Review Article Open Access
Iron Deficiency and Anaemia in Pregnant Women in Malaysia - Still a Significant and Challenging Health Problem
| Nils Milman* | |
| Departments of Clinical Biochemistry and Obstetrics, Naestved Hospital, University of Copenhagen, Naestved, Denmark | |
| Corresponding Author : | Nils Milman Lindevangen 87B DK-2830 Denmark Tel: +45 20103577 Fax: +45 32719401 E-mail: nils.milman@webspeed.dk |
| Received April 08, 2014; Accepted May 22, 2015; Published May 27, 2015 | |
| Citation: Milman N (2015) Iron Deficiency and Anaemia in Pregnant Women in Malaysia – Still a Significant and Challenging Health Problem. J Preg Child Health 2:168. doi: 10.4172/2376-127X.1000168 | |
| Copyright: © 2015 Milman N, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Journal of Pregnancy and Child Health
Abstract
Background: Iron deficiency (ID) is the most frequent cause of anaemia (IDA) in women of reproductive age and pregnant women. Recommended cut-off value for anaemia in women of reproductive age is haemoglobin (Hb) <12.0 g/dL and in pregnancy Hb <11.0 g/dL. Serum ferritin <15-20 μg/L is consistent with ID; Hb below cut-off values and ferritin <12-15 μg/L is consistent with IDA. Methods: Literature survey on publications and guidelines on the frequency of ID and IDA in Malaysia compared with Western countries. Results: Prevalence of anaemia in women of reproductive age is ~30% and in pregnant women ~40%. With advancing pregnancy the frequency of anaemia increases, due to deficiency of iron and hematinic vitamins (folate, vitamin B12). Iron demands in pregnancy cannot be fulfilled by dietary iron intake but require oral iron supplementation. If the mother has IDA, the newborn has a high risk of ID and IDA. ID and IDA have multiple negative effects in both infants and adults. Oral iron prophylaxis should start at 10-12 weeks gestation. Among pregnant women in Malaysia 80-90%, have low iron status and 38-42% develop anaemia. Consequently, the prophylactic oral iron dose should be 100 mg ferrous iron/day. IDA is treated with oral ferrous iron 180-200 mg/day and Hb should be checked after 2-3 weeks. If there is no increase in Hb, due to poor compliance and/or impaired iron absorption, or the anaemia is severe (Hb <8.0 g/dL) treatment with intravenous iron is indicated. Conclusions: In Malaysia, anaemia in pregnant women still constitute a major and challenging health problem. We are aware of the causes for anaemia and we know the solutions, so efforts should focus on better implementation of early oral iron and vitamin prophylaxis, early diagnosis of anaemia, and on increasing the low compliance of the women to the prophylaxis programs.
| Keywords |
| Anemia; Ferritin; Hemoglobins; Iron-deficiency; Pregnancy; Supplement |
| Abbreviations |
| ATP: Adenosine Triphosphate; DNA: Deoxyribonucleic Acid; Hb: Haemoglobin; ID: Iron Deficiency; IDA: Iron Deficiency Anaemia; TIBC: Total Iron Binding Capacity; WHO: World Health Organization |
| Introduction |
| Iron is an essential metal for humans. The human body operates several hundred iron-containing metalloenzymes in which iron is indispensable. Amongst the most important are ribonuclotide reductase, being necessary for DNA synthesis and cell proliferation, the cytochromes, being involved in electron transport and ATP synthesis. Important iron containing proteins are haemoglobin (Hb), which provides transport of oxygen from the lungs to the body tissues and myoglobin, which serves as an oxygen reserve in the muscles. Consequently, iron is important for a normal function of all cells and organs in the human body. |
| In women who become pregnant, a favorable iron status is necessary for a good course of pregnancy, for the well-being of the mother and for a normal development of the foetus and maturity of the newborn infant [1]. Iron deficiency (ID), even in the absence of iron deficiency anaemia (IDA), has a negative impact on women of reproductive age, causing impaired cognitive abilities and decreased physical performance. In pregnant women, IDA is associated with fatigue, emotional instability, depression, stress, low cognitive performance tests as well as impaired physical performance, palpitations, shortness of breath, increased susceptibility to infections and reduced quality of life. Furthermore, IDA during pregnancy has negative implications for the foetus/newborn, being associated with impaired brain development, premature birth, a low birth weight for gestational age and birth complications. In addition, prepartum IDA is followed by anaemia after delivery, postpartum anaemia [2,3] which is associated with increased peripartum maternal mortality. |
| In Malaysia, there are approximately 500 000 live birth per year. In addition, the prevalence of ID and IDA is high both among women of reproductive age and in pregnant women. The high prevalence of untreated anaemia has a significant negative impact on the productivity of the community and the incidence is related to the socioeconomic status of the population. This poses considerable demands for directing resources to the antenatal care health system and furthermore stresses the importance of keeping focus on the prophylaxis of ID and vitamin deficiencies in pregnant women by promoting an efficient nutritional supplementation policy in the female population. |
| Definition of Iron Deficiency and Anaemia |
| Iron deficiency without anaemia is ascertained by the presence of a low serum ferritin <15-30 μg/L and often a low serum transferrin saturation <20%. |
| In the definition of anaemia, it is practical to use the cut-off values for the Hb concentration recommended by the World Health Organization (WHO), <12.0 g/dL in women of reproductive age and <11.0 g/dL in pregnant women in the first and third trimester and 10.5 g/dL in the second trimester [1,4,5]. Hb can be analyzed on the Hemocue® device or on automated blood analysis machines, which in addition can provide red blood cell indices, reticulocytes, leucocytes and differential count, usually termed “a full blood count”. Iron deficiency anaemia is ascertained by the presence of anaemia and a low serum ferritin <12-15 μg/L usually with a low serum transferrin saturation <15-16%. |
| Causes for Anaemia in Women |
| Iron deficiency |
