0.103; mean ± SD) were seined from 250 m2 semi–concrete pond and were taken to concrete tank (50 m2) for conditioning for 48 hours. Thereafter, 20 fish were randomly sampled and their morphometric measures (weight, standard length and total length) taken as described by Skelton [15] before being placed in each hapa. The remaining fish were kept in the same concrete tank for possible replacements upon mortality that occurred within the first fourteen days of the experiment.
Isonitrogenous (30%) and isocaloric (10%) soya bean based feed (soya bean 54.6%, maize meal 43.4%, vitamin starter 1% and di – calcium phosphate 1%) fortified with DL–methionine feed grade 99% (Sumitomo Chemical Limited, Japan) levels at 2%, 4% and 6% was prepared and provided to fish at 3% live body weight twice a day with an exception of Saturdays and Sundays.
Selected water quality parameters (dissolved oxygen, water temperature, conductivity and Chlorophyll a) were taken once daily three times in a week (Monday, Wednesday and Friday) at 10 hours. Mortality was also monitored every day throughout the study.
At the end of the experiment, all the fish were weighed and the length measured. Three fish were randomly selected from each treatment and analyzed for dry matter and ash content according to the procedures of AOAC [19]. The ash was determined as total inorganic matter by incineration of the sample in an Advantech electric furnace at 550°C for 5 hours. The remaining inorganic material was reduced to their stable form, oxides or sulphates were considered as ash. Then the ash was calculated as follows:
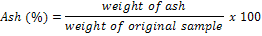
Moisture was determined by drying samples in an Advantech electric furnace maintained at 105°C for 5 hours. The difference between the initial weight of the sample and that after drying was recorded and moisture calculated as follows:
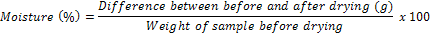
The remainder of the fish was then placed in buckets filled with 10% formalin for 10 days. After this period, the fish were dissected and the gonads recovered. Upon recovery, the maturity stage of the gonad was identified as described by Balarin [20]. The gonads were then weighed on sensitive analytical balance.
Statistical analysis
Growth of the fish was calculated following the equations; Weight gain (g)=Final fish weight (g)–Initial fish weight (g); Specific growth rate =100(LnWT1 – LnWT0)/t where, Ln is natural logarithm, WT0 is the initial weight (g), WT1 is the final fish weight (g) and t is time in days. Feed convention efficiency (FCE%) was calculated as follows: 100(body weight gain/feed taken). Condition factor was calculated as follows: 100,000W/L3 where W is weight (g) while L is standard length (mm) of fish [21]. The gonad somatic index (GSI) was calculated as follows: 100(weight (g) of gonads/weight (g) of fish).
Analysis of variance (ANOVA) planned contrasts were performed to determine the differences between use and non-use of methionine and among methionine levels means which were deemed significant at P<0.05 for parametric data. Before analysis, parametric data were tested for normality using Shapiro–Wilk test and the homogeneity of variance using Levene’s test for Equality of Variances. Contrasts tests not assuming equal variances were used if the assumption of equal variance was violated.
The stages of maturity of fish were analyzed using the multinomial logistic regression models where size (weight), sex and methionine entered as factors and stages of gonads as dependent variable. In order to eliminate cells with zero frequencies, the gonadal stages where grouped into three categories. These were immature, inactive (inactive and inactive–active) and ripe (active, ripe and ripe running) only. The ripe gonads were used as a reference group. The size (weight) was collapsed into 10 g intervals. Interactions between and among the factors were tested after the main effects were maintained in the model if significant (P<0.05), and similarly factors not having significant (P>0.05) effect were taken out from the model.
The model used was follows:
Y=a + b1X1 + b2X2 + b3X3+ b4X4 + Ɛ
Where:
Y=Dependent variable (stages of gonads)
X1 to X4=Independent variables
X1=Sex of fish
X2=Weight of fish (g)
X3=Methionine level (%)
X4=interaction effect
b1 to b4=regression coefficients
a=Constant
Ɛ=Residual
Cross tabulations and Chi-square (χ2) were used to examine the associations between the reproductive status of fish and sex, and methionine levels.
