Investigation of Water-Soluble Silver Ion and Silver Nanoparticles in Wound Dressing using ICP-MS
Received: 15-Feb-2019 / Accepted Date: 11-Mar-2019 / Published Date: 18-Mar-2019 DOI: 10.4172/2155-9872.1000414
Abstract
Due to its anti-microbial properties, silver has been used in wound treatment for many decades. In some cases, the wound is covered a week or longer with a foam dressing that contains silver as an anti-microbial agent. Although more expensive, silver laden dressings have been found to be more effective at reducing the microbial load, thereby allowing more rapid wound healing. However, the exact mechanism of silver’s anti-microbial property is still unclear and the form of the silver that is most effective is unknown. Even though many manufacturers have begun to incorporate silver nanoparticles in wound dressings, there currently exists no U.S. regulation as to which form is to be used, nor at what concentration. This paper explores the water-soluble form of silver in several commercially available wound dressings both through size characterization of the ionic and nanoparticle forms and measurement of each form’s silver concentrations by ICP-MS. The method as a whole represents the beginning of a larger project to investigate the most effective form and optimum concentration of silver for wound dressing inclusion to promote maximum healing.
Keywords: Silver ion; Silver nanoparticle; Wound dressing; ICP-MS
Introduction
Chronic wounds often do not heal due to the severity of the wound, other health issues, or the age of the patient. In the case of a recalcitrant wound that is not healing or in the case of a very complex wound, medical personnel will sometimes employ negative wound therapy. In this practice, the wound is manually scraped so that the blood vessels and tissue may grow again. Once performed, the wound is subjected to mechanical suction, covered with a foam dressing, (because it can handle more fluid than typical gauze and often contains silver), and this is kept in place for 7-10 days [1].
The greatest barrier to healing is colonizing microorganisms growing in the biofilm phenotypic state [2]. Silver has long been used in medicine as an antibiotic for certain bacteria and has more recently been incorporated into wound dressings as a means of reducing bacterial growth in dermal wounds of various causes. It has been reported that silver ion facilitates healing by killing bacteria that might otherwise infiltrate into the tissue, causing further infection [3]. Currently, manufacturers are producing dressings and other medical devices which distribute silver ion and/or silver nanoparticles to the wound in a sustained release [4,5]. However, the exact mechanism and most active form of silver in wound healing are still unclear. Literature reports describe several means by which silver can destroy microbial cells:
(1) Binding to the cell’s DNA, thus preventing cell reproduction [6].
(2) Binding to the cell’s enzymes that control respiration [7-9].
(3) Attaching to the cell wall, causing cell lysis [10,11].
Many manufacturers report the inclusion of both silver nanoparticle and silver ion (+1 oxidation state, generally in the form of silver sulfate or silver alginate) in their dressings. However, it is unknown whether the silver nanoparticle or silver ion is bioactive. It has been speculated that the degree of oxidation of the silver nanoparticle in situ is dependent on its environmental conditions [12]. In this proposed mechanism, an oxidizing agent would expedite dissolution whereas other constituents might serve to coat and protect the nanoparticle. Others theorize a dissolution equilibrium with either a low solubility silver salt or silver nanoparticles that might offer a maximum sustained delivery. However, the extent of silver penetration into the wound has not yet been studied.
The concentration of silver is also an important consideration, both when considering cost and overall toxicity. Inclusion of silver in the wound dressing increases the cost and researchers have documented that excessive silver may retard healing [13]. Although a lifetime limit of 10 g has been set for human safety by the World health organization, silver is a toxin in the environment, requiring proper disposal of used dressings to prevent effects to other organisms [14,15]. Summarily, the ideal dressing would include an adequate amount of silver to promote effective wound healing and lower cost without leaving large concentrations unused for disposal.
