Research Article Open Access
Irregular External Gastric Stimulation is Associated with Suppression of Serum Ghrelin Levels and Prolonged Decrease in Weight: A Novel Method for Sustaining Weight Loss
Khoury T, Elbaz A, Rotnemer-Golinkin D, Shabat Y, Zolotarovya L and Ilan Y*Gastroenterology and Liver Units, Department of Medicine, Hadassah-Hebrew University Medical Center, Jerusalem, Israel
- *Corresponding Author:
- Yaron Ilan, MD
Gastroenterology and Liver Units
Department of Medicine
Hadassah-Hebrew University Medical Center
Jerusalem, POB 1200, IL-91120, Israel
Tel: 972-2-6778231
E-mail: ilan@hadassah.org.il
Received date: October 21, 2016; Accepted date: December 03, 2016;; Published date: December 06, 2016
Citation: Khoury T, Elbaz A, Rotnemer-Golinkin D, Shabat Y, Zolotarovya L, et al. (2016) Irregular External Gastric Stimulation is Associated with Suppression of Serum Ghrelin Levels and Prolonged Decrease in Weight: A Novel Method for Sustaining Weight Loss. J Obes Weight Loss Ther 6:327. doi:10.4172/2165-7904.1000327
Copyright: © 2016 Khoury T, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Obesity & Weight Loss Therapy
Abstract
Introduction: Weight regain is a major obstacle for most dietary procedures. Gastric stimulation has been shown to affect hormone levels.
Aim: The aim of the present study was to determine the effect of irregular external gastric stimulation on longterm weight reduction.
Methods: External gastric stimulation was performed by holding a rotator device on the abdomen of mice for 5 minutes in a regular or irregular manner. Mice were assessed for body weight and serum ghrelin levels. Results: External gastric stimulation was associated with a significant decrease in serum ghrelin levels. Irregular external gastric stimulation was associated with a prolonged effect on body weight reduction compared with regular stimulation. By the end of week 5, the body weight of untreated control mice had increased compared with a slight weight increase in mice treated with regular stimulation, while there was a significant continued decrease in the body weight of mice treated with irregular stimulation.
Conclusions: Irregular gastric stimulation is associated with a prolonged effect on weight loss and provides a method for overcoming the brain-gut axis accommodation of weight loss.
Keywords
Ghrelin; Weight regain; Gastric stimulation
Introduction
Weight regain following dietary procedures is a major obstacle for long-term weight reduction [1-3]. Strategies for the maintenance of long-term weight loss are therefore needed [4]. Gastric electrical stimulation (GES) using both external and internal devices is being evaluated for the treatment of GI motility disorders, including gastroparesis, and for weight loss [5]. While initial clinical studies failed to show an effect on body weight, there is a renewed interest in this method as a minimally invasive and low-risk intervention that may assist some patients with obesity [6].
Appetite regulation is a complex system that involves a number of orexigenic and anorexigenic peptide hormones [7,8]. Ghrelin is the only circulating orexigenic gut hormone. Although ghrelin-producing cells are found throughout the gastrointestinal tract, the enteroendocrine cells of the gastric fundus are the main source of its production [9]. Ghrelin regulates energy metabolism and acts as a signal of hunger [10,11]. Ghrelin administration increases energy intake and induces weight gain. In an acute setting, ghrelin levels are elevated by fasting and suppressed following a meal or after an oral glucose tolerance test [12]. In a chronic state, ghrelin levels are high in obese subjects and low in lean subjects [13]. The ghrelin axis plays a role in energy homeostasis, adipogenesis, and insulin regulation, and in the reward associated with food in stress-induced food intake behavior [7,14].
The vagal afferent pathway is the neural path by which information about ingested nutrients reaches the central nervous system (CNS) to influence feeding behavior [15]. Ghrelin signals the presence of gut nutrients to the CNS and up-regulates food intake while lowering energy expenditure. In the CNS, ghrelin acts on the hypothalamus and limbic system, areas that regulate appetite and energy expenditure. The effects of ghrelin in the hypothalamus are mediated by homeostatic pathways to signal hunger and increase food intake and adiposity, promoting weight gain [16,17]. Ghrelin exerts its effect through a network of neuroendocrine links, including the melanocortin and endocannabinoid systems [18]. Hypothalamic nuclei, the hippocampus, the amygdala, the caudal brain stem, and midbrain dopaminergic neurons play roles in the orexigenic actions of ghrelin [19]. The only known ghrelin receptor is the growth hormone secretagogue receptor, which is located in several distinct regions of the CNS [20].
