Investigation of Vitamins as Potential Dual Inhibitors of Acetylcholinesterase and β-secretase
Received: 29-Aug-2022 / Manuscript No. JADP-22-73227 / Editor assigned: 01-Sep-2022 / PreQC No. JADP-22-73227 (PQ) / Reviewed: 15-Sep-2022 / QC No. JADP-22-73227 / Revised: 22-Sep-2022 / Manuscript No. JADP-22-73227 (R) / Published Date: 30-Sep-2022 DOI: 10.4172/2161-0460.1000552 QI No. / JADP-22-73227
Abstract
Background: Alzheimer's Disease (AD) is a chronic neurodegenerative disease affecting primarily people with 65 years or older. The current therapy of AD involves the combination of at least 2 drug options to target different aspects of the disease. The study purpose is to investigate the capability of vitamins to act as dual inhibitors of acetylcholinesterase (AChE) as well as β-secretase (BACE) in silico.
Methods: The whole set of vitamins structures were docked against AChE and BACE using the AutoDock vina interface within UCSF Chimera. In addition, ADME parameters were predicted via SwissADME server.
Results: Two vitamins (vitamin K2 and B9) proved efficacious dual binding to both targets in comparison with the standard inhibitors of the examined targets. Nevertheless, their ADME properties were found to suffer from at least one violation to Lipinski's rule of five.
Conclusion: Vitamins K2 and B9 are superior candidates as dual inhibitors for AD.
Keywords: Alzheimer's disease; Vitamins; ADME; MTDL; Docking
Abbreviations
MW: Molecular Weight; RB: Rotatable Bonds; HA: Hydrogen Acceptors; HD: Hydrogen Donors; GI: Gastrointestinal Tract; BBB: Blood Brain Barrier
Introduction
It is estimated that in America about 6 million live with Alzheimer's disease (AD). The disease appears most commonly in the elderly although younger individuals come into play in recent years. Beyond age of 65, the incidence of AD doubles. As of 2060, the number is suggested to raise 3-folds (expected for reaching 14 million in US alone) [1]. Of those, more than 121.000 deaths occurred in 2019 ranking AD the sixth leading cause of mortality in US [2]. AD is the most common type of dementia characterized by gradual loss of memory that ultimately hampers the simple routine activities. The factors governing such duration of continuum include genetics, age and gender [3].
Two proteins are now well-studied to be firmly associated with the decline in cognitive abilities seen in AD patients: β-amyloid (Aβ) and tau protein [4]. Aβ forms a plaque in the extracellular medium and inducing inflammation reaction therein, whilst tau protein accumulates within neurons leading consequently to the formation of neurofibrillary tangles that result in neurodegeneration. On the contrary of what was thought, Aβ pathology is now recognized as a preceding step to tauopathy [5].
Excessive production accompanied by diminished clearance of Aβ monomers are the first event in Aβ plaque formation. Aβ overproduction is traced to abnormal activity of β-secretase (BACE) as well as γ-secretase in the brain [6]. In addition, neurofibrillary tangles formation and aggregation is another target of AD therapy. Besides, cholinergic neurons have been found to be lost within basal forebrain. Therefore, combination of BACE and acetylcholinesterase (AChE) inhibition do alleviate cognitive manifestations of AD patients [7]. Recently, a novel appraoch has been exploited to simultaneously affect multiple targets known as Multi-target Directed Ligand (MTDL) strategy as depicted in Figure 1. Plenty of ligands have been reported to have multi-modal binding and, hence, overriding the monotargetmonoligand concept [8].
A large body of evidence has linked the intake of single or multiple vitamins with the lower risk of AD pathogenesis [9–13]. According to our knowledge, the investigation of the capability of vitamins to act as dual inhibitors of AD targets has not yet been studied. So, in line with the MTDL concept, this study aims to dock vitamins against AChE and BACE and predict the pharmacokinetics of vitamins.
Materials and Methods
Preparation of the receptors
Two hotspot targets of AD therapy were retrieved from PDB: acetylcholinesterase (PDB ID#4EY7) and β-secretase (PDB ID#2OHM) as PDB files. The 3D structure of the two receptors were elucidated via X-ray crystallography with a resolution 2.35 Å and 2.70 Å respectively as shown in Figure 2 [14,15]. 4EY7 is a homodimeric protein with a sequence length of 542 amino acids while 2OHM is a monomer consisting of 402 amino acids.
