Review Article Open Access
Involvement of PKR in Alzheimer's Disease
Jacques Hugon1,2*, François Mouton-Liger2, Julien Dumurgier1,2, Pauline Lapalus1, Magali Prévôt1, Sandrine Indart1, Jean Louis Laplanche3 and Claire Paquet1,2
1Memory Centre, Saint Louis Lariboisiere Fernand Widal Hospital APHP, University Paris Diderot Paris, France
2Inserm Unit 942, Saint Louis Lariboisiere Fernand Widal Hospital APHP, University Paris Diderot Paris, France
3Department of Biochemistry, Saint Louis Lariboisiere Fernand Widal Hospital APHP, University Paris Diderot Paris, France
- Corresponding Author:
- Jacques Hugon
Memory Centre, Saint Louis Lariboisiere Fernand Widal Hospital APHP
University Paris Diderot Paris, France
Tel: 33 1 40054313
E-mail: jacques.hugon@inserm.fr
Received date: March 10, 2014; Accepted date: June 30, 2014; Published date: July 30, 2014
Citation: Hugon J, Mouton-Liger F, Dumurgier J, Lapalus P, Prevot M, et al. (2014) Involvement of PKR in Alzheimer’s Disease. J Alzheimers Dis Parkinsonism 4: 154. doi:10.4172/2161-0460.1000154
Copyright: © 2014 Hugon J, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Alzheimers Disease & Parkinsonism
Abstract
Alzheimer’s disease (AD) is characterized by memory troubles followed by aphasia apraxia and agnosia associated with behavioral disturbances. Neuropathological lesions include senile plaques formed by Aβ peptide, neurofibrillary tangles made of hyperphosphorylated tau and neuronal loss. The cause of AD is unknown but Aβ peptide could be responsible for neuronal degeneration. PKR is a stress and pro-apoptotic kinase that controls protein translation via the phosphorylation of the eukariotic initiation factor 2α (eIF2α). Activated PKR accumulates in affected neurons in AD brains and the phosphorylation of PKR can be induced by Aβ peptide. We have found increased levels of PKR in the cerebrospinal fluid of AD patients and PKR level is a good predictor of the cognitive decline. In addition PKR can modulate the levels of BACE1, an APP cleaving enzyme, and can influence tau phosphorylation. Altogether, PKR represents a potential new biomarker and a valid new therapeutic target for neuroprotection in AD.
Keywords
Alzheimer’s disease; Apraxia; Agnosia; Neuropathological lesions; Senile plaques
Introduction
Alzheimer’s disease (AD) is becoming a major question of public health in developed countries because of population aging. This disease is clinically characterized by progressive memory disturbances followed by aphasia, apraxia and agnosia associated with behavioral symptoms. The dementia stage is very often preceded by a prodromal phase called mild cognitive impairment (MCI) in patients with light cognitive alteration and no repercussion of symptoms in the daily life. It is clinically difficult to determine if the MCI phase is associated with AD but this has been very much improved with the use of biological markers of the cerebrospinal fluid. The pathology of AD is made of senile plaques with accumulated Aβ peptide, neurofibrillary tangles with abnormally phosphorylated tau protein and synaptic and neuronal losses. The cause of AD is not established but the Aβ peptide could be toxic to neurons and other brain cells, leading to the amyloid cascade hypothesis [1]. The cause of Aβ accumulation in sporadic forms of AD is not fully elucidated. Aβ is formed after cleavage of APP (amyloid precursor protein) by β secretase (BACE1) followed by the cleaving action of γ secretase. Aβ production is prevented when α secretase cleaves APP. According to the amyloid cascade hypothesis the accumulation of Aβ or oligomeric forms of Aβ could be responsible for the deleterious consequences detected in AD brains leading to neuronal loss and dementia. This accumulation could be linked to an increased Aβ production due to enhanced BACE1 and γ secretase activities or to a reduced degradation [2]
PKR
PKR was identified as a protein kinase activated by double-stranded RNA, which plays a major role in the defense against virus. PKR is a stress and pro-apoptotic kinase also activated by interferon, TNFα, calcium, Aβ peptide and other agents [3]. One of its main mechanisms of action is to block protein synthesis by phosphorylation and inhibition of eIF2α, thereby decreasing or preventing viral replication. However, PKR has many other activators and targets. PKR is activated through a complex mechanism which combines displacement of an N-terminal inhibitory domain, dimerization, and autophosphorylation of the activation loop on 2 residues (Thr-446 and Thr-451). PKR is implicated in the production of inflammatory cytokines through NFκB activation and cell apoptosis and can participate in the activation of the inflammasome.