| In every case of anaemia the diagnostic set-up should be focused on revealing the cause(s) of anaemia, which may be a single cause or perhaps several causes working in synergy, for example concomitant folate, vitamin B12 or vitamin B-complex deficiency. ID, according to WHO, is the most common nutritional deficiency in the world. On a global scale as well as in Malaysia, IDA is solely or partly responsible for 75-80% of all anaemias in women of reproductive age. |
| Hb cannot be used as a marker of iron reserves because the Hb concentration remains constant over a wide range of serum ferritin concentrations. Only when iron reserves are completely exhausted, Hb starts to decline as IDA develops. Therefore, in order to assess whether the anaemia is caused by ID or by other causes, analysis of serum ferritin is very helpful. In healthy subjects, ferritin is a valuable biomarker for body iron status, being an indicator for the size of body iron reserves and is used as a routine analysis in the Western countries. A ferritin <30 μg/L indicates small or absent iron reserves and values <12-15 μg/L are consistent with ID and with IDA if Hb is low. Serum iron as single parameter is of practically no value in the assessment of iron status, but must be combined with serum total iron binding capacity (TIBC) or serum transferrin in order to calculate the TIBC or serum transferrin saturation percent. Transferrin saturation values below 15-16% indicate an insufficient iron supply to the red blood cells in the bone marrow and an insufficient tissue iron supply. Functional iron is assessed by the Hb concentration; a low Hb concentration decreases the functional (physical) working capacity. |
| Main causes for iron deficiency |
| Women of reproductive age have monthly menstrual blood and thereby iron losses, during approximately 40 years and in addition women in Malaysia may have 3-4 pregnancies and child deliveries, which cause further iron losses due to iron depletion during pregnancy (when no supplementary iron is taken) and iron losses due to bleeding at delivery. This is the fundamental cause for the high frequency of ID and IDA in women in Malaysia. In addition, young women in Malaysia have a low mean dietary iron intake around 10 mg/day [6,7], being markedly below the recommended intake of 20-29 mg/day by the Malaysian health authorities [8]. Factors affecting the prevalence of anaemia are socioeconomic status, ethnicity and urban vs. rural location. |
| We distinguish between non-heme iron and heme iron. Nonheme iron is mainly of vegetable origin, it is rather poorly absorbed and the absorption is depending on the balance between enhancers and inhibitors in the diet. Strong enhancers of iron absorption are the “meat factors” (see below) and vitamin C, whereas tea, coffee and calcium are strong inhibitors. Heme iron is present in foods of animal origin, meat, poultry and fish, it has a much better absorption profile than non-heme iron and is to a large extent independent of the presence of enhancers and inhibitors of absorption in the diet. Furthermore, foods of animal origin contain strong enhancers of non-heme iron absorption called “meat factors”. Therefore, taking animal foods together with a vegetable diet provides the best conditions for iron absorption. Alcohol is also an enhancer of iron absorption, however, in Malaysia alcohol consumption is low while tea drinking is widespread due to a strong cultural tradition. |
| In women in Malaysia, the main part of dietary iron, around 75%, is of vegetable origin [6,7], which has a poor bioavailability, so that only 5-10% is absorbed; 25% of dietary iron is of animal origin with a good bioavailability of 20-25%. |
| Other causes for anaemia’s |
| Additional nutritional causes for anaemia can be deficiencies of folate, vitamin B12, the vitamin B complex and ascorbic acid. |
| Malaysia is located in the so called “Thalassaemia Belt”. Thalassaemia occurs with a high prevalence in Malaysia and is a significant cause of anaemia. However, one should be aware of that thalassaemia minor and IDA may co-exist in the same patients, which is diagnosed by both a low Hb and a low serum ferritin (see below). |
| Other causes of anaemia comprise pathological conditions for example anaemia of inflammatory diseases, malignant blood diseases, pathologic blood losses including gynaecological diseases (menorrhagia, metrorrahgia), gastrointestinal disorders (peptic ulcers, inflammatory bowel diseases) gastrointestinal parasitic infestations and infections as well as malabsorption, for example tropical sprue. |
| Anaemia in Women of Reproductive Age and in Pregnant Women in Malaysia is a Significant Health Problem! |
| When does anaemia in a population group become a health problem for the society? The figures are shown in Table 1. In Denmark, the prevalence of anaemia in otherwise healthy young women is 4-5%, which is low and is in fact no problem. However, in Malaysia, the frequency of anaemia is 30-42% and therefore constitutes a moderate to severe public health problem (Table 1). |
| Women of reproductive age |
| In Malaysia, around 30% of women of reproductive age (corresponding to approximately 2 million women) have anaemia according to the WHO surveys [9]. In a recent study of young women from Sabah [6,7] 32% were anaemic, and in 88% the anaemia was due to ID. |
| Eventually, most women of reproductive age in Malaysia will end up becoming pregnant and having children. When non-pregnant women with IDA become pregnant, they have a poor starting position for a good pregnancy and for a good outcome of pregnancy, unless they take adequate iron supplements from early pregnancy. |
| Pregnant women |