Preparation of ligands
The whole set of vitamins (both fat-soluble and water-soluble) were retrived from PubChem dataset in Table 1, using the corresponding PubChem ID in UCSF Chimera 1.16 program. The downloaded structures had their energy minimized prior to docking.
| No. | Ligand | Scientific name |
|---|---|---|
| 1. | Vitamin A | Retinol |
| 2. | Vitamin D3 | Cholecalciferol |
| 3. | Vitamin E | α-Tocopherol |
| 4. | Vitamin K1 | Phylloquinone |
| 5. | Vitamin K2 | Menaquinone |
| 6. | Vitamin C | Ascorbic acid |
| 7. | Vitamin B1 | Thiamine |
| 8. | Vitamin B2 | Riboflavin |
| 9. | Vitamin B3 | Niacin |
| 10. | Vitamin B5 | Pantothenic acid |
| 11. | Vitamin B6 | Pyridoxine |
| 12. | Vitamin B7 | Biotin |
| 13. | Vitamin B9 | Folic acid |
Table 1: The screened ligands for docking in the current study.
Docking
Protein and ligand preparation as well as molecular docking were performed using UCSF Chimera software (version 1.16) and its builtin AutoDock vina interface [16,17]. We performed a blind docking with a grid box covering all the AChE receptor structure with dimensions 53 × 52 × 69 Å and centred at -2.851, -38.1235 and 33.4475 of X, Y and Z coordinates. With respect to BACE, 50 × 62 × 40 Å are the dimensions of grid box centered at 64.5245, 46.71 and -0.649 of coordinates. Moreover, the polar hydrogens as well as charge were added.
The 3D view and 2D diagram of the best poses were illustrated by Discovery studio client 21 software.
ADME properties
With every docking study, it is advisable to predict the absorption, distribution, metabolism and excretion (ADME) of the screened ligands. For the current study, ADME parameters were predicted using SwissADME webserver [18]. The parameters tested included molecular weight, hydrogen bond acceptors, hydrogen bond donors, rotatable bonds and LogP (Lipinski's rule of five). Besides, gastrointestinal tract absorption and blood-brain barrier permeability were also evaluated.
Results and Discussion
The present work describes the possibility of vitamins to serve as dual inhibitors of two of the most common targets of AD, i.e. AChE and BACE using molecular docking. Moreover, the pharmacokinetics of the whole set of vitamins were also obtained.
Analysis of docking
Apparently from Table 2, fat-soluble vitamins performed well as inhibitors of AChE with free energy ranges from -9.7 for vitamin A to -11.6 kcal/mole for vitamin K2. In contrast, water-soluble vitamins are less likely to block AChE except for folic acid which had a binding affinity of -11.2 kcal/mole (ranked 2nd right after vitamin K2 as AChE inhibitors). This was in the same line with Rehman et al. [19] who proved the efficacy of folate in blocking AChE as shown in Table 2.
| Ligand | AChE | BACE |
|---|---|---|
| Binding affinity | Binding affinity | |
| Reference inhibitor | -11.8 | -6.3 |
| Vitamin A | -9.7 | -6.4 |
| Vitamin D3 | -8.9 | -8.7 |
| Vitamin E | -10.4 | -6.7 |
| Vitamin K1 | -10.9 | -6.6 |
| Vitamin K2 | -11.6 | -7.1 |
| Vitamin C | -6.0 | -5.4 |
| Vitamin B1 | -8.5 | -6.0 |
| Vitamin B2 | -8.4 | -6.7 |
| Vitamin B3 | -6.0 | -4.6 |
| Vitamin B5 | -6.9 | -5.0 |
| Vitamin B6 | -6.0 | -5.2 |
| Vitamin B7 | -6.0 | -5.3 |
| Vitamin B9 | -11.2 | -8.1 |
Table 2: The binding affinities of the screened ligands against both AChE and BACE.
Similarly, vitamin K2 and vitamin B9 are the best scoring vitamins toward BACE inhibition with a free energy of -7.1 and -8.1 kcal/mole respectively even better than the reference inhibitor of BACE enzyme (vitamin D3 ranked top).
Collectively, Vitamins K2 and B9, and to a lesser extent vitamin D3, are superior candidate dual inhibitors of both AChE and BACE.
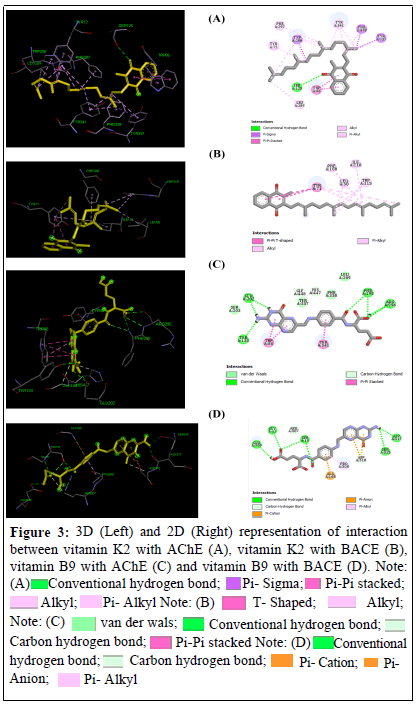
The interaction between best ligands (vitamins K2 and B9) with the target enzymes (AChE and BACE) involved both hydrogen bonds as well as VdW interactions as depicted in Figures 3a-3d.