PKR and Memory
Several recent reports have shown that PKR plays a role in memory formation. In a conditional transgenic mouse strain in which PKR was activated in hippocampus by the administration of a compound AP20187, the authors have revealed that PKR activation and eIF2α phosphorylation in CA1 hippocampal region, impaired late phase LTP and memory consolidation [4]. In 2011, Zhu et al. have observed that the lack of PKR in PKR-/- mice enhanced learning and memory in several tasks by increasing network excitability [5]. In 2013, another report has shown that the blockade of PKR enhances positive and negative forms of cortex dependent taste memory [6]. Finally another data reported that Aβ oligomers can lead to the production of TNFα and to PKR-dependent memory impairment in mice and monkeys [7].
PKR and AD
In 2002, we have demonstrated that the pro-apoptotic and stress kinase PKR that can phosphorylates eIF2α, accumulates in degenerating neurons of AD brains. Activated PKR accumulations were sometimes associated with neurofibrillary tangles [8]. In addition we have also shown that PKR could be activated in vitro by Aβ peptide leading to neuronal death. This neurotoxicity could be reduced by primary neuronal cultures from PKR-/- mice exposed to Aβ peptide [9]. Subsequently, we have revealed that the Aβ peptide-induced PKR activation in neural cell cultures was linked to caspase 3 activity associated with the release of ER calcium produced by Aβ peptide [10,11]. Recently we found that levels of the PKR activator PACT correlates with activated PKR levels in AD brains and transgenic mice [12].
Cellular stresses can activate PKR and several of these stresses are observed in senescent cells and aging is the major risk factor for AD. Aging can also enhance PKR expression [13] and could render neurons more vulnerable to the initial molecular changes leading to AD pathological lesions. PKR gene polymorphisms were recently associated with AD [14]. The time of occurrence of the lesions in AD brains is not known but they could start several years before the onset of clinical signs marked by memory disturbances. The analysis of the levels of activated PKR and eIf2 α in human body fluids such as CSF or blood has represented an interesting way to detect early biological changes in patients affected by Mild Cognitive Impairment (MCI) or Subjective Cognitive Impairment (SCI) occurring before MCI. We have shown that phosphorylated PKR could also be a good blood biomarker linked to the cognitive decline in AD patients but some overlaps were found between the PKR levels found in AD patients and in controls [15]. Therefore we decided to analyze the levels of phosphorylated PKR and total PKR in the CSF of AD patients and neurological controls. In a prospective study the CSF levels of Aβ 1-42, tau, phosphorylated 181 tau, phosphorylated PKR and total PKR were assessed in 45 AD patients, 11 patients with Mild Cognitive Impairment (MCI) and 35 neurological controls. The mean level of phosphorylated PKR was increased by 300 % in AD patients compared to controls. The phosphorylated PKR levels were also enhanced in MCI patients. In AD patients the levels of total and phosphorylated PKR correlated with the levels of phosphorylated 181 tau [16]. We have followed our patients for a period of 2 to 3 years and used a mixed linear model to explore the usefulness of PKR as a predictive biomarker [17]. The results showed that phosphorylated PKR levels were associated with longitudinal MMSE scores during the follow-up period. Patients with high CSF phosphorylated PKR levels have had a more rapid cognitive decline. Altogether PKR accumulated in AD brains and is released into the CSF and could be a good diagnostic and prognostic marker and further confirmatory studies will be needed in the future. PKR can also be part of the pathophysiological pathways associated with AD brain lesions.