Methods are well known that use single particle ICP-MS (spICPMS) in the separation and characterization of silver ion and nanoparticles. Tuoriniemi reported on size discrimination when using spICP-MS to measure nanoparticles (effective for particles >20 nM) and Mitrano and Laborda successfully used spICP-MS for yielding both particle size and concentration of silver nanoparticles within a range of 20-80 nM with the simultaneous measurement of silver ion concentration [16-18]. Several papers note the care that must be exercised in matrices in which the chloride ion is present as dissolved silver may form AgCl nanoparticles or microparticles [19].
In this research, the authors sought to develop a methodology to quantify silver particles and silver ions and to apply that same methodology to explore the form of water-soluble silver in several commercially available wound dressings. Water soluble silver would include silver ion and silver nanoparticles as well as unionized silver sulfate particulates and silver alginate complexes as nanoparticles. Firstly, each wound dressing was analyzed for total silver. An extraction procedure involving centrifugation was developed to analyze watersoluble silver ion separately from water-soluble silver nanoparticles. DI water was used as opposed to a saline solution to eliminate the possibility of AgCl precipitation [20,21]. The protocol was tested with known mixtures with promising results. The final procedure was applied to three commercially available dressings and the results were compared to advertised values.
Materials and Methods
Reagents
Trace metal-grade nitric acid (Thermo Fisher Scientific, Waltham, MA) was used in sample preparation. Calibration curves were prepared in 2% nitric acid from stock elemental solutions (Thermo Fisher Scientific, Waltham, MA). Indium (Thermo Fisher Scientific, Waltham, MA) was used as internal standard in all analyses. Ultrapure water (18 MΩ) for the preparation of all samples was provided by a water purification system (Barnstead E-Pure system, Thermo Fisher Scientific, Waltham, MA). Standard nanoparticles of silver with mean particle size of 20 nM were purchased from Sigma (St. Louis, MO).
The investigation of mixtures of known concentrations of silver ion and silver nanoparticles was conducted using stock solutions with concentrations of 10. μg L-1 prepared from silver nitrate salts and silver nanoparticles. The mixtures were centrifuged at 4000 rpm for 20 minutes to separate particles from soluble ions. The pellet was resuspended in deionized water to reconstitute the suspension of particles. The stock mixtures and supernatant and pellet suspensions were subjected to ICP-MS analysis. This data was used to validate the methodology of silver measurements for ionic and particulate forms.
Three commercially available wound dressings were purchased from a local drugstore. All were advertised as containing silver to aid in the healing process. Only mesh and fiber dressings were considered as they incorporate silver on the surface of the material, making it readily available. Pad or fibrous dressings are typically embedded with silver and therefore, moisture must first be absorbed to mobilize the antimicrobial element. Product A was a mesh dressing with Ag2SO4 listed as the active ingredient and silver was listed at a concentration of 8000 ng/g of dressing. Product B was advertised as a silver alginate (anionic polysaccharide) containing an ionic silver complex (silver sodium hydrogen zirconium phosphate) as the active ingredient and silver concentration of 2000-5000 ng/g of dressing was documented. Product C listed silver nanoparticles as the active ingredient with no amount given.
Determination of total silver
The wound dressings were punched with an 8 mm sterile punch. Samples were collected to a total mass of approximately 0.02 g of dressing. The dressing punches were placed in clean glass vials and acidified with 2.0 mL of 2% nitric acid and sonicated for 20 minutes. One mL of liquid was removed from each vial and set aside and a second 1 mL volume of 2% HNO3 was added to the vial and sonicated for 20 minutes. This was repeated once more for a total of 3.0 mL of extracted liquid from the dressing sample vials. A volume 1.5 mL of the extracted liquid was centrifuged in a 3 mL glass centrifuge tube for 20 minutes at 4000 rpm. A volume of 750 μL of the supernatant was extracted from each of the centrifuge tubes and diluted for ICP-MS analysis. Indium was added as internal standard. The entire process was repeated for a total of 4 trials for each of the three wound dressings tested.