The interactions involving vitamin B9 with receptors revealed the multi-hydrogen bond nature. Conversely, only one H-bond was found in the interaction between vitamin K2 and AChE (no Hbonds were found with BACE). This can be explained by the high polarity of folic acid given that it is a water-soluble vitamin while vitamin K2 is a fat-soluble one [20].
ADME properties
In order to assess the druglikeness of the screened ligands, SwissADME webserver was utilized (Table 2). According to ADME results, there was only one violation to Lipinski's rule of the fat-soluble vitamins. The violation is that their LogP were >5. Likewise, folic acid suffered from 2 violations. Focusing on vitamin K2 and B9, GI absorption and BBB permeability were low, reflecting the reduced possibility of reaching the brain to be inhibitors of AD targets. However, this was in disagreement with the empirical results proving the permeability of menaquinone to the brain [21] and exert many beneficial actions therein [9]. Adding oil or fat to the diet or salad dressings have been shown to enhance the absorption of fat-soluble vitamins [22]. Folic acid is 2-fold better in GI absorption than folate. Also, the gut microbiota is another source provide the body with folic acid [23,24]. These considerations would overcome the low GI absorption of vitamin K2 and B9 as shown in Table 3.
| Ligands | MW | RB | HA | HD | LogP | Lipinski violations | GI absorption | BBB permeant |
|---|---|---|---|---|---|---|---|---|
| Vitamin A | 286.45 | 5 | 1 | 1 | 5.51 | 1 | High | Yes |
| Vitamin D3 | 384.64 | 6 | 1 | 1 | 7.62 | 1 | Low | No |
| Vitamin E | 430.71 | 12 | 2 | 1 | 8.84 | 1 | Low | No |
| Vitamin K1 | 450.7 | 14 | 2 | 0 | 9.16 | 1 | Low | No |
| Vitamin K2 | 444.65 | 11 | 2 | 0 | 8.92 | 1 | Low | No |
| Vitamin C | 176.12 | 2 | 6 | 4 | -1.41 | 0 | High | No |
| Vitamin B1 | 300.81 | 4 | 3 | 2 | -2.38 | 0 | Low | No |
| Vitamin B2 | 376.36 | 5 | 8 | 5 | -1.68 | 0 | Low | No |
| Vitamin B3 | 123.11 | 1 | 3 | 1 | 0.78 | 0 | High | Yes |
| Vitamin B5 | 219.23 | 7 | 5 | 4 | -1.04 | 0 | High | No |
| Vitamin B6 | 169.18 | 2 | 4 | 3 | -0.22 | 0 | High | No |
| Vitamin B7 | 244.31 | 5 | 3 | 3 | 0.04 | 0 | High | No |
| Vitamin B9 | 441.4 | 10 | 9 | 6 | -0.38 | 2 | Low | No |
Table 3: Predicted ADME properties of the screened ligands and their compatibility to Lipinski's rule of five.
Conclusion
In summary, the current study demonstrates the applicability of vitamin K2 and B9 and to a lesser extent vitamin D3 in simultaneous blockade of AChE and BACE (as dual inhibitors). In addition, they have good ADME properties making them superior nutraceuticals for the patients suffering AD. Nonetheless, in vitro and subsequent in vivo assays confirmation should be performed of the vitamins at appropriate doses to be good candidates to manage AD patients.
References
- Matthews KA, Xu W, Gaglioti AH, Holt JB, Croft JB, et al. (2019) Racial and ethnic estimates of Alzheimer’s disease and related dementias in the United States (2015–2060) in adults aged≥ 65 years. Alzheimers Dement 15:17–24.
- Racial and ethnic estimates of Alzheimer’s disease and related dementias in the United States (2015–2060) in adults aged≥ 65 years
- Vermunt L, Sikkes SAM, Hout A, Handels R, Bos I, et al. (2019) Duration of preclinical, prodromal, and dementia stages of Alzheimer’s disease in relation to age, sex, and APOE genotype. Alzheimers Dement 15(7):888-898.
- Selkoe DJ, Hardy J (2016) The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol Med 8(6):595-608.
[Crossref][Google Scholar][PubMed].