| In Malaysia, 38% of pregnant women have anaemia according to the WHO survey [9]. Table 2 shows the surveys of anaemia in women of reproductive age and in pregnant women in Malaysia. In Maternity Hospital, Kuala Lumpur, the prevalence of anaemia in pregnancy was 43.8% [10] and in a subsequent report 50% had IDA [5]. In a retrospective study from 102 health clinics in Kelantan, the prevalence of anaemia and ID at booking was 47.5% [11]. University Malaya Medical Centre, Kuala Lumpur reported a prevalence of anaemia of 42.5%; the prevalence of mild anaemia (Hb 9.5-10.9 g/dL) was 33%; 65.3% of anaemia was classified as IDA with serum ferritin <12 μg/L [12]. There was a gradual decrease in Hb during pregnancy, mean Hb in the first, second and third trimester was 11.8 g/dL, 11.0 g/dL and 10.9 g/dL, respectively. Subsequent studies from Malaysia have reported a frequency of 35-42% in Selangor, Johor and Kelantan, being high in both urban and rural areas [13-15]. A nationwide, cross-sectional study of more than 1000 pregnant women, 20-30 years of age, found that the prevalence of anaemia increased with increasing gestational age, being 12% in the first, 32% in the second and 43% in the third trimester [14]. As most Malaysian studies on anaemia during pregnancy have been performed in the first trimester, the real prevalence of anaemia could well be significantly underestimated. |
| For comparison, in Singapore, a cross-sectional study showed a lower prevalence of anaemia of 15.3% at delivery [16]. The most common cause of anaemia was ID in 81.3%. The occurrence of anaemia in pregnancy was associated with the socio-economic status of the women. Multiparous women of the lower socio-economic class who tended to book late in pregnancy had the highest risk of anaemia [16]. |
| Developing countries have a higher prevalence of anaemia in pregnant women than developed countries. However, even in the European countries, anaemia is still a significant problem when the women are not taking iron supplements. A study in Danish pregnant women showed that among women taking placebo instead of iron, 50% developed ID and 25% developed IDA during pregnancy [17]. |
| The most vulnerable groups for ID and IDA comprise adolescent girls who had their menarche, women of reproductive age, pregnant women and postpartum lactating women. Efforts should be concentrated on measures, which improve iron status and decrease the prevalence of ID and IDA in these groups of women. |
| Iron status in women prior to pregnancy |
| A poor iron status with small or absent iron reserves before pregnancy will increase the risk of ID and IDA during pregnancy. Systematic epidemiologic studies of iron status in women in Malaysia are not available. However, as 30% of women of reproductive age in Malaysia have IDA, the estimated prevalence of ID (serum ferritin <15 μg/L), is 50-60% and the estimated prevalence of small or absent iron reserves (ferritin <30 μg/L) is 90%. |
| For comparison, among healthy ethnic Danish women of reproductive age, 42% have small or absent iron reserves (ferritin <30 μg/L), 10% have ID and 5% have IDA [18]. However, among female immigrants from the Middle East, Far East and South-East Asia, the prevalence of ID and IDA is markedly higher than among Danish women [19]. This is probably also the situation among immigrants/ refugees entering Malaysia from its neighbouring countries, especially from Indonesia. |
| The demands for iron increase drastically during pregnancy |
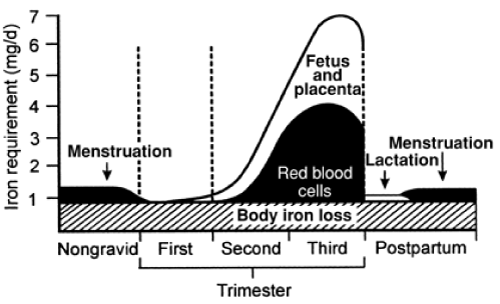
| Pregnancy is the period in a woman’s life where the physiological needs for iron are exceptionally high. The demands for absorbed iron increase gradually from 0.8-1.0 mg/day in the first trimester to 7.5 mg/day in the last trimester (Figure 1) [20]. The gross totals demands for absorbed iron is approximately 1200 mg. Iron is used to expand the mothers red cell mass, for the formation of the placenta and for foetal growth. After delivery, the mothers red cell mass decreases and approximately 600 mg iron from disintegrated Hb in the red blood cells is returned to the mother’s body iron reserves. Thus the net total demands for iron is approximately 600 mg during a normal pregnancy. In addition, there are iron losses during delivery due to blood losses, normally varying from 144 mg (300 ml blood) to 240 mg (500 ml blood). Blood losses in excess of 500 ml are inevitably associated with postpartum ID and IDA. |
| Iron deficiency and anaemia, consequences for the pregnant woman |
| ID and IDA are associated with symptoms, which have a negative effect on the quality of life. It should be recognized that ID without IDA in itself produces symptoms, which affect the daily life. The consequences comprise a reduced exercise capacity [21] and impaired cognitive functions [22], which both respond to treatment with oral iron. |
| IDA may cause psychological changes with insomnia, emotional instability, behavioural disturbances, depression [23], restless legs [24], malfunction of epithelial tissues (hair, skin, nails, tongue, mouth, pharynx) and frequent infections [25]. |
| Furthermore, IDA in late third trimester significantly increases the risk of complications at delivery as well as the risk for maternal perinatal death [26]. |
| Iron deficiency anaemia in pregnant woman, consequences for the foetus and newborn |
| There is a significant correlation between serum ferritin in the mother and the newborn, which shows that the iron status of the newborn is dependent of the iron status of the mother [17]. Iron is essential for a normal development of the brain because ironcontaining metalloenzymes are involved in metabolic processes in the brain. Animal experiments have shown that there is a critical time window in early pregnancy where ID impairs brain development, which cannot be reversed by later intervention with iron treatment. A study in Chilenean children showed that IDA in early childhood, even when sufficiently treated with iron, was associated with compromised recognition memory at both 4 and 10 years of age [27]. A study in Malaysian primary school children demonstrated that both ID and IDA was accociated with negatives effects on processing speed and visual motor coordination [28]. Therefore, inadequate iron supply may have a negative impact on the growing foetal brain and permanently impair the psychomotor development of the newborn child [29]. |
| Studies from developing countries as well as from Singapore, Hong Kong and USA have shown that the presence of IDA in the mother significantly increases the frequency of preterm birth, prematurity and low birth weight for gestational age [16,30,31]. Likewise, a large study from China documented that low Hb values prior to conception in expecting mothers are predictive of a low birth weight [32]. |
| How Shall We Manage Prophylaxis of Iron Deficiency and Anaemia in Pregnancy? |