Determination of water-soluble silver ion fraction
New punches (mass of approximately 0.02 g) were placed in a clean glass vial and 3 mL of DI water were added. The vials were sonicated for one hour after which they were centrifuged in 3 mL glass centrifuge tubes for 20 minutes at 4000 rpm. The supernatant was removed and 500 μL was diluted and acidified appropriately for analysis. Indium was added as internal standard. The entire process was repeated for a total of 4 trials for each of the three wound dressings tested.
Determination of silver nanoparticle fraction
For each trial, the pellet remaining was re-suspended in 2% nitric acid [22]. After dissolution, samples were diluted appropriately for analysis [23,24]. Indium was added as internal standard. For particle analysis, pellets were suspended in DI water only.
Instrumental Analysis
The particle size analyzer that was used was the Microtrac Inc. “Zetatrac” Nanotrac Particle Analyzer.
An Agilent 7700 Inductively Coupled Plasma−Mass Spectrometer equipped with an Octopole Reaction System as the collision cell, was used for analysis. Ultra-High Purity Argon (>99.999% Purity) was used for the plasma and Ultra High Purity He (>99.999% Purity) was used as the collision gas, and the 7700. Series Mass Hunter software was employed for data manipulation. Samples were introduced with an integrated auto sampler (Model I-AS). Specific instrument conditions are as given in Table 1.
| Forward power | 1550 W |
| Plasma gas flow | 15.0 L min-1 |
| Carrier gas | 0.90 L min-1 |
| Makeup gas | 0.12 L min-1 |
| Nebulizer type | Micromist |
| Nebulizer pump | 0.10 rps |
| Spray chamber temperature | 2°C |
| Isotopes monitored | 107Ag, 109Ag |
Table 1: ICP-MS operating conditions.
Particle size analysis
A solution of nanoparticles of known size (20 nM) were analyzed at a 1x, 10x, and 100x dilution to validate particle measurements. Each of the dressing products was punched with an 8 mm sterile punch until samples were collected to a total mass of approximately 0.02 g of dressing. The samples were placed in clean glass vials, sonicated with DI water, and analyzed with the particle analyzer following appropriate dilution. After the centrifugation process was completed, the supernatant was also analyzed using the particle size analyzer.
Results And Discussion
An extraction protocol was designed to allow for the determination of water-soluble silver ion and silver nanoparticle (detailed in Experimental) and the procedure was performed on a known mixture of silver ion and nanoparticle. Results are shown in Tables 2A and 2B. These results demonstrate that the mixture of the pure ionic silver and pure silver nanoparticle samples exhibit congruity and agree with the theoretical value of 20.0 μg L-1. This procedure was repeated with 20.0 μg L-1 mixtures of both the pure silver ion and the pure silver particle solutions (40.0 μg L-1 theoretical mixture value) and similar results were obtained.
| Trial Number | Concentration of Pure Ionic Solution (µg L-1) | Concentration of Particle Solution (µg L-1) | Concentration of Mixture of the Solutions (µg L-1) | Percent Recovery |
|---|---|---|---|---|
| 1 | 9.3 | 9.1 | 18.4 | 92.00% |
| 2 | 9.2 | 8.9 | 18.1 | 90.50% |
| 3 | 10.9 | 9.4 | 20.3 | 101% |
Table 2A: Recoveries of Solutions with Known Concentrations of Silver Ions and Silver Nanoparticles (20.0 µg L-1).
| Trial Number | Concentration of Pure Ionic Solution (µg L-1) | Concentration of Particle Solution (µg L-1) | Concentration of Mixture of the Solutions (µg L-1) | Percent Recovery |
|---|---|---|---|---|
| 1 | 13.4 | 25.9 | 39.3 | 98.20% |
| 2 | 17.7 | 20.6 | 38.3 | 95.80% |
| 3 | 18 | 20.1 | 38.1 | 95.20% |
Table 2B: Recoveries of solutions with known concentrations of silver ions and silver nanoparticles (40.0 µg L-1).
