Research Article Open Access
Investigation of the Effect of Co-Metabolism on Removal of Dodecane by Microbial Consortium from Soil in a Slurry Sequencing Bioreactor
| Majid Nozari, Mohammad Reza Samaei* and Mansooreh Dehghani | ||
| Department of Environmental Health Engineering, School of Health, Shiraz University of Medical Sciences, Iran | ||
| Corresponding Author : | Mohammad Reza Samaei Department of Environmental Health Engineerin School of Health Shiraz University of Medical Sciences, Iran Tel/Fax: +98-9177320737 E-mail: mrsamaei@sums.ac.ir |
|
| Received August 18, 2014; Accepted October 17, 2014; Published October 20, 2014 | ||
| Citation: Nozari M, Samaei MR, Dehghani M (2014) Investigation of the Effect of Co-Metabolism on Removal of Dodecane by Microbial Consortium from Soil in a Slurry Sequencing Bioreactor. J Bioremed Biodeg 5:253. doi:10.4172/2155-6199.1000253 | ||
| Copyright: © 2014 Nozari M, et al. This is an open-a ccess article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | ||
Related article at Pubmed Pubmed  Scholar Google Scholar Google |
||
Visit for more related articles at Journal of Bioremediation & Biodegradation
Abstract
Background and Objectives: Biodegradation techniques are nowadays widely used for cleaning soil contaminated with oil. The aim of this study is to evaluate the effects of cell metabolism (glucose) on Dodecane removal in slurry sequencing batch reactors (SBR) by a bacterial consortium.
Materials and Methods: In this study, a Slurry Sequencing Batch Reactor (SSBR) was used as a pilot by a bacterial consortium, including bacterium Acinetobacter radioresistens, Bacillus subtilis, and Pseudomonas aeruginosa, in order to remove different concentrations of dodecane (1, 4, 7, and 10 percent). Sampling was performed for four times during the sedimentation step. Then, the samples were analyzed by GC-FID and the results were analyzed statistically.
Results: The results showed that dodecane removal (%) by the microbial consortium was higher in lower initial concentrations; such a way that the biological removal of dodecane was respectively 47.39%, 38.41%, 28.03%, and 17.46% in the concentrations of 1%, 4%, 7%, and 10% on the third day. Moreover, Adding an external carbon source (glucose) in the first and second day increased Dodecane biological removal by 17.12 and 17.41 respectively, which are 1.2 and 1.94 times the amount of biological Dodecane removal in non-cometabolism conditions.
Conclusion: The results showed that SSBR could be used as an exit-situation effective method for dodecane removal in low concentrations through considering the effective factors in its function, such as dissolved oxygen, pH, and temperature. Also, adding the secondary carbon source could be effective in dodecane removal from the soil. Yet, this effect might be different on various days.
| Keywords |
| Soil; Dodecane; Slurry Sequencing Batch Reactor (SSBR); Microbial consortium; Co-metabolism |
| Introduction |
| Linear hydrocarbons are including alkanes, alkenes and alkynes, and alkanes with moderate length are the most important pollutants of soil [1]. Among Alkanes, N-Alkanes with medium chain have been identified as the most important contaminants of soil [2,3]. Dodecane (C12H26) with low solubility in water (0.0037 mgl-1 at 25°C) is one of medium-chain n-alkanes [4]. In some studies, Dodecane has been used as a representative hydrocarbon for liquid alkanes in various hydrocarbon mixtures [5]. Several studies have shown that a wide range of micro-organisms are capable of degenerating diesel fuel [6-8] and n-alkanes [9-11] without co-substrate. Petroleum hydrocarbons are decomposed by microorganisms like bacteria, yeast and fungi that can use crude oil as a source of carbon and energy [12-15]. Aerobic decomposition of alkanes with varying chain lengths has been widely studied and documented [16-19]. |
| Biodegradation is an option capable of removing and destroying toxic contaminants using natural biological activities. By definition, biodegradation is use of living organisms, primarily micro-organisms, to break down environmental pollutants to less toxic forms. Biodegradation use natural plants, fungi or bacteria to break down or detoxify substances hazardous to human health or the environment [20]. Micro-organisms may be native to the contaminated region or they may be taken from elsewhere to the contaminated sites. Polluting substances change shape by living organisms through reactions as part of the metabolic processes within them. Biodegradation of a combination is often a result of reactions by multiple organisms. When micro-organisms enter a contaminated site, decomposition increases, a process known as bio-Augmentation [14]. Biodegradation is more cost-effective than typical physical and chemical thermal remediation like burning [21,22]. Biodegradation processes are classified into three broad categories based on location, transport and the soil treated including in situ, ad situ and ex situ. The second and third categories of these processes are used to remediate four groups of contaminated soils and sites: (a) contaminated sludge, soils or sediments with high concentrations of recalcitrant contaminants like poly-nuclear aromatic hydrocarbons [23,24], diesel [25,26], explosives [27], pesticides and chlorinated organic contaminants [2,28] and petrochemical oily sludge [29,30]; (b) clay and stratified soil with low hydraulic conductivity, low permeability and high levels of organic matter [2,31]; (c) soils in areas where environmental conditions are harmful to biological processes, for example, those having low temperatures which have a negative effect on the rate of biodegradation [32] and (d) contaminated sites that need to be remediated immediately due to regulatory and other pressures [33,34]. |