| Due to the low mean dietary iron intake of approximately 10 mg/ day in women in Malaysia, which is comparable to the mean intake of 9 mg/day in Danish women, iron prophylaxis is inevitable if ID and IDA shall be avoided. |
| Dietary measures are inadequate |
| In order to fulfil iron requirements, the daily diet should contain at least 27 mg iron having 25% bioavailability. This would imply drastic changes in the daily diet, which would be impossible to implement in women in Malaysia. Of course a higher intake of iron from foods of animal origin will improve absorption and have a positive effect on iron status, but dietary studies before and during pregnancy have shown that most women do not change their diet significantly when they become pregnant. The Nordic Nutrition Recommendations 2013, which is the official nutritional organ for the Nordic countries consequently advocate oral iron prophylaxis to all pregnant women, except to those who have iron reserves above 500 mg, which corresponds to serum ferritin levels above 70-80 μg/L. Among healthy Danish women, only 18% have ferritin levels of this magnitude, whereas in healthy women in Malaysia, the estimate is very low, about 4-7%. |
| Oral iron supplements |
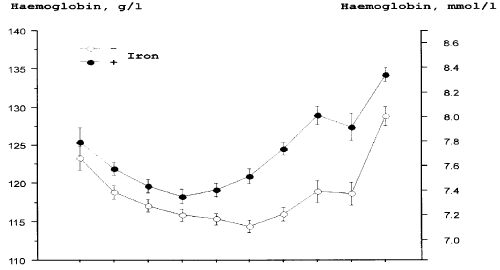
| Oral iron supplements are physiological and easy to administer. Placebo-controlled studies in pregnant women show that oral iron in adequate doses is an efficient prophylaxis against ID and IDA (Figure 2) [17] (Table 3). Amongst women taking 66 mg elemental iron/day, none displayed IDA during pregnancy, whereas amongst women taking placebo, 25% developed IDA [17]. A study from Singapore [16] demonstrated that gestational iron prophylaxis, Hb level at booking, race and previous history of anaemia in earlier pregnancy were important predictors of anaemia at delivery. Women not taking iron prophylaxis had an 11 times higher frequency of anaemia that those taking prophylactic iron [16]. A recent large meta-analysis reported that use of iron supplements during pregnancy was associated with a 50-60% reduction in the risk of ID and IDA. Furthermore, use of iron supplements was associated with a 16% lower risk of preterm birth [33] (Table 3). |
| How should oral iron be taken? |
| Preferably, iron supplements should be taken between meals or at bedtime in order to obtain the highest rate of absorption, because the absorption of standard ferrous salts (sulphate, fumarate, gluconate, succinate) in fast release tablets is diminished by 30-40% when taken with a meal [34], and further diminished if tea is consumed together with the meal. However, the absorption of the slow release iron formula Ferro Duretter®, may to a lesser extent be affected by food than fast release iron formulas [35]. |
| Which dose of iron is appropriate? |
| The adequate dose of iron used for prophylaxis should be tailored according to the iron status of the female population of reproductive age [36]. In developing countries, WHO recommends general oral iron prophylaxis to pregnant women consisting of 60 mg ferrous iron and 5 mg folic acid taken once or twice daily [37]. The prophylaxis should be given for at least 6 months in countries having a prevalence of anaemia below 40%. In Malaysia, 30% of women of reproductive age have IDA, so an estimated 60-70% has ID and an estimated 90-95% has low iron status (ferritin <30 μg/L). Thaneemali et al. [12] reported that 65.3% of pregnant women had serum ferritin <12 μg/L so we therefore estimate that a high percentage has levels <30 μg/L. Ideally, iron supplements should be adjusted according to iron status (serum ferritin), prior to pregnancy or in early pregnancy (Table 4) [38]. The low iron status in women in Malaysia indicates that an adequate iron supplement dose should be close to 100 mg elemental ferrous iron/day (Table 4). |
| The Malaysian Clinical Practice Guidelines “The Management of Anaemia in Pregnancy and Chronic Kidney Disease” [5] advocates: “For prophylaxis, 100 mg of elemental iron with 5 mg folic acid is recommended”. The National Guidelines in Malaysia state:” Iron supplementation, 100 mg iron/day is recommended for pregnant women” [8] and the Malaysian Perinatal Care Manual likewise advocate 100 mg elemental iron/day for prophylaxis in pregnant women [39]. |
| For comparison, in Denmark, where iron status in women of reproductive age is much better than in Malaysia (see above), a ferrous iron dose of 40 mg/day is adequate to prevent IDA in 95% of the pregnant Danish women [40]. In addition, the National Health Authorities recommend supplements with 0.4 mg folic acid/day from at least 2 weeks before planned pregnancy until delivery in order to prevent neural tube defects and ensure a normal development of the foetus [41]. |
| When should iron supplements be started? |
| It is essential, that iron supplements should be started before IDA has developed. Under ideal circumstances, due to the high prevalence of ID and IDA in Malaysia, the women’s iron status should be improved before they become pregnant [42]. Observational studies report that iron supplements need to be started some weeks before pregnancy in order to prevent low birth weight and preterm delivery [43]. This is only possible in a subset of the women, and in the majority of women, the recommendations in Denmark and USA are that iron supplements should be started in early pregnancy at 10 weeks gestation or at the first visit to the antenatal clinic/general practitioner/midwife. |
| Other arguments for an early onset of iron supplement are that the pregnant woman’s red cell mass starts to increase already before 12 weeks gestation and that iron is essential for the very early development of the foetal brain. |
| When should iron supplements be discontinued? |
| When the woman’s iron status is low during pregnancy and at delivery with ID or IDA, and/or peripartum blood losses are 500 ml or more, iron should be continued during the lactation period or for at least three months. If in doubt whether to continue iron or not, check serum ferritin two weeks after delivery and continue supplements if iron status is low (serum ferritin <30 μg/L). WHO recommends that iron supplements should be continued for at least 3 months postpartum in countries with a prevalence of anaemia above 40% [37]. |
| Iron formulas: fast and slow release formulas |