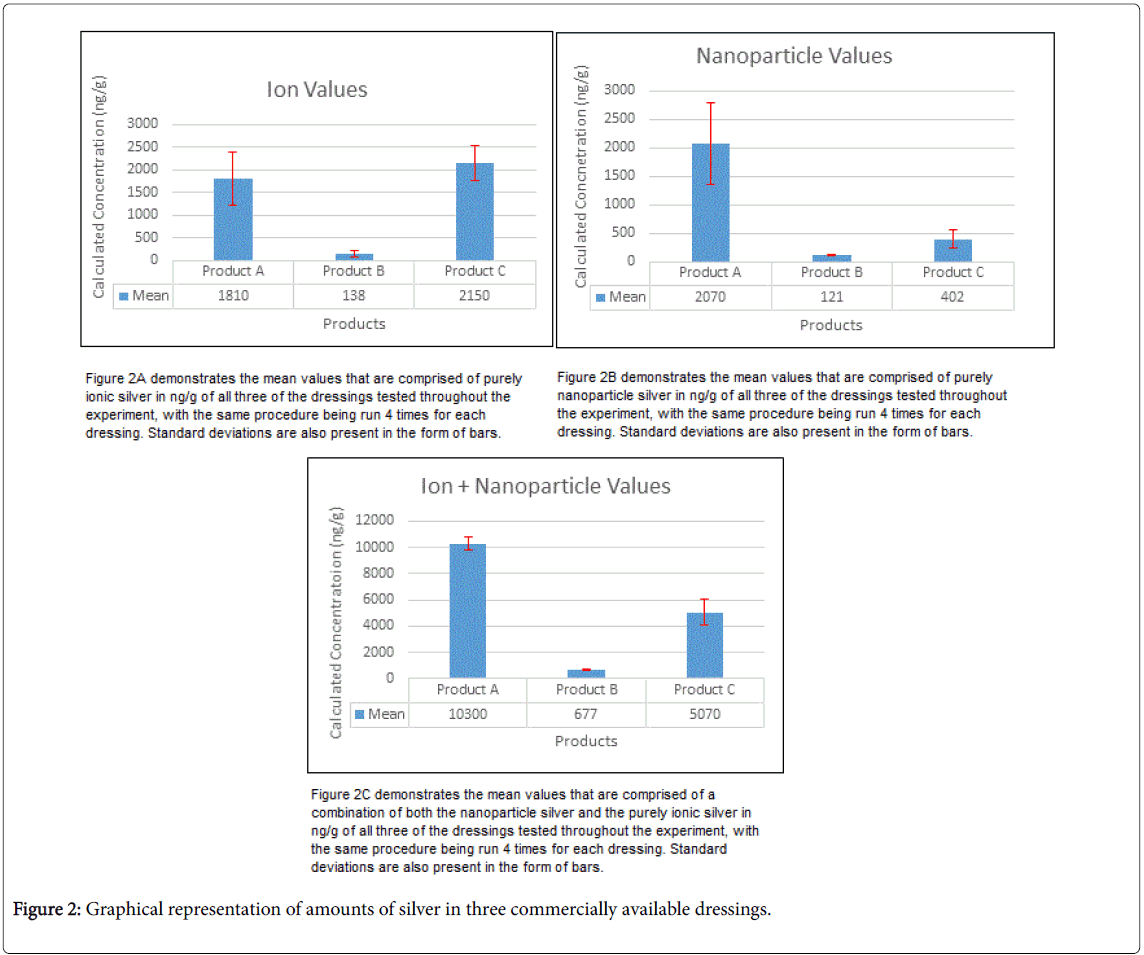
The total silver concentration results of commercially purchased dressings are shown in Table 3. Product A demonstrated an average value of 10300 ± 500 ng g-1 total silver, which is in good agreement with the advertised value of 8000 ng g-1. Product B showed an average total silver of 680 ± 60 ng g-1 and was somewhat lower than the manufacturer’s listed content of 2000-5000 ng g-1. Finally, Product C showed an average of 5100 ± 900 ng g-1. No manufacturer specifications were given with respect to silver concentration in this particular-brand.