As ghrelin is the only peripheral hormone known to transmit satiety signals, inhibition of its signaling has being evaluated as an antiobesity strategy [10,21-23]. The efficacy of ghrelin has been tested in diseases involving anorexia, negative energy balance, systemic inflammation, gastroparesis, cancer, cachexia, cardiovascular disorders, chronic heart failure, chronic renal failure, chemotherapy, arthritis, and inflammatory bowel disease [9,24]. Ghrelin agonists have been developed for the treatment of hypomotility disorders, and the peptidomimetic TZP-102 is in clinical trials for the treatment of diabetic gastroparesis [25].
Body weight homeostasis involves the gut-brain axis, a complex and highly coordinated system of peripheral appetite hormones, and centrally mediated neuronal regulation [26]. A balance between the level of orexigenic ghrelin and the levels of anorexigenic glucagon-like peptide 1 (GLP-1), cholecystokinin (CCK), peptide YY (PYY), which are produced in the gastrointestinal tract, and leptin, which is produced in adipocytes plays a role in this process. The gut-brain axis in obese individuals is different to that in lean individuals. Both fasting and postprandial levels of gut hormones change in obese individuals who lose weight [26]. Therefore, accommodation of the brain-gut axis was suggested to underlie part of the mechanism responsible for weight regain.
The aim of the present study was to determine the effect of irregular external gastric stimulation on serum ghrelin levels as a strategy for the long-term maintenance of weight reduction.
Materials and Methods
Animals: Male C57BL/6 mice (11-12 weeks old) were obtained from Harlan Laboratories (Jerusalem, Israel) and maintained in the Animal Core of the Hadassah-Hebrew University Medical School. Mice received standard laboratory chow and water ad libitum and were housed with a 12 hour light/dark cycle. Animal experiments were performed according to the guidelines and with the approval of the Hebrew University-Hadassah Institutional Committee for Care and Use of Laboratory Animals.
Assessment of the effects of external gastric stimulation on the serum ghrelin levels
Experimental groups: Male mice (approximately 11-12 weeks old) were purchased from Harlan Laboratories (Jerusalem, Israel). External gastric stimulation was performed by holding a rotator device on the abdomen of mice following 6 hours of fasting. Three groups were included in this study, and each group comprised 4 mice. Group A was the control; group B received regular stimulation by manually applying the rotator for 5 minutes; group C received irregular stimulation by manually applying the rotator for 1 minute followed by a 2 minute break and an additional 1 minute of stimulation.
Assessment of the effect of external gastric stimulation on long-term weight reduction
Experimental groups: Three groups were examined, and each group comprised 5 mice. Group A was the control group: the mice were kept on a standard diet without gastric stimulation for 5 weeks. The mice in group B were exposed to regular external gastric stimulation by manually applying an external rotator for 5 minutes every day, for 5 consecutive days per week, for 5 weeks. Group C received irregular stimulation for 2 minutes followed by a 1 minute break, and an additional 1 minute of stimulation for '5 consecutive days per week, for 5 weeks.
Measurement of ghrelin levels: Serum ghrelin levels were assessed using a rat/mouse ghrelin sandwich ELISA kit with a 96-well plate (Cat. EZRGRA-90K, EMD Millipore Corporation, Missouri 63304 USA). Ghrelin molecules (active form) in the sample were captured by anti-ghrelin IgG antibodies, and the resulting complex was immobilized in the wells of a microtiter plate coated with a pre-titered amount of anchor antibodies, which simultaneously bound a second biotinylated antibody to ghrelin. Then, the unbound materials were washed away, followed by conjugation of horseradish peroxidase to the immobilized biotinylated antibodies. Free enzyme was washed away, and the immobilized antibody-enzyme conjugates were quantified by monitoring horseradish peroxidase activities in the presence of the substrate 3,3Ë?,5,5Ë?-tetra-methylbenzidine. The enzyme activity was measured spectrophotometrically at 450 nm and corrected from the absorbance at 590 nm after acidification of the products that formed.
Statistical analysis: All analyses were performed using Excel (Microsoft, Redmond, WA, United States). The variables are expressed as the mean ± the SD. Two independent groups were compared by performing a Student’s t-test. All the tests applied were two-tailed. A p value of 0.05 or less was considered to be statistically significant.