PKR and BACE1
So far very little has been done on the possible control of BACE1 expression and Aβ production by PKR activation. The cleavage of APP by BACE1 is the key step limiting enzyme for Aβ production [18]. BACE1 levels are increased in AD brains and AD transgenic mice exposed to acute energy inhibition. PKR can generally block protein translation via the phosphorylation of eIF2α on serine 51 but this activation can, for some specific mRNAs with particular Open Reading Frame (ORF), including BACE1, induce an increased translation. A recent report in 2008 has shown that BACE1 expression is increased by the phosphorylation of eIF2α [19]. Because the levels of BACE1 are controlled by the eIF2α phosphorylation and because PKR can modulate this phosphorylation, we have explored if activated PKR that is increased in AD brains could be responsible for augmented neuronal degeneration and enhanced BACE1 levels. We have shown that BACE1 levels correlated with phosphorylated eIF2α levels in AD brains and in the brain of APP/PS1 transgenic mice. In addition in neuroblastoma cell cultures exposed to oxidative stress, we have revealed that phosphorylated PKR, eIF2α and BACE1 levels were increased and that the pharmacological inhibition of PKR reduced BACE1 levels in exposed cells [20]. These findings suggest that the activation of PKR could control the levels of BACE1 in stressed cells.
PKR and tau
Phosphorylated tau is the main component of neurofibrillary tangles. Tau can be phosphorylated by many kinases including GSK3β. In the brain of AD patients we have reported a co-localization of phosphorylated tau and PKR [8]. We have analyzed the links between PKR, GSK3β and tau protein [21]. In AD brains the two activated kinases co-localized with phosphorylated tau. In neural cell cultures, Aβ induced the phosphorylation of PKR, GSK3β and tau phosphorylation. The pharmacological inhibition of PKR reduced GSK3 and tau phosphorylations suggesting that PKR could indirectly controlled the abnormal formation of tangles.
Conclusion
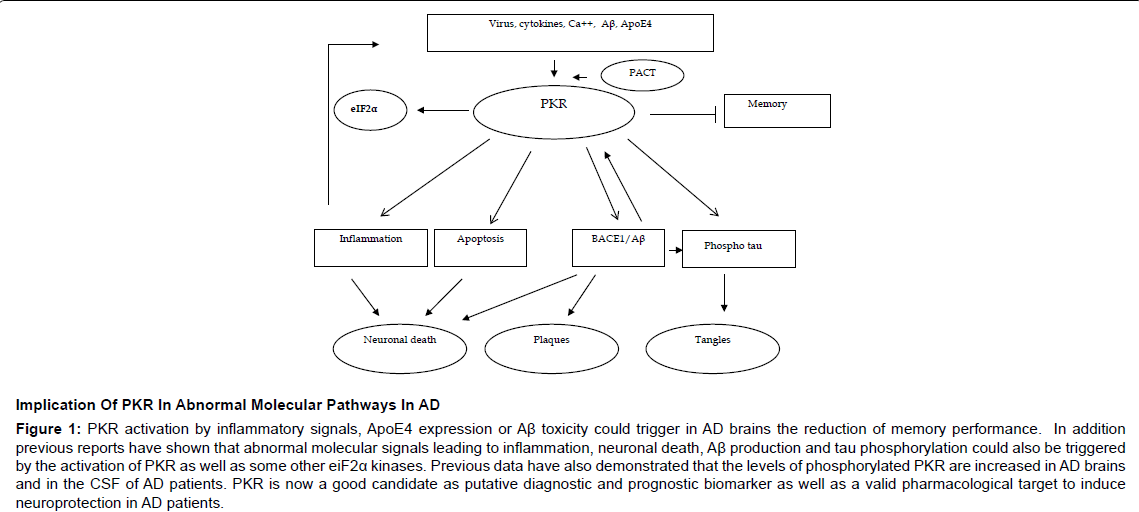
All these findings suggest that PKR and eIF2α could play a role in the events leading to abnormal molecular signals at the origin of neurodegeneration in AD as well as in memory disturbances. PKR is a potential biomarker of this disease and is also a molecular target in AD. The inhibition of PKR activation could modify the pathological pathways linked to neuronal demise and afford neuroprotection in AD [22-24] (Figure 1).