The water-soluble silver ion fraction and silver nanoparticle fraction results are listed in Table 3. While the method developed differentiated silver ion (+1) and uncharged silver (0) particles, in its application the particulate forms included insoluble colloidal silver salts. For simplicity, we identified ions and dispersed colloidal silver or silver salts as soluble silver. Product A exhibited 1800 ± 600 ng g-1 in the form of silver ion and a greater amount, 2100± 700 ng g-1, in the form of dispersed silver sulfate nanoparticles. The total water-soluble silver was calculated by the summation of the average ion and particle values amongst the 4 trials. The mean value was 5140 ng g-1, demonstrating that 50 ± 11% of the silver in the dressing was water soluble and available to the wound. Product B showed 140 ± 80 ng g-1 in the form of silver ion and a slightly less amount, 121 ± 0 3 ng g-1, in the form of silver nanoparticle. Total water-soluble silver was averaged to be 315 ng g-1, demonstrating that 47 ± 14% of the silver in the dressing was water soluble and available to the wound. Finally, Product C showed 2100 ± 400 ng g-1 in the form of silver ion and a lesser amount, 400 ± 150 ng g-1, in the form of silver nanoparticle. Total water-soluble silver was 2790 ng g-1, demonstrating that 55 ± 7% of the silver in the dressing was water soluble and available to the wound.
| Trial Number | Concentration of Pure Ionic Solution (µg L-1) | Concentration of Particle Solution (µg L-1) | Concentration of Mixture of the Solutions (µg L-1) | Percent Recovery |
|---|---|---|---|---|
| 1 | 13.4 | 25.9 | 39.3 | 98.20% |
| 2 | 17.7 | 20.6 | 38.3 | 95.80% |
| 3 | 18 | 20.1 | 38.1 | 95.20% |
Table 3: Total silver and water-soluble silver ion and silver nanoparticle in 3 commercial wound dressings.
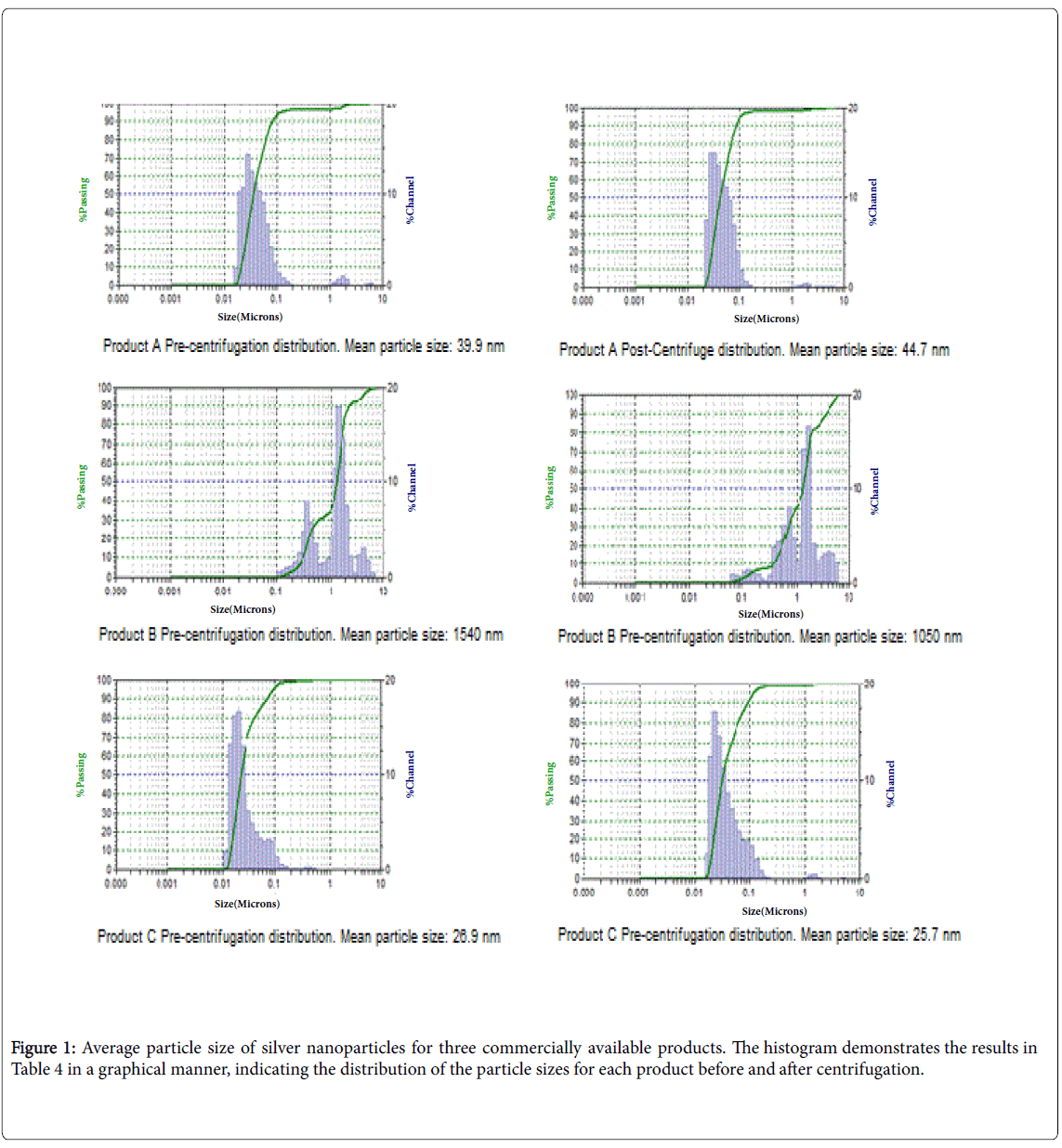
Particle size and mass distributions are very important for assessing environmental effects and the risk associated with the use of these materials in consumer products. A known standard (Sigma) nanoparticles of silver was analyzed using a particle size analyzer following 1x, 10x and 100x dilutions. The Sigma samples yielded the mean value of 20.2 nM with a standard deviation of 1.3 nM.