Results
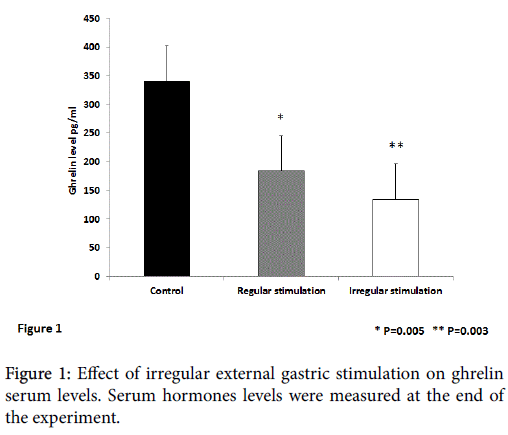
Figure 1 shows the effect of irregular external gastric stimulation on the serum levels of ghrelin. A statistically significant decrease in the ghrelin serum levels was observed in mice treated with regular and irregular external stimulation compared with untreated controls (p=0.005 and 0.003, for regular and irregular stimulations, respectively). Irregular gastric stimulation was associated with a trend for a more profound decrease in ghrelin levels.
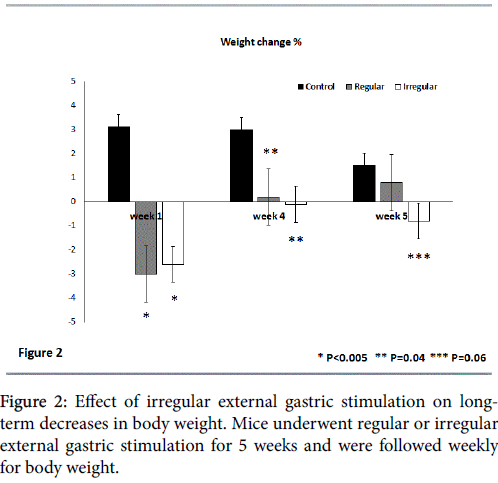
Figure 2 shows the effect of external gastric stimulation on longterm weight reduction by following the change in weight at the end of the first, fourth, and fifth week of the study compared to body weight at the end of the preceding week. At the end of the first week, a significant weight decrease was noted in both regular and irregular external gastric stimulation-treated groups compared with the controls. There was a 3.0% and 2.6% decrease in weight for the regular and irregular stimulated groups, respectively, compared with an increase of 3.1% in the untreated controls (p<0.005 for both treated groups vs. untreated controls).
At the end of the fourth week, neither group showed a significant change in body weight (an increase of 0.2 and 0% for regular and irregular stimulation-treated groups, respectively) compared with an additional increase of 3% in the untreated controls (p=0.04 for treated vs. untreated mice).
However, a long-term effect on body weight was noted for the irregular gastric stimulation-treated mice. These mice manifested an additional decrease of 0.8% in their body weight over the preceding week compared with an increase of 0.8% in the regular external stimulation treated-group and an increase of 1.5% in the untreated controls (p=0.06 for group A vs. group C and 0.3 for group A vs. group B).
Discussion
Currently used medical and non-medical therapies have limited effects on long-term weight loss [6]. A method to prevent weight regain is required for patients undergoing any type of obesity-directed therapy. While standard bariatric surgery is effective, it is associated with morbidity and mortality. The data from the present study suggests that irregular external gastric stimulation could be used as a method to overcome the problem of weight regain. Irregular external gastric stimulation resulted in increased suppression of serum ghrelin levels and exerted a long-term effect on the maintenance of weight loss.
Although dietary restriction often results in initial weight loss, the majority of obese dieters fail to maintain their reduced weight. Weight loss causes changes in appetite and energy expenditure that promote weight regain [27]. Diet-induced weight loss results in a compensatory increase of hunger and decreased ghrelin suppression that encourage weight regain [28]. Compensatory metabolic changes that accompany weight loss, including increased ghrelin levels, contribute to weight regain and difficulty in long-term weight loss maintenance [29]. It has been suggested that ghrelin resistance is a mechanism to maintain a higher body weight set-point during times of food availability [30].