Statistical Package for Social Scientist (SPSS) 15.0 (SPSS Inc) softwares were used in analyzing the data. Microsoft excel was used in the production of figures and graphs. Untransformed data are presented to facilitate interpretation.
Results
Planned comparisons revealed a significant effect (P<0.05) of methionine on SGR. Trend analysis revealed that SGR (%) increased proportionally (F =30.504, P=0.0001) with methionine level. Similar trend was observed in FCE (%) (F=13.725, P =0.0001). The final mean weight, condition factor (K) and standard length (mm) did not differ significantly (P>0.05) among the treatments. However, the highest final mean weight was recorded at 4% methionine level. Specific growth rate (SGR) differed significantly (P <0.05) among methionine levels. However, the control did not differ (P>0.05) from that of 2% while the 4% methionine level was similar (P>0.05) to that of 6%. The mean body weight gain differed significantly (P<0.05) with the higher levels of methionine recording the highest body weight gain. The 4% and 6% methionine did not differ significantly (P>0.05). Similarly the control and the lowest methionine level (2%) used did not differ significantly (P> 0.05). The same trend was observed in the feed conversion efficiency (%) (Table 1).
There were no significant differences (P>0.05) observed in the ash and dry matter of the fish among the methionine levels used in the experiment. However, the 4% methionine level recorded the lowest ash and highest dry matter. Dry matter was lowest in the control group (Table 1).
Gonadosomatic index (GSI) increased linearly with methionine levels and this was significant (F=3.358, P=0.02). The highest methionine level used produced fish with the highest GSI and this was significant different (P<0.05) from the other treatments including the control. There was a relationship (P<0.05) between the independent variables (sex, fish size and methionine levels) and the stages of maturity of the gonads. However, only size and sex had a significant relationship to the maturity stage of the fish and therefore, it was excluded from the final model. The females were less likely to be immature (Odds Ratio=0.069) or inactive Odds Ratio=0.271) compared to males. Additionally small fish were more likely to be immature (Odds Ratio=42.99) or inactive Odds Ratio=4.959) than the bigger fish. However, the medium sized fish did not differ (P>0.05) from the reference group which is large fish. This shows that the females are more likely to be in advanced stage of maturity than males indicating that females matured earlier than males. Consequently, large fish seemed to be advanced in maturity status (Table 2).
Significant differences (P<0.05) were observed in the stages of maturity with ripe stage recording the highest reproductive stage although this was not significant different (P>0.05) from the immature group. Inactive group was the lowest but was not significant different (P>0.05) from the immature group (Figure 1).
According to sex, there were more immature males (79.4%) than females and this was significant (P<0.05). Although the inactive group was not significant (P>0.05) between the sexes, the females (46.8%) were fewer than the males. There number of females in the ripe stage were significantly (P<0.05) more than the females (Figure 2).
There were no significant differences (P>0.05) observed in the selected water quality parameters determined (Table 3).
Discussion
Protein requirements are defined as the minimum amount needed to meet requirements for amino acids and to achieve maximum growth [22]. There have several varying results reported by authors probably due to variances in experimental conditions. Wilson [23] estimated 1.8 to 4.0% dietary methionine of the dietary protein as the requirement for commonly cultured species of fish. Jackson and Capper [23] reported that O.mossambicus appears to have a lower than 0.53% dietary methionine that may provide a satisfactory growth. In the current experiment, a practical diet fortified with methionine did not produce fish with different final mean weight. However, SGR (%) day-1, weight gain and FCE (%) differed significantly. However, the 4% and 6% methionine produced similar but superior to the control and 2% methionine level. The insignificant final (P>0.05) weight exhibited by the fish subjected to all the treatments may be due to shorter experimental period. The rate at which fish grow is dependent on a number of factors including species, age, genetic potential, water temperature, health, and quantity and quality of food. Specific growth rate has been used as a suitable index for determining fish growth. In the current experiment SGR (%) day-1 was found to be highest at the (P>0.05) from that of 4%. This was similar to the weight gain (g) and FCE (%). However, the final mean weight was not significant (P>0.05) although the 4% methionine level produced the fish with the highest final mean weight. Similar results were found by Nguyeni and Davis [24] who did not find any positive effect of methionine addition to soya bean based feed on the final weight on Red tilapia and O. niloticus. However, they did not report on SGR (%) day-1. Osman et al. [9] found that the dietary requirements of methionine for O. niloticus fingerlings to be 3.05%of the dietary crude protein. Our results are, therefore, consistent with the finding of Wilson [23] Osman et al. [9].