Particle distribution histograms were generated using the MicroTrac Inc. FLEX application software 10.6.2, which analyzes the results from the particle size analyzer and generates a histogram, are presented in Figure 1. The average particle sizes of pre and post centrifugation extracts of the dressing products are shown in Table 4. A comparison of particle size pre and post centrifugation indicated the silver nanoparticles did not agglomerate during the centrifugation process. As shown, Product A has a tight range of size with an average diameter of approximately 40 nM both pre-centrifugation and postcentrifugation, with over 87% of the overall solution producing these values pre-centrifugation and 98% post-centrifugation. For Product B samples, the particle sizes are for alginate complexes, which consist of populations of particles over greater than two orders of magnitude in size difference. The “majority” of the particles had an average diameter of approximately 1540 nM. It is to be noted that 52% of the sample precentrifugation and 70% of the sample post-centrifugation were of approximately this size. These results indicate that there are uneven levels of agglomeration of the particles in extracts of Product B and this could be associated with the property of the alginate polymer. Since over 50% of the silver in this dressing is outside of the definition of a nanoparticle (less than 100 nM), it is better classified as colloidal silver [25,26]. Finally, results for Product C indicate that most of the particles have an average size of approximately 26 nM in both pre and post centrifugation extracts.
| Wound dressing | Mean Particle Size Pre-Centrifugation (nM) | % Volume (% present in the solution) | Mean Particle Size Post- Centrifugation (nM) | % Volume (% present in the solution) |
|---|---|---|---|---|
| Product A | 39.9 | 97.7 | 44.7 | 87.9 |
| Product B | 1540 | 52 | 1050 | 70 |
| Product C | 26.9 | 100 | 25.7 | 100 |
Table 4: Silver particle size for the commercially available products.