In a one year randomized controlled trial, weight loss was achieved through a reduced calorie diet or exercise and was associated with increased ghrelin levels in overweight or obese postmenopausal women [29]. In a randomized l-year trial with a 12 month follow-up period, obese Mexican-American women using interventions including diet, exercise and orlistat showed increased ghrelin levels at 6 months, but the levels returned to the baseline at 12 months in the weight loss group. Baseline ghrelin concentrations were directly related to the degree of weight loss achieved after 12 months [31], suggesting that ghrelin rises in response to weight loss as a counter regulatory mechanism [31]. In a prospective study of 43 patients treated with a BioEnterics® intragastric balloon, the ghrelin hyper-response in nonmorbidly obese patients was associated with greater short-term treatment efficiency and weight regain to obesity [32]. Patients that had undergone gastric bypass surgery showed a diet-induced weight loss of 17% of the initial body weight, which was associated with a 24% increase in the area under the curve of the 24 hour ghrelin profile [27]. In a follow-up of 45 patients, patients with higher preoperative ghrelin levels were found to be more susceptible to weight regain after gastric bypass than those patients with lower levels [33]. Population-based studies suggest that repetitive cycles of weight loss and regain may be associated with future weight gain [34]. A higher degree of weight cycling, characterized by an increased frequency of intentionally losing more than 10 lb, was associated with higher concentrations of ghrelin [34].
Electrical stimulation can alter the gastric motor function or modulate afferent signaling to the brain. In animal studies, various methods of chronic GES achieved weight loss by reducing food intake, fat tissue weight, and gastric emptying. Altered levels of neuropeptide Y (NPY), alpha-melanocyte-stimulating hormone (alpha-MSH), orexin (OX), and oxytocin and their receptors in the hypothalamus, adipose tissue and stomach were observed [35].
In a recent review of 31 studies, weight loss was achieved during the first 12 months. However, only a few studies had a follow-up period of more than 12 months. The Transcend® (Implantable Gastric Stimulation) device showed a relatively longer period of significant weight loss. Other significant results included changes in appetite/ satiety, gastric emptying rate, blood pressure and neurohormone levels or biochemical markers, such as ghrelin or HbA1c [36,37]. In a study of 27 obese patients, GES using the Ability® system, featuring a transgastric sensor that detects food intake, resulted in a 49% excess weight loss (EWL) [38]. In a 12 month study of 34 obese subjects, the mean EWL was 28.7% and the mean reduction in BMI was 4.8. The effect was stable for up to 27 months [39]. GES performed using the DIAMOND® device in 61 patients with type 2 diabetes led to a 5.7% decrease in body weight and a 0.9% decrease in mean HbA1c [40]. The meal-initiated implantable gastric contractility modulation (GCM) device was tested in obese type 2 diabetes patients. At 12 months, body weight and HBA1C levels had both decreased [41]. The SHAPE (Screened Health Assessment and Pacer Evaluation) trial was a 24 month randomized multicenter placebo-controlled study to determine the efficacy of using an implantable gastric stimulator (IGS) for weight loss. At 24 months, the control group showed weight gain from the baseline that was significantly different from the weight loss in the treatment group. At 12 months, fasting ghrelin levels were significantly increased in the treatment group but not in the control. These data suggested that IGS does not prevent the increase in fasting plasma ghrelin levels associated with weight loss [42].
The present study provides preliminary results as to the potential use of irregular gastric stimulation for alteration of the brain-gut axis in a way that can overcome accommodation to stimuli. Long-term trials are required for further evaluating this effect. Further studies of additional hormones and metabolic parameters are required for elucidation of the mechanism of action.
In summary, minimally invasive gastric manipulation methods promoting safe, reliable and long-term sustainable weight loss are needed. The data from the present study suggest that an irregular external gastric stimulation-based algorithm is associated with suppression of ghrelin levels, enabling long-term weight loss. This method may be a strategy for overcoming the brain-gut axis accommodation.
References
- Montesi L, El Ghoch M, Brodosi L, Calugi S, Marchesini G, et al. (2016) Long-term weight loss maintenance for obesity: a multidisciplinary approach. Diabetes MetabSyndrObes 9: 37-46.
- McGrice M, Don Paul K (2015) Interventions to improve long-term weight loss in patients following bariatric surgery: challenges and solutions. Diabetes MetabSyndrObes 8: 263-274.
- Leibel RL, Seeley RJ, Darsow T, Berg EG, Smith SR, et al. (2015) Biologic Responses to Weight Loss and Weight Regain: Report From an American Diabetes Association Research Symposium. Diabetes 64: 2299-2309.
- MacLean PS, Wing RR, Davidson T, Epstein L, Goodpaster B, et al. (2015) NIH working group report: Innovative research to improve maintenance of weight loss. Obesity (Silver Spring) 23: 7-15.