To the best of our knowledge there have been no studies conducted on the effect of methionine on the reproduction of O. andersonii. Therefore, our results cannot be referred to any study but can be a benchmark for future studies. Although, the level of methionine did not affect the rate of maturity of O. andersonii, there was a positive linear relationship between the GSI and methionine level. The increase in GSI as the methionine increases indicates an elevated gonad mass due to supplementation of the soya bean feed with methionine. This shows a high investment in reproduction as the methionine increases. We have not come across any study that has directly implicated the methionine to reproduction efficiency in fish. Therefore, the increase in gonad mass of the fish with the highest methionine may be attributed to the satisfying the amino acid composition of the feed as high protein level has been associated with high GSI [25,26]. However, sex and size of fish appears to affect the maturity of the O. andersonii. The females were found to mature earlier than males and larger fish were more at an advanced maturity stage than small fish. Similar results were observed by Kefi [26]. This observation emphasizes the need to culture all males only if growth before maturity is to be maximized.
The study reveals that 4% and 6% methionine level give similar but superior results on the growth rate of O. andersonii. However, methionine seemed to have increased GSI of O. andersonii. Therefore, 4% methionine level inclusion in soybean based fish feed would be recommended in the growth enhancement of O. andersonii. However, there is need to conduct an economic analysis of utilization of methionine in the soya bean based fish feeds. Furthermore, there is need to study the fortification of the soya bean based fish feeds with methionine for a longer period in aquaculture facility being used in the production of fish for commercial purposes.
Acknowledgements
Authors wish to thank Indian Council of medical research (ICMR), New Delhi for financial support. We also want to thank Shilpa Virdi, Gaurav Sharma and Lekhraj in collection and processing of samples.
References
- NRC (2012) Nutrient Requirements of Swine. (11th rev edn ) Natl. Acad. Press, Washington DC.
- NRC (1993) Nutrient requirements of fish. National Research Council, National Academy Press, Washington, D.C.
- Deguara S (1998) Effect of supplementary enzymes on the growth and feed utilisation of gilthead sea bream, Sparus aurata. PhD Thesis, University of Stirling, Stirling, Scotland.
- Cremer MC, Enhua Z, Jian Z, O’Keefe T (2008) Soy protein concentrate replaces fishmeal in fingerling test diets in China. American Soybean Association, St. Louis, Missouri 63141 USA.
- Martin R, Ruberte R (1980) Legume. In technique and plants for tropical subsistence farm. United States Department of Agriculture 19-20
- Fafioye OO, Fagade SO, Adebisi AA, Oni J, Omoyinmi GAK (2005) Effect of dietary soybean (Glycine max (L) Merr) on growth and body composition of African Catfish (Clarias gariepinus, Burchell) fingerlings. Turk J Fish Aquat Sc 5: 31-35.
- Guillaume J, Kaushik S, Bergot P, Metailler R (2001) Nutrition and feeding of fish and crustaceans, Praxis, Cornwall, United Kingdom
- Gatlin DM. III, Barrows FT, Braown P, Dabrowski K, Gaylord TG et al. (2007) Expanding the utilization of sustainable plant products in aquafeeds: a review. Aquacult Res 38: 551-579.
- Osman FM, Amer MA, Mahfouz SA, Wasly KM (2008) Reviewing the optimal level of methionine and lysine for tilapia fingerlings (Oreochromis niloticus). Proceedings of 8th International Symposium on Tilapia in Aquaculture, 903-919.
- Davies OA, Ezenwa NC (2004) Groundnut cake as alternative protein source in the diet of Clarias gariepinus fry. International Journal of Science and Nature 1: 73-76.
- Wu G, Davis DD (2005) Interrelationship among methionine, choline and betaine in Channel Catfish Ictalurus punctatus. J. World Aquac. Soc 36: 337-345.