Of importance to the physician, a few statements can be made regarding the three commercially available products tested as shown graphically in Figure 2. Product A clearly demonstrated the greatest amount of silver as it was the product with the highest total silver concentration as well as highest water-soluble silver ion and silver nanoparticle concentrations. Product B clearly offered the least amount of total and water-soluble silver as it was statistically the least in all fractions. All of the products had very similar percentages of water-soluble silver when compared to the total silver incorporated into the product (Table 3). With respect to Product C, (the formulation reported to contain silver nanoparticles), the water-soluble silver nanoparticle concentration is much less than that of the water-soluble silver ion. It may be that this formulation uses sheets of silver that were not dispersed when DI extraction was conducted. In contrast, silver complexes or low solubility silver appear to be much more efficient for transfer to the aqueous phase.
Conclusion
A simple method for the determination of water-soluble silver ion and silver nanoparticle in silver-containing wound dressings was developed. Results of this research indicate that approximately 50% of all silver incorporated was water-soluble regardless of the initial concentration and that silver complexes or low solubility silver appear to be more efficiently transferred to the aqueous phase. Next steps include testing the silver available to the wound in the presence of wound exudate. This may pose a different scenario as opposed to water as the exudate many contain other constituents that might either inhibit or facilitate the silver available to the wound.
Acknowledgement
Authors would like to acknowledge valuable critique and input to this manuscript by Dr. Patrick J Slonecker, Adjunct Associate Professor of the Department of Chemistry, University of Cincinnati.
References
- Fleck CA, Frizzell LD (2004) When negative is positive: A review of negative pressure wound therapy. Extended Care Product News 92: 20-25.
- Percival SL, McCarty SM (2015) Silver and alginates: Role in wound healing and biofilm control. Advances in wound care. 4: 407-414.
- Navarrete R, Abernathy L (2018) A student-friendly guide for the selection of wound care dressings for the foot and ankle. Podiatry is our focus p: 91.
- Sussman EM, Jayanti P, Dair BJ, Casey BJ (2015) Assessment of total silver and silver nanoparticle extraction from medical devices. Food and Chemical Toxicology 85: 10-19.
- Stevens KN, Crespo-Biel O, van den Bosch EE, Dias AA, Knetsch ML, et al. (2009) The relationship between the antimicrobial effect of catheter coatings containing silver nanoparticles and the coagulation of contacting blood. Biomaterials 30: 3682-3690.
- Russell AD, Hugo WB (1994) 7 antimicrobial activity and action of silver. In Progress in Medicinal Chemistry 31: 351-370.
- Hostýnek JJ, Hinz RS, Lorence CR, Price M, Guy RH (1993) Metals and the skin. Critical Reviews in Toxicology 23: 171-235.
- Schreurs WJ, Rosenberg H (1982) Effect of silver ions on transport and retention of phosphate by Escherichia coli. Journal of Bacteriology 152: 7-13.
- Bragg PD, Rainnie DJ (1974) The effect of silver ions on the respiratory chain of Escherichia coli. Canadian journal of Microbiology 20: 883-889.
- Clement JL, Jarrett PS (1994) Antibacterial silver. Metal Based Drugs 1: 467-482.
- Dibrov P, Dzioba J, Gosink KK, Häse CC (2002) Chemiosmotic mechanism of antimicrobial activity of Ag+ in Vibrio cholerae. Antimicrob Agents Chemother 46: 2668-2670.