Figure 1: PKR activation by inflammatory signals, ApoE4 expression or Aß toxicity could trigger in AD brains the reduction of memory performance. In addition previous reports have shown that abnormal molecular signals leading to inflammation, neuronal death, Aß production and tau phosphorylation could also be triggered by the activation of PKR as well as some other eiF2a kinases. Previous data have also demonstrated that the levels of phosphorylated PKR are increased in AD brains and in the CSF of AD patients. PKR is now a good candidate as putative diagnostic and prognostic biomarker as well as a valid pharmacological target to induce neuroprotection in AD patients.
References
- Hardy J, Selkoe DJ (2002) The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science 297: 353-356.
- Mao P, Reddy PH (2011) Aging and amyloid beta-induced oxidative DNA damage and mitochondrial dysfunction in Alzheimer's disease: implications for early intervention and therapeutics. Biochim Biophys Acta 1812: 1359-1370.
- Marchal JA, Lopez GJ, Peran M, Comino A, Delgado JR, et al. (2014) The impact of PKR activation: from neurodegeneration to cancer. FASEB J 28: 1965-1974.
- Jiang Z, Belforte JE, Lu Y, Yabe Y, Pickel J, et al. (2010) eIF2alpha Phosphorylation-dependent translation in CA1 pyramidal cells impairs hippocampal memory consolidation without affecting general translation. J Neurosci 30: 2582-2594.
- Zhu PJ, Huang W, Kalikulov D, Yoo JW, Placzek AN, et al. (2011) Suppression of PKR promotes network excitability and enhanced cognition by interferon-γ-mediated disinhibition. Cell 147: 1384-1396.
- Stern E, Chinnakkaruppan A, David O, Sonenberg N, Rosenblum K (2013) Blocking the eIF2α kinase (PKR) enhances positive and negative forms of cortex-dependent taste memory. J Neurosci 33: 2517-2525.
- T Lourenco MV, Clarke JR, Frozza RL, Bomfim TR, Forny-Germano L, et al. (2013) TNF-a mediates PKR-dependent memory impairment and brain IRS-1 inhibition induced by Alzheimer's ß-amyloid oligomers in mice and monkeys. Cell Metab. 18: 831-843.
- Chang RC, Wong AK, Ng HK, Hugon J (2002) Phosphorylation of eukaryotic initiation factor-2alpha (eIF2alpha) is associated with neuronal degeneration in Alzheimer's disease. Neuroreport 13: 2429-2432.
- Chang RC, Suen KC, Ma CH, Elyaman W, Ng HK, et al. (2002) Involvement of double-stranded RNA-dependent protein kinase and phosphorylation of eukaryotic initiation factor-2alpha in neuronal degeneration. J Neurochem 83: 1215-1225.
- Suen KC, Yu MS, So KF, Chang RC, Hugon J (2003) Upstream signaling pathways leading to the activation of double-stranded RNA-dependent serine/threonine protein kinase in beta-amyloid peptide neurotoxicity. J Biol Chem 278: 49819-49827.
- A Page G, Rioux Bilan A, Ingrand S, Lafay-Chebassier C, Pain S, et al. (2006) Perault Pochat MC, Bouras C, Bayer T, Hugon J. Activated double-stranded RNA-dependent protein kinase and neuronal death in models of Alzheimer's disease.Neuroscience.139: 1343-1354.