- Busche MA, Hyman BT (2020) Synergy between amyloid-β and tau in Alzheimer’s disease. Nat Neurosci 23(10):1183-1193.
- Takahashi RH, Nagao T, Gouras GK (2017) Plaque formation and the intraneuronal accumulation of β-amyloid in Alzheimer’s disease: Intraneuronal accumulation of β-amyloid. Pathol Int 67(4):185-193.
- Mathew A, Balaji EV, Pai SRK, Kishore A, Pai V, et al. (2022) Current Drug Targets in Alzheimer’s Associated Memory Impairment: A Comprehensive Review. CNS Neurol Disord Drug Targets 2022.
- Rossi M, Freschi M, de Camargo Nascente L, Salerno A, et al. (2021) Sustainable Drug Discovery of Multi-Target-Directed Ligands for Alzheimer’s Disease. J Med Chem 64:4972–4990.
- Popescu A, German M (2021) Vitamin K2 Holds Promise for Alzheimer’s Prevention and Treatment Nutrients 13(7):2206.
- Browne D, McGuinness B, Woodside JV, McKay GJ. Vitamin E and Alzheimer’s disease: what do we know so far? Clin Interv Aging 2019;14:1303.
- D’Cunha NM, Georgousopoulou EN, Boyd L, Veysey M, Sturm J, et al. (2019) Relationship between B-vitamin biomarkers and dietary intake with apolipoprotein E є4 in Alzheimer’s disease. J Nutr Gerontol Geriatr 38(2):173-195.
- Mehta V, Desai N, Perwez A, Nemade D, Dawoodi S, et al. (2017) ACE Alzheimer’s: The Role of Vitamin A, C and E (ACE) in Oxidative Stress induced Alzheimer’s Disease. J Med Res Innov 2:e000086.
- Mielech A, Puścion-Jakubik A, Markiewicz-Żukowska R, Socha K (2020) Vitamins in Alzheimer’s disease—Review of the latest reports. Nutrients 12(11):3458.
- Cheung J, Rudolph MJ, Burshteyn F, Cassidy MS, Gary EN et al. (2012) Structures of Human Acetylcholinesterase in Complex with Pharmacologically Important Ligands. J Med Chem 55(22):10282-10286.
- Murray CW, Callaghan O, Chessari G, Cleasby A, Congreve M, et al. (2007) Application of Fragment Screening by X-ray Crystallography to β-Secretase. J Med Chem 50:1116–1123.
- Murray CW, Callaghan O, Chessari G, Cleasby A, Congreve M, et al. (2007) Application of Fragment Screening by X-ray Crystallography to β-Secretase. J Med Chem 50:1116–1123.
- Butt SS, Badshah Y, Shabbir M, Rafiq M. Molecular docking using chimera and autodock vina software for nonbioinformaticians. JMIR Bioinforma Biotechnol 2020;1:e14232.
- Li Q, Shah S. Structure-based virtual screening (2017) Methods Mol Biol 1558:111-124.
- Daina A, Michielin O, Zoete V (2017) SwissADME: a free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci Rep 7:1–13.
- Rehman S, Ali Ashfaq U, Sufyan M, Shahid I, Ijaz B, et al. (2022) The Insight of In Silico and In Vitro evaluation of Beta vulgaris phytochemicals against Alzheimer’s disease targeting acetylcholinesterase. PLOS ONE 17:e0264074.
[Crossref][Google Scholar][PubMed]
- Gironés-Vilaplana A, Villaño D, Marhuenda J, Moreno DA, García-Viguera C (2017) Vitamins. In: Galanakis CM, editor. Nutraceutical Funct. Food Compon 2017.
[Crossref]
- Carrié I, Portoukalian J, Vicaretti R, Rochford J, Potvin S, et al. (2004) Menaquinone-4 concentration is correlated with sphingolipid concentrations in rat brain. J Nutr 134:167–72.
- Moran NE, Johnson EJ (2017) Closer to clarity on the effect of lipid consumption on fat-soluble vitamin and carotenoid absorption: do we need to close in further? Am J Clin Nutr 106:969–70.
- Milman N (2012) Intestinal absorption of folic acid - new physiologic & molecular aspects. Indian J Med Res 136:725–8.
Citation: Al-Madhagi HA (2022) Investigation of Vitamins as Potential Dual Inhibitors of Acetylcholinesterase and β-secretase. J Alzheimers Dis Parkinsonism 12: 552. DOI: 10.4172/2161-0460.1000552
Copyright: © 2022 Al-Madhagi HA. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 3185
- [From(publication date): 0-2022 - Oct 19, 2025]
- Breakdown by view type
- HTML page views: 2717
- PDF downloads: 468