| Iron tablets are produced in two different formulas - as standard, fast release iron salt formulas and as slow release iron formulas. The slow release Durette® principle is used in Duoferon Duretter® = Ferro Duretter® iron formulas, whereas the slow release Gradumet® technology is contained in Ferro-Gradumet® and Ferro-Grad Folic® = IberetFolic® iron formulas. |
| When iron doses of 100 mg or more are prescribed, there may be advantages using slow release instead of fast release formulas. There are several slow release preparations available on the market, but in majority of these, the bioavailability (absorption rate) of the iron is not adequately documented. It is therefore important to use formulas with a documented iron biovailability which should be equal to or preferably higher than fast release formulas. As a consequence of this inadequate documentation, the use of slow release iron formulas is proscribed by the UK National Health Service [44]. |
| In the Nordic Countries, the slow release ferrous iron formula Ferro Duretter® has been widely used with success in the past 30 years. The absorption rate from Ferro Duretter® is approximately 29% higher than from standard ferrous sulphate tablets in blood donors and in subjects with iron deficiency anaemia [35]. The slow release formula, Ferro-Gradumet® is equal to or more effective than fast release tablets in pregnant women [45]. In contrast, another slow release (Ciba Slow Fe®) iron formula showed similar absorption rate as fast release tablets [46]. Ferro-Grad Folic® = IberetFolic®), which in addition to iron contain other haematinics, folic acid, vitamin B12,vitamin B complex and the iron absorption enhancer ascorbic acid, yield higher serum iron, red cell count, hematocrit, Hb and folic acid levels in pregnant women, than Ferro-Gradumet®, which contains only iron [45]. |
| Oral iron may produce gastrointestinal discomfort in some women. These side-effects are dose dependent and become overt at ferrous iron doses above 100-180 mg/day. However, placebo controlled studies in pregnant women have shown that ferrous iron doses of 105 mg/day has a side effect profile, which is not significantly different from placebo [47,48]. The slow release iron formulas Ferro Duretter®, IberetFolic® and ferrous sulphate with mucoproteose (Tardyferon®) have fewer gastrointestinal side-effects (nausea, epigastric pain, constipation) than fast release formulas [45,49,50]. Actually, Ferro Duretter® has a side effect profile being comparable to placebo tablets [49]. |
| Fast release iron tablets yields a high concentrations of iron ions locally in the proximal intestines, which may generate free radicals and damage the intestinal epithelium. Slow release iron tablets generate a lower concentration of free iron in the intestinal lumen, because the iron content of the tablets is released and diluted over a longer segment of the small intestine [44]. |
| The issue of compliance |
| In order to launch an efficient iron prophylaxis program, it is crucial to have a high compliance in the target populations. In pregnant women in Malaysia, the compliance to daily vitamin and/or mineral supplements is only 49% [15]. Compliance is 47% in women in urban Selangor and 52% in women in rural Johor, so there is no difference between urban vs. rural areas. For comparison, the compliance in Danish pregnant women is above 80%, due to an extended education program of health personal promoted by the Danish National Board of Health. |
| It is important to prioritize the improvement of compliance to nutritional supplements in women in Malaysia through health education and adequate information and motivation. Compliance can be enhanced by simplifying the administration of the supplements, for example by restricting the number of tablets to one tablet daily, which should contain adequate amounts of iron, folic acid, vitamin B12 and ascorbic acid. |
| Treatment of Iron Deficiency Anaemia in Pregnancy |
| It is better to prevent than to cure! When a pregnant woman presents with IDA, it is too late for prophylaxis, but it is time for treatment. Guidelines for treatment of IDA are shown in Table 5. Mild to moderate IDA (Hb 8.0-10.5 g/dL) in the first and second trimester should as first choice be treated with oral ferrous iron 180-200 mg elemental iron/day as well as folic acid, vitamin B12 and ascorbic acid [39]. These haematinics are included in a slow-release iron formula (IberetFolic®), which besides prophylaxis for ID/IDA, also can be used for treatment of IDA. Treatment should be continued until Hb has increased to normal level and serum ferritin is above 30-50 μg/L. It is important to check the Hb concentration after two weeks in order to see whether the treatment works. If Hb does not increase by 0.3-1.0 g/dL per week, treatment has not succeeded, either due to poor compliance or due to poor absorption of oral iron. In these cases, parenteral iron treatment should be considered. Ferric iron sucrose can be administered intravenously in doses of 200 mg elemental iron up to a total of 1000 mg iron. Low molecular weight iron dextran can be given intravenously in doses of 500-1000 mg iron. Another intravenous iron option is ferric iron carboxymaltose, which is given as a total dose infusion of 500-1000 mg elemental iron. Intravenous iron should be administered at clinics or in a hospital regimen. |
| In severe IDA (Hb <8.0 g/dL) in the first and second trimester, oral iron treatment (see above) should be tried first. If there is no increase in Hb after two weeks, then proceed to intravenous iron. |
| When a woman presents with IDA in the third trimester, it is important to alleviate or at least reduce anaemia prior to delivery, in order to reduce peripartum complications. Oral iron treatment works slowly and do not have enough time to be efficient. Therefore, intravenous iron should be administered in order to treat anaemia and reduce the need for blood transfusions in late pregnancy and peripartum. Often these women need referral to hospital clinics for assessment for intravenous iron and/or blood transfusion. In women with severe anaemia who are symptomatic and are beyond 36 weeks gestation, blood transfusions are indicated in order to correct anaemia. Preferably, a Hb level of 9.5- 10.0 g/dL should be obtained prior to delivery. Women who refuse blood transfusion can be treated with intravenous iron and human recombinant erythropoietin 10.000 IU subcutaneously for 3-5 days in order to further stimulate erythropoiesis (Table 5). |