- Toncelli C, Mylona K, Kalantzi I, Tsiola A, Pitta P, et al. (2017) Silver nanoparticles in seawater: A dynamic mass balance at part per trillion silver concentrations. Sci Total Environ 601: 15-20.
- Innes ME, Umraw N, Fish JS, Gomez M, Cartotto RC (2001) The use of silver coated dressings on donor site wounds: A prospective, controlled matched pair study. Burns 27: 621-627.
- World Health Organization (1996) Guidelines for drinking-water quality: Health criteria and other supporting information. (2nd edn), World Health Organization, Geneva, Switzerland.
- Yin Y, Xu W, Tan Z, Li Y, Wang W, et al. (2017) Photo-and thermo-chemical transformation of AgCl and Ag2S in environmental matrices and its implication. Environ Pollut 220: 955-962.
- Tuoriniemi J, Cornelis G, Hassellöv M (2012) Size discrimination and detection capabilities of single-particle ICPMS for environmental analysis of silver nanoparticles. Anal Chem 84: 3965-3972.
- Mitrano DM, Lesher EK, Bednar A, Monserud J, Higgins CP, et al. (2012) Detecting nanoparticulate silver using singleâ€particle inductively coupled plasma-mass spectrometry. Environ Toxicol Chem 31: 115-121.
- Laborda F, Jiménez-Lamana J, Bolea E, Castillo JR (2011) Selective identification, characterization and determination of dissolved silver (I) and silver nanoparticles based on single particle detection by inductively coupled plasma mass spectrometry. J Anal At Spectrom 26: 1362-1371.
- Zook JM, Long SE, Cleveland D, Geronimo CLA, MacCuspie RI (2011) Measuring silver nanoparticle dissolution in complex biological and environmental matrices using UV–visible absorbance. Anal Bioanal Chem 401: 1993.
- Dong F, Valsami-Jones E, Kreft JU (2016) New, rapid method to measure dissolved silver concentration in silver nanoparticle suspensions by aggregation combined with centrifugation. J Nanoparticle Res 18: 259.
- Majedi SM, Lee HK (2016) Recent advances in the separation and quantification of metallic nanoparticles and ions in the environment. Trends Analyt Chem 75: 183-196.
- Fabricius AL, Duester L, Meermann B, Ternes TA (2014) ICP-MS-based characterization of inorganic nanoparticles—sample preparation and off-line fractionation strategies. Anal Bioanal Chem 406: 467-479.
- Misra SK, Dybowska A, Berhanu D, Luoma SN, Valsami-Jones E (2012) The complexity of nanoparticle dissolution and its importance in nanotoxicological studies. Sci Total Environ 438: 225-232.
- Elzey S, Grassian VH (2010) Agglomeration, isolation and dissolution of commercially manufactured silver nanoparticles in aqueous environments. J Nanoparticle Res 12: 1945-1958.
- Rogers KR, Navratilova J, Stefaniak A, Bowers L, Knepp A, et al. (2018) Characterization of engineered nanoparticles in commercially available spray disinfectant products advertised to contain colloidal silver. Sci Total Environ 619: 1375-1384.
- Loza K, Diendorf J, Sengstock C, Ruiz-Gonzalez L, Gonzalez-Calbet JM, et al. (2014) The dissolution and biological effects of silver nanoparticles in biological media. J Mater Chem 2: 1634-1643.
Citation: Vonderheide AP, Chatterjee R, Walsh M, Savu A (2019) Investigation of Water-Soluble Silver Ion and Silver Nanoparticles in Wound Dressing using ICP-MS. J Anal Bioanal Tech 10:414. DOI: 10.4172/2155-9872.1000414
Copyright: © 2019 Vonderheide AP, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Open Access Journals
Article Tools
Article Usage
- Total views: 4246
- [From(publication date): 0-2019 - Apr 11, 2025]
- Breakdown by view type
- HTML page views: 3340
- PDF downloads: 906