- Mizrahi M, Ben Ya'acov A, Ilan Y (2012) Gastric stimulation for weight loss. World J Gastroenterol 18: 2309-2319.
- Chiu JD, Soffer E (2015) Gastric electrical stimulation for obesity. CurrGastroenterol Rep 17: 424.
- Seim I, El-Salhy M, Hausken T, Gundersen D, Chopin L (2012) Ghrelin and the brain-gut axis as a pharmacological target for appetite control. Curr Pharm Des 18: 768-775.
- Muller TD, Nogueiras R, Andermann ML, Andrews ZB, Anker SD, et al. (2015) Ghrelin. MolMetab 4: 437-460.
- Jeffery P, McDonald V, Tippett E, McGuckin M (2011) Ghrelin in gastrointestinal disease. Mol Cell Endocrinol 340: 35-43.
- Patterson M, Bloom SR, Gardiner JV (2011) Ghrelin and appetite control in humans--potential application in the treatment of obesity. Peptides 32: 2290-2294.
- Menzies JR, Skibicka KP, Leng G, Dickson SL (2013) Ghrelin, reward and motivation. EndocrDev 25: 101-111.
- Prodam F, Monzani A, Ricotti R, Marolda A, Bellone S, et al. (2014) Systematic review of ghrelin response to food intake in pediatric age, from neonates to adolescents. J ClinEndocrinolMetab 99:1556-1568.
- Iwakura H, Kangawa K, Nakao K (2015) The regulation of circulating ghrelin - with recent updates from cell-based assays. Endocr J 62: 107-122.
- Schellekens H, Finger BC, Dinan TG, Cryan JF (2012) Ghrelin signalling and obesity: at the interface of stress, mood and food reward. PharmacolTher 135: 316-326.
- Ronveaux CC, Tome D, Raybould HE (2015) Glucagon-like peptide 1 interacts with ghrelin and leptin to regulate glucose metabolism and food intake through vagal afferent neuron signaling. J Nutr 145: 672-680.
- Muller TD, Tschop MH (2013) Ghrelin - a key pleiotropic hormone-regulating systemic energy metabolism. EndocrDev 25: 91-100.
- Dieguez C, Vazquez MJ, Romero A, López M, Nogueiras R, et al. (2011) Hypothalamic control of lipid metabolism: focus on leptin, ghrelin and melanocortins. Neuroendocrinology 94: 1-11.
- Kirsz K, Zieba DA (2011) Ghrelin-mediated appetite regulation in the central nervous system. Peptides 32: 2256-2264.
- Zigman JM, Bouret SG, Andrews ZB (2016) Obesity Impairs the Action of the Neuroendocrine Ghrelin System. Trends EndocrinolMetab 27: 54-63.
- Mason BL, Wang Q, Zigman JM (2014) The central nervous system sites mediating the orexigenic actions of ghrelin. Annu Rev Physiol 76: 519-533.
- Sato T, Ida T, Nakamura Y, Shiimura Y, Kangawa K et al. (2014) Physiological roles of ghrelin on obesity. Obes Res ClinPract 8: e405-e413.
- Sato T, Nakamura Y, Shiimura Y, Ohgusu H, Kangawa K, et al. (2012) Structure, regulation and function of ghrelin. J Biochem 151: 119-128.
- Kirchner H, Heppner KM, Tschop MH (2012) The role of ghrelin in the control of energy balance. HandbExpPharmacol 209:161-184.
- DeBoer MD (2012) The use of ghrelin and ghrelin receptor agonists as a treatment for animal models of disease: efficacy and mechanism. Curr Pharm Des 18: 4779-4799.
- Peeters TL (2013) Ghrelin and the gut. EndocrDev 25: 41-48.
- Lean ME, Malkova D (2016) Altered gut and adipose tissue hormones in overweight and obese individuals: cause or consequence? Int J Obes (Lond) 40: 622-632.
- Cummings DE, Weigle DS, Frayo RS, et al. (2002) Plasma ghrelin levels after diet-induced weight loss or gastric bypass surgery. N Engl J Med 346: 1623-1630.
- Jakubowicz D, Froy O, Wainstein J, Boaz M (2012) Meal timing and composition influence ghrelin levels, appetite scores and weight loss maintenance in overweight and obese adults. Steroids 77: 323-331.
- Mason C, Xiao L, Imayama I, Duggan CR, Campbell KL, et al. (2015) The effects of separate and combined dietary weight loss and exercise on fasting ghrelin concentrations in overweight and obese women: a randomized controlled trial. ClinEndocrinol (Oxf) 82: 369-376.