| Iron Deficiency and Anaemia in Late Pregnancy and Postpartum |
| The aspects of prepartum and postpartum anaemia constitute further arguments for iron prophylaxis during pregnancy. There is a significant correlation between maternal mortality rate and the prevalence of anaemia [52]. In severe anaemia, maternal death may occur in the last trimester, during labour, immediately after delivery and during puerperium due to cardiac failure or pulmonary embolism [53]. Anaemia in association with delivery increases the frequency of birth complications [2,3,26]. Therefore, a reduction in the prevalence of prepartum and postpartum anaemia will also reduce maternal mortality rate. Postpartum anaemia depends on two main factors: I. The woman’s prepartum iron status, presence of ID or IDA at delivery; II. The magnitude of blood losses in association with delivery. Therefore, a good prepartum iron status and moderate blood losses of 200-400 ml will reduce the risk of peripartum anaemia and thus of maternal death. In postpartum IDA, oral iron and folate therapy must be continued during the lactation period for at least three months [54]. |
| Haemoglobinopathies, Pregnancy and Iron Supplements? |
| Th e populations in Malaysia display high prevalences of haemoglobinopathies. The most prevalent are α-thalassaemia, β-thalassaemia, Hb E and Hb Constant Spring. Thalassemias are genetic disorders, with an autosomal recessive inheritance, which cause in impaired production of the haemoglobin molecule. As 4.5% of the Malays and Chinese in Malaysia are heterozygous β-thalassemia carriers, this constitutes an important health issue in Malaysia. Homozygous β-thalassemia major produces severe anaemia and the patients require blood transfusions in order to survive. Thalassemia is seldom in Indians in Malaysia [55]. Prevention and control of thalassaemias should include population screening for heterozygotes, genetic counselling and fetal diagnosis with selective abortion of affected pregnancies. In addition, Indians in Malaysia may be carriers of the sickle cell trait and some even have sickle cell anaemia, which may be combined with α-thalassemia. |
| The severe forms of thalassaemias need to be treated with blood transfusions and many patients develop significant iron overload, confirmed by measurements of serum ferritin, which may be highly elevated above 300-1000 μg/L depending on the number of transfusions. Iron overload may cause dysfunction of the organs, especially the heart, and necessitate treatment with iron chelators [56]. In this category of patients, implementation of pregnancy is a complicated and controversial issue and iron supplements are contraindicated. |
| However, the majority of women in Malaysia with thalassaemias have the “minor” variants, they are heterozygotes, and have no or few symptoms. We assume that women in Malaysia with thalassaemia minor have an iron status, which is comparable to normal women, indicating that many may have ID and IDA in addition to thalassaemia minor. A study from Thailand showed that among pregnant women with anaemia and haemoglobinopathy, 23% had IDA [57]. However, some of the women with non-transfusion-dependent thalassaemia minor, as well as women with thalassaemia intermedia have ineffective erythropoiesis, which generates increased gastrointestinal iron absorption and increased iron stores, which may progress into clinical iron overload with markedly elevated serum ferritin. Therefore, when a pregnant woman with thalassaemia minor or thalassaemia intermedia attends the antenatal care clinic, the serum ferritin should always be checked before iron supplements are prescribed. If the woman presents with slight to moderate anaemia, it can either be due to thalassaemia minor/intermedia or to IDA or both. To discriminate between these two disorders it is necessary to measure serum ferritin and if available also serum transferrin saturation. If ferritin is above 80-100 μg/L, iron supplements are not necessary. A ferritin below 15-30 μg/L indicates ID/IDA and iron supplements or oral iron treatment should be prescribed and subsequently Hb should be checked after 2 weeks in order to evaluate whether the therapeutic response is adequate. Antenatal care of women with haemoglobinopathies requires special clinical knowledge concerning iron metabolism in the context of the various forms of thalassaemias. |
| Conclusion |
| In Malaysia and in many other countries in South East Asia, ID/ IDA in pregnant women constitute a major health problem. The negative consequences of ID/IDA impair quality of life for both women and newborn babies. ID/IDA contributes to perinatal complications, postpartum anaemia, increased maternal perinatal mortality, as well as prematurity and low birth weight of the newborns. We are well aware of the causes for ID/IDA and we know the solutions, so efforts should focus on diagnosing ID/IDA, implementing oral iron prophylaxis and increasing the compliance of the pregnant women. |
References
- Milman N(2008) Prepartum anaemia: prevention and treatment.Ann Hematol 87: 949-959.
- Milman N (2011) Postpartum anemia I: definition, prevalence, causes, and consequences.Ann Hematol 90: 1247-1253.
- Milman N (2012) Postpartum anemia II: prevention and treatment.Ann Hematol 91: 143-154.
- Milman N, Bergholt T, Byg KE, Eriksen L, Hvas AM (2007) Reference intervals for haematological variables during normal pregnancy and postpartum in 434 healthy Danish women.Eur J Haematol 79: 39-46.
- Anemia Working Group Malaysia (2007) Clinical Practice Guidelines. The management of anemia in pregnancy and chronic kidney disease.
- Foo LH, Khor GL, Tee ES, Prabakaran D (2004) Iron status and dietary iron intake of adolescents from a rural community in Sabah, Malaysia. Asia Pacific J ClinNutr 13:48-55.
- Foo LH, Khor GL, Tee ES, Dhanaraj P (2004) Determinants of iron status in Malaysian adolescents from a rural community.Int J Food SciNutr 55: 517-525.
- Recommended Nutrient Intakes for Malaysia (2005). Recommended Nutrient Intakes for Malaysia.