- Alam MD, Teshima SI, Ishikawa M, Koshio, S (2000) Methionine requirement of juvenile Japanese flounder, Paralichthys olivaceus. J. World Aquac. Soc 31: 618-626.
- Mai K, Wan J, Ai Q, Xu W, Liufu Z et al. (2006) Dietary methionine requirement of large yellow croaker, Pseudoscia enacrocea. Aquaculture 253: 564-572.
- Bae JY, Ok IH, Lee S, Hung SSO, Min TS et al. (2011) Re-evaluation of Dietary Methionine Requirement by Plasma Methionine and Ammonia Concentrations in Surgically Modified Rainbow Trout, Oncorhynchus mykiss. Asian Australas J Anim Sci 7: 974-981.
- Skelton P (2001) A Complete Guide to the Freshwater Fishes of Southern Africa, Struik, Cape Town, South Africa.
- http://www.fishbase.org/summary/Oreochromis-andersonii.html.
- Kefi AS, Kang’ombe J, Kassam D, Katongo C (2013) Effect of Dietary Soyabean (Glycine max (L.) Merr.) Protein Level on Growth and Feed Utilization of Oreochromis andersonii (Castelnau, 1861). Pak J Nutr 12: 990–995.
- Kefi AS, Kang’ombe J, Kassam D, Katongo C (2013) Optimal Dietary Plant Based Lipid on Growth of Oreochromis andersonii (Castelnau, 1861). Turk J Fish Aquat Sc 13: 503–508.
- AOAC (Association of Official Analytical Chemists) (2002) Methods of Analysis of the Association of Official Analytical Chemists. Association of Official Analytical Chemists, Inc., Arlington, U. S. A.
- Balarin JD (1983)A guide to Tilapia breeding: the Baobab Hatchery Technique. Tilapia culture section, Baobab Farm Limited, Mombasa, Kenya.
- Williams JE (2000) The Coefficient of Condition of Fish. Chapter 13 in Schneider, James C. Manual of fisheries survey methods II: with periodic updates. Michigan Department of Natural Resources, Fisheries Special Report 25, Ann Arbor.
- Jackson A, Capper J (1982)Investigation into the requirements of the tilapia (Sarotherodon mossambicus) for dietary methionine, lysine and argnine in semi – synthetic diets. Aquaculture, 29: 289-287.
- Wilson RP (2002)Amino acids and proteins. In: Fish Nutrition, (3rd Ed.) Ed. J. E. Halver and R. W. Hardy. Academic Press, Inc., New York, USA 143-179.
- Nguyen TN, Davis DA (2009) Evaluation of Alternative Protein Sources to Replace Fish Meal in Practical Diets for Juvenile Tilapia, Oreochromis spp. J World Aquac Soc 40:113–121.
- Kithsiri HM, Sharma P, Zaidi SGS, Pal AK, Venkateshwarlu G (2010) Growth and reproductive performance of female guppy, Poecilia reticulate (Peters) fed diets with different nutrient levels. Indian J Fish, 57: 65–71.
- Kefi AS (2014) Influence of dietary soya beans (glycine max (l.) merr.) protein and lipid combination, and androgen (17 α – methyl testosterone) levels on the growth and reproduction of Oreochromis andersonii (Castelnau, 1861). PhD thesis, Bunda College of Agriculture, University of Malawi, Lilongwe, Malawi.
Relevant Topics
- Algal Blooms
- Blue Carbon Sequestration
- Brackish Water
- Catfish
- Coral Bleaching
- Coral Reefs
- Deep Sea Fish
- Deep Sea Mining
- Ichthyoplankton
- Mangrove Ecosystem
- Marine Engineering
- Marine Fisheries
- Marine Mammal Research
- Marine Microbiome Analysis
- Marine Pollution
- Marine Reptiles
- Marine Science
- Ocean Currents
- Photoendosymbiosis
- Reef Biology
- Sea Food
- Sea Grass
- Sea Transportation
- Seaweed
Recommended Journals
Article Tools
Article Usage
- Total views: 14176
- [From(publication date):
December-2014 - Jul 02, 2025] - Breakdown by view type
- HTML page views : 9620
- PDF downloads : 4556