- Strohacker K, McCaffery JM, MacLean PS, Wing RR (2014) Adaptations of leptin, ghrelin or insulin during weight loss as predictors of weight regain: a review of current literature. Int J Obes (Lond) 38: 388-396.
- Garcia JM, Iyer D, Poston WS, et al. (2006) Rise of plasma ghrelin with weight loss is not sustained during weight maintenance. Obesity (Silver Spring) 14: 1716-1723.
- Nikolic M, Boban M, Ljubicic N, Supanc V, Mirosevic G, et al. (2011) Morbidly obese are ghrelin and leptinhyporesponders with lesser intragastric balloon treatment efficiency : ghrelin and leptin changes in relation to obesity treatment. ObesSurg 21: 1597-1604.
- Tamboli RA, Breitman I, Marks-Shulman PA, Jabbour K, Melvin W, et al. (2014) Early weight regain after gastric bypass does not affect insulin sensitivity but is associated with elevated ghrelin. Obesity (Silver Spring) 22: 1617-1622.
- Hooper LE, Foster-Schubert KE, Weigle DS, Sorensen B, Ulrich CM, et al. (2010) Frequent intentional weight loss is associated with higher ghrelin and lower glucose and androgen levels in postmenopausal women. Nutr Res 30: 163-170.
- Yan Y, Tian L, Xiang X, Ding W, Song G, et al. (2016) Chronic gastric electrical stimulation leads to weight loss via modulating multiple tissue neuropeptide Y, orexin, alpha-melanocyte-stimulating hormone and oxytocin in obese rats. Scand J Gastroenterol 51: 157-167.
- Cha R, Marescaux J, Diana M (2014) Updates on gastric electrical stimulation to treat obesity: Systematic review and future perspectives. World J GastrointestEndosc 6: 419-431.
- Mintchev MP (2013) Gastric electrical stimulation for the treatment of obesity: from entrainment to bezoars-a functional review. ISRN Gastroenterol 2013: 434706.
- Miras M, Serrano M, Duran C, Valiño C, Canton S (2015) Early experience with customized, meal-triggered gastric electrical stimulation in obese patients. ObesSurg 25:174-179.
- Horbach T, Thalheimer A, Seyfried F, Eschenbacher F, Schuhmann P, et al. (2015) Abiliti Closed-Loop Gastric Electrical Stimulation System for Treatment of Obesity: Clinical Results with a 27-Month Follow-Up. ObesSurg 25: 1779-1787.
- Lebovitz HE, Ludvik B, Yaniv I, et al. (2015) Treatment of Patients with Obese Type 2 Diabetes with Tantalus-DIAMOND(R) Gastric Electrical Stimulation: Normal Triglycerides Predict Durable Effects for at Least 3 Years. HormMetab Res 47: 456-462.
- Wong SK, Kong AP, Luk AO, Ozaki R, Ng VW, et al. (2015) A pilot study to compare meal-triggered gastric electrical stimulation and insulin treatment in Chinese obese type 2 diabetes. Diabetes TechnolTher 17: 283-290.
- Korner J, Nandi A, Wright SM, Waitman J, McMohan DJ, et al. (2011) Implantable gastric stimulator does not prevent the increase in plasma ghrelin levels that occurs with weight loss. Obesity (Silver Spring) 19: 1935-1939.
Relevant Topics
- Android Obesity
- Anti Obesity Medication
- Bariatric Surgery
- Best Ways to Lose Weight
- Body Mass Index (BMI)
- Child Obesity Statistics
- Comorbidities of Obesity
- Diabetes and Obesity
- Diabetic Diet
- Diet
- Etiology of Obesity
- Exogenous Obesity
- Fat Burning Foods
- Gastric By-pass Surgery
- Genetics of Obesity
- Global Obesity Statistics
- Gynoid Obesity
- Junk Food and Childhood Obesity
- Obesity
- Obesity and Cancer
- Obesity and Nutrition
- Obesity and Sleep Apnea
- Obesity Complications
- Obesity in Pregnancy
- Obesity in United States
- Visceral Obesity
- Weight Loss
- Weight Loss Clinics
- Weight Loss Supplements
- Weight Management Programs
Recommended Journals
Article Tools
Article Usage
- Total views: 2858
- [From(publication date):
December-2016 - Dec 21, 2024] - Breakdown by view type
- HTML page views : 2202
- PDF downloads : 656