- McLean E, Cogswell M, Egli I, Wojdyla D, de Benoist B (2009) Worldwide prevalence of anaemia, WHO Vitamin and Mineral Nutrition Information System, 1993-2005.Public Health Nutr 12: 444-454.
- Tee E Siong, Kandiah M, Ali J, Kandiah V, Zahari MR, et al. (1984) Nutritional anemia in pregnancy: a study at the maternity hospital, Kuala Lumpur.Malays J Reprod Health 2: 32-50.
- Ahmad Z Jr, Jaafar R, Mohd Hassan M, Othman M, Hashim A (1997) Anaemia during pregnancy in rural Kelantan.Malays J Nutr 3: 83-90.
- Thaneemali J, Jamiyah H (2005) Incidence of anemia in pregnancy – University Malaya Medical Centre. Mal J ObstetGynecol 8:74-75.
- Hassan R, Abdullah WZ, NikHussain NH (2005) Anemia and iron status of Malay women attending an antenatal clinic in KubangKerian, Kelantan, Malaysia.Southeast Asian J Trop Med Public Health 36: 1304-1307.
- Haniff J, Das A, Onn LT, Sun CW, Nordin NM, et al. (2007) Anemia in pregnancy in Malaysia: a cross-sectional survey.Asia Pac J ClinNutr 16: 527-536.
- Thirukkanesh S, ZaharaAM (2010) Compliance to vitamin and mineral supplementation among pregnant women in urban and rural areas in Malaysia. Pac J Nutr 9:744-750.
- Singh K, Fong YF, Arulkumaran S (1998) Anaemia in pregnancy--a cross-sectional study in Singapore.Eur J ClinNutr 52: 65-70.
- Milman N, Agger OA, Nielsen OJ (1991) Iron supplementation during pregnancy. Effect on iron status markers, serum erythropoietin and human placental lactogen. A placebo controlled study in 207 Danish women. Dan Med Bull 38: 471–476.
- Milman N, Byg K-E, Ovesen L, Kirchhoff M, Jürgensen K S-L (2003) Iron status in Danish women, 1984-1994: a cohort comparison of changes in iron stores and the prevalence of iron deficiency and iron overload. Eur J Haematol 71: 51-61.
- Nybo M, Friis-Hansen L, Felding P, Milman N (2007) Higher prevalence of anemia among pregnant immigrant women compared to pregnant ethnic Danish women.Ann Hematol 86: 647-651.
- Milman N (2006) Iron and pregnancy--a delicate balance.Ann Hematol 85: 559-565.
- Brownlie T 4th, Utermohlen V, Hinton PS, Giordano C, Haas JD (2002) Marginal iron deficiency without anemia impairs aerobic adaptation among previously untrained women.Am J ClinNutr 75: 734-742.
- Murray-Kolb LE, Beard JL (2007) Iron treatment normalizes cognitive functioning in young women.Am J ClinNutr 85: 778-787.
- Beard JL, Hendricks MK, Perez EM, Murray-Kolb LE, Berg A, et al. (2005) Maternal iron deficiency anaemia affects postpartum emotions and cognition. J Nutr 135: 267-272.
- Birgegård G, Schneider K, Ulfberg J (2010) High incidence of iron depletion and restless leg syndrome (RLS) in regular blood donors: intravenous iron sucrose substitution more effective than oral iron.Vox Sang 99: 354-361.
- Kumar V, Choudhry VP (2010) Iron deficiency and infection.Indian J Pediatr 77: 789-793.
- Chan KK, Chan BC, Lam KF, Tam S, Lao TT (2009) Iron supplement in pregnancy and development of gestational diabetes--a randomised placebo-controlled trial.BJOG 116: 789-797.
- Congdon EL, Westerlund A, Algarin CR, Peirano PD, Gregas M, et al. (2012) Iron deficiency in infancy is associated with altered neural correlates of recognition memory at 10 years.J Pediatr 160: 1027-1033.
- Hamid Jan JM Jr, Amal KM, Rohani A, Norimah AK (2010) Association of Iron Deficiency with or without Anaemia and Cognitive Functions among Primary School Children in Malaysia.Malays J Nutr 16: 261-270.
- Grantham-McGregor S, Ani C (2001) A review of studies on the effect of iron deficiency on cognitive development in children.J Nutr 131: 649S-666S.
- Ramakrishnan U(2004) Nutrition and low birth weight: from research to practice.Am J ClinNutr 79: 17-21.
- Rasmussen KM, Stoltzfus RJ (2003) New evidence that iron supplementation during pregnancy improves birth weight: new scientific questions.Am J ClinNutr 78: 673-674.
- Ronnenberg AG, Wood RJ, Wang X, Xing H, Chen C, et al. (2004) Preconception hemoglobin and ferritin concentrations are associated with pregnancy outcome in a prospective cohort of Chinese women.J Nutr 134: 2586-2591.
- Haider BA, Olofin I, Wang M, Spiegelman D, Ezzati M, et al. (2013) Nutrition Impact Model Study Group (anaemia). Anaemia, prenatal iron use, and risk of adverse pregnancy outcomes: systematic review and meta-analysis. BMJ 346: f3443
- Brise H (1962) Influence of meals on iron absorption in oral iron therapy.Acta Med ScandSuppl 376: 39-45.
- Boye Nielsen J ,lkkala E. Sölvell L. Björn-Rasmussen E. Ekenved G (1976) Absorption of iron from slow-release and rapidly-disintegrating tablets - A comparative study in normal subjects, blood donors and subjects with iron deficiency anemia. Scand J Haematol 28: 89-97.
- Milman N (2012) Oral iron prophylaxis in pregnancy: not too little and not too much!J Pregnancy 2012: 514345.
- Stoltzfus R, Dreyfuss ML (1998) Guidelines for the use of iron supplements to prevent and treat iron deficiency anemia. INAGG, WHO, UNICEF. Geneva.