- Paquet C, Mouton-Liger F, Meurs EF, Mazot P, Bouras C, et al. (2012) The PKR activator PACT is induced by Aß: involvement in Alzheimer's disease. Brain Pathol 22: 219-229.
- Ladiges W, Morton J, Blakely C, Gale M (2000) Tissue specific expression of PKR protein kinase in aging B6D2F1 mice. Mech Ageing Dev. 114: 123-132.
- Bullido MJ, MartÃnez-GarcÃa A, Tenorio R, Sastre I, Muñoz DG, et al. (2008) Double stranded RNA activated EIF2 alpha kinase (EIF2AK2; PKR) is associated with Alzheimer's disease. Neurobiol Aging 29: 1160-1166.
- Paccalin M, Pain-Barc S, Pluchon C, Paul C, Besson MN, et al. (2006) Activated mTOR and PKR kinases in lymphocytes correlate with memory and cognitive decline in Alzheimer's disease. Dement Geriatr Cogn Disord 22: 320-326.
- Paccalin M, Pain-Barc S, Pluchon C, Paul C, Besson MN, et al. (2006) Activated mTOR and PKR kinases in lymphocytes correlate with memory and cognitive decline in Alzheimer's disease. Dement Geriatr Cogn Disord 22: 320-326.
- Mouton-Liger F, Paquet C, Dumurgier J, Lapalus P, Gray F, et al. (2012) Groupe d'Investigation du Liquide Céphalorachidien Study Network.Increased cerebrospinal fluid levels of double-stranded RNA-dependant protein kinase in Alzheimer's disease. Biol Psychiatry. 71: 829-835.
- Dumurgier J, Mouton-Liger F, Lapalus P, Prevot M, Laplanche JL, et al. (2013) Groupe d’Investigation du Liquide Cephalorachidien (GIL) Study Network. Cerebrospinal fluid PKR level predicts cognitive decline in Alzheimer's disease. PLoS One. 8: e53587.
- Velliquette RA, O'Connor T, Vassar R (2005) Energy inhibition elevates beta-secretase levels and activity and is potentially amyloidogenic in APP transgenic mice: possible early events in Alzheimer's disease pathogenesis. J Neurosci 25: 10874-10883.
- O'Connor T, Sadleir KR, Maus E, Velliquette RA, Zhao J, et al. (2008) Phosphorylation of the translation initiation factor eIF2alpha increases BACE1 levels and promotes amyloidogenesis. Neuron 60: 988-1009.
- O'Connor T, Sadleir KR, Maus E, Velliquette RA, Zhao J, et al. (2008) Phosphorylation of the translation initiation factor eIF2alpha increases BACE1 levels and promotes amyloidogenesis. Neuron 60: 988-1009.
- Mouton-Liger F, Paquet C, Dumurgier J, Bouras C, Pradier L, et al. (2012) Oxidative stress increases BACE1 protein levels through activation of the PKR-eIF2α pathway. Biochim Biophys Acta 1822: 885-896.
- Bose A, Mouton-Liger F, Paquet C, Mazot P, Vigny M, et al. (2011) Modulation of tau phosphorylation by the kinase PKR: implications in Alzheimer's disease. Brain Pathol 21: 189-200.
- Hugon J, Paquet C, Chang RC (2009) Could PKR inhibition modulate human neurodegeneration? Expert Rev Neurother 9: 1455-1457.
Relevant Topics
- Advanced Parkinson Treatment
- Advances in Alzheimers Therapy
- Alzheimers Medicine
- Alzheimers Products & Market Analysis
- Alzheimers Symptoms
- Degenerative Disorders
- Diagnostic Alzheimer
- Parkinson
- Parkinsonism Diagnosis
- Parkinsonism Gene Therapy
- Parkinsonism Stages and Treatment
- Stem cell Treatment Parkinson
Recommended Journals
Article Tools
Article Usage
- Total views: 14469
- [From(publication date):
August-2014 - Jul 08, 2025] - Breakdown by view type
- HTML page views : 9848
- PDF downloads : 4621