- Milman N, Byg KE, Bergholt T, Eriksen L, Hvas AM (2006) Body iron and individual iron prophylaxis in pregnancy--should the iron dose be adjusted according to serum ferritin?Ann Hematol 85: 567-573.
- Perinatal Care Manual. Antenatal Care, Section 2. Division of Family Health Development. Ministry of Health Malaysia, 2nd ed. 2010.
- Milman N, Bergholt T, Eriksen L, Byg KE, Graudal N, et al. (2005) Iron prophylaxis during pregnancy -- how much iron is needed? A randomized dose- response study of 20-80 mg ferrous iron daily in pregnant women.ActaObstetGynecolScand 84: 238-247.
- Milman N (2012) Intestinal absorption of folic acid - new physiologic & molecular aspects.Indian J Med Res 136: 725-728.
- Peña-Rosas JP, De-Regil LM, Dowswell T, Viteri FE (2012) Daily oral iron supplementation during pregnancy.Cochrane Database Syst Rev 12: CD004736.
- Scholl TO (2011) Maternal iron status: relation to fetal growth, length of gestation, and iron endowment of the neonate.Nutr Rev 69 Suppl 1: S23-29.
- Joint Formulary Committee (2012) British National Formulary. 63rd edition. London: British Medical Association and Royal Pharmaceutical Society of Great Britain.
- Morrison J, Bell J, Chang AM, Larkin PK (1977) A comparative trial of haematinic supplements in pregnancy.Med J Aust 1: 482-484.
- Baird IM, Walters RL, Sutton DR (1974) Absorption of slow-release iron and effects of ascorbic acid in normal subjects and after partial gastrectomy.Br Med J 4: 505-508.
- Kerr DN, Davidson S (1958) The prophylaxis of iron-deficiency anaemia in pregnancy.Lancet 2: 483-488.
- Milman N, Byg KE, Bergholt T, Eriksen L (2006) Side effects of oral iron prophylaxis in pregnancy--myth or reality?ActaHaematol 115: 53-57.
- Rybo G, Sölvell L (1971) Side-effect studies on a new sustained release iron preparation.Scand J Haematol 8: 257-264.
- Cancelo-Hidalgo MJ, Castelo-Branco C, Palacios S, Haya-Palazuelos J, Ciria-Recasens M, et al. (2013) Tolerability of different oral iron supplements: a systematic review.Curr Med Res Opin 29: 291-303.
- Network for Advancement of Transfusion Alternatives (NATA): (2007) Guidelines on intravenous iron supplementation in surgery and obstetrics/gynecology. Transfusion Alternatives in Transfusion Medicine 9:29.
- Brabin BJ, Hakimi M, Pelletier D (2001) An analysis of anemia and pregnancy-related maternal mortality.J Nutr 131: 604S-614S.
- Sharma JB (1999) Iron deficiency anemia in pregnancy – still a major cause of maternal mortality and morbidity in India. ObsGynae Today IV:693-701.
- Krafft A, Perewusnyk G, Hänseler E, Quack K, Huch R, et al. (2005) Effect of postpartum iron supplementation on red cell and iron parameters in non-anaemic iron-deficient women: a randomised placebo-controlled study.BJOG 112: 445-450.
- Tan JA, Lee PC, Wee YC, Tan KL, Mahali NF, et al. (2010) High prevalence of alpha- and beta-Thalassemia in the Kadazandusuns in East Malaysia: challenges in providing effective health care for an indigenous group. J Biomed Biotechnol 2010. Article ID 706872.
- Taher AT, Porter JB, Viprakasit V, Kattamis A, Chuncharunee S, et al. (2013) Deferasirox effectively reduces iron overload in non-transfusion-dependent thalassaemia (NTDT) patients: 1-year extension results from the THALASSA study. Ann Hematol 92:1485-1493.
- Thinkhamrop J, Apiwantanakul S, Lumbiganon P, Buppasiri P (2003) Iron status in anemic pregnant women.J ObstetGynaecol Res 29: 160-163.
Tables and Figures at a glance
| Table 1 | Table 2 | Table 3 | Table 4 | Table 5 |
Figures at a glance
 |
 |
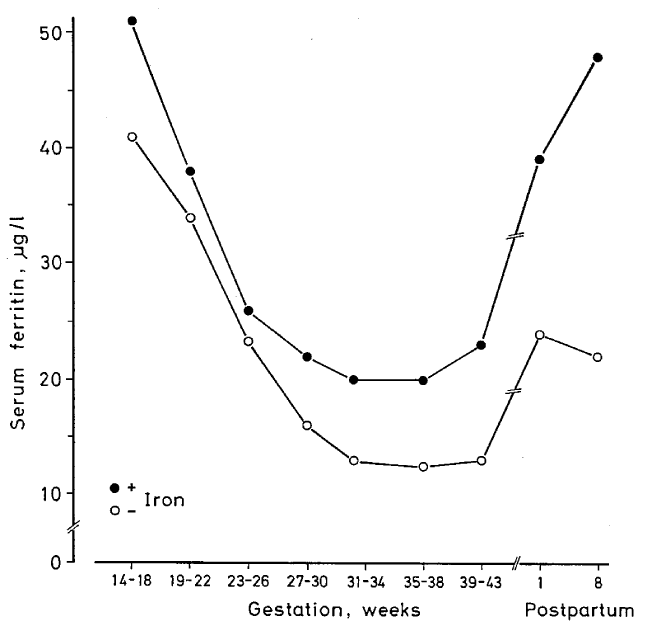 |
| Figure 1 | Figure 2a | Figure 2b |
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 40873
- [From(publication date):
June-2015 - Jun 30, 2025] - Breakdown by view type
- HTML page views : 34308
- PDF downloads : 6565