| Slurry Bioreactors (SBs) form one of the most important in situ and ex situ techniques. Treatment of soils and sediments by slurry bioreactors is becoming one of the good options for bioremediation of soils contaminated by recalcitrant contaminants under controlled environmental conditions [35-37]. SBs are often used to assess the feasibility and actual potential of biological strategies in the final restoration of contaminated soil or sites [25,28,38]. The pollutant depletion rate under slurry conditions depends primarily on the degradation activity by microorganisms in the system [34]. Generally, the obtained results show the soil's actual potential in biological depuration [1,39]. The SB technology is an engineered complex that usually includes four stages: installations for handling and treating polluted soil, bioreactor battery, installations for handling and disposing treated soil, and auxiliary equipment for treatment of process by-streams [33,34]. In terms of operation, SBs are classified as batch, semi-continuous, and continuous. Another classification is based on the main electron acceptor that is used in the biodegradation process includes: aerobic (molecular oxygen), anoxic (nitrate and some metal cations), anaerobic (sulfate-reducing, methanogenic, fermentation) [2], and mixed or combined electron acceptors [40,41]. Aerobic SBs have been widely used and anaerobic SBs are flourishing in the area of research and development [36]. |
| SBs have some remarkable and distinctive features. They include the fact that oil is treated in aqueous suspension of 10 to 30% w/v and they provide mechanical or pneumatic mixing. Process advantages of these features include: (a) an increase in mass transfer rates and contact microorganisms, pollutant and nutrients; (b) an increase in pollutant biodegradation rate over in situ bioremediation or ad situ solid phase biotreatment; (c) shorter treatment times; (d) likelihood of using diverse electron acceptors (O2 ,SO4-2 ,CO2 ,NO3-) ; (e) effective use of biostimulation and bioaugmentaion; (f) control and use of several environmental parameters such as temperature, pH, etc. and (g) increased desorption and availability of pollutant by adding surfactants and solvents [2,14,26,42]. |
| For more than 20 years, Cometabolic bioremediation has been applied to some of recalcitrant contaminants like polychlorethylene, trichlorethylene, TNT, dioxins, Atrazine, aromatic hydrocarbons, chlorinated alkenes, halogenated aliphatic and etc. In many systems the essential nutrients needed for complete biodegradation are not available and therefore biodegradation is limited [43]. Several studies have shown that environmental compatibility of microorganisms and their potential for biodegradation can be increased by adding nutrients like yeast extract and glucose [20,25]. Also, in many studies, lactose [44], sucrose [45] and molasses [44,46,47] have been used as co-substrates. In some other studies, glucose has been used as co-substrate [44,48]. But, glucose as co-substrate has not been used for removal of medium-chain alkanes such as Dodecane. The aim of this study is to evaluate the use of glucose as an external carbon source (Co-substrate) to enhance the decomposition of organic contaminants, particularly medium-chain petroleum hydrocarbons (Dodecane). Experiments to study the effect of glucose on aerobic decomposition of Dodecane in slurry sequencing batch reactor by measuring Dodecane concentrations with and without co-substrates. |
| Materials and Methods |
| Materials Specifications |
| Chemical materials used in this study including Hexadecane, 1,2,4-trchlorobenzene, acetone, glucose, HCl, H2SO4, NaOH, NaN3, Na2SO4, NH4Cl, NaCl, MgSO4, FeCl3.6H2O, CaCl2 and MnCl2.4 H2O. All chemicals material used in this study were 99.7% purity and purchased from Merck, Germany. |
| Preparing the soil |
| The soil used in this study was agricultural soil collected from Paskoohak region 40 Km from Shiraz, Iran. The physicochemical analysis of the soil has been presented in Table 1. In order to prepare the soil, first the soil was sieved with a 10 mesh (2mm) sieve for screening the soil and reaching uniformity. Then, it was soaked with distilled water and autoclaved for 15 minutes at 121°C. At the end, it was located in the oven at 160°C to make it sterile and dry and reach its primary weight. After being dried, it was sieved with a 10-mesh (2 mm) sieve. At the end, it was transferred to a 1-liter container and contaminated in different concentrations. In order to artificially contaminate the soil with dodecane at 1, 4, 7, and 10% concentrations, first the necessary amount of dodecane was dissolved in 30 ml hexane. Then, the obtained solution was added to the soil. In order for uniform distribution of dodecane in the soil, the soil was completely submerged in the solution. Then, the soil was regularly mixed in short time intervals and it was permitted to dry completely under the vent at room’s temperature. At the end, a one-week period was considered for absorption of dodecane by the soil. |
| Preparing the essential nutrients |
| In order for the SSBR to operate, in addition to contaminating the soil with dodecane, essential nutrients and water are also needed for the microorganisms. In this study, tap water was used to prepare the essential nutrients. The specifications of tap water were measured using polaroagraphy. The nutrients included 2.5 g L-1 NH4Cl, 0.5 g L-1 NaCl, 0.3 g L-1 MgSO4, 0.3 g L-1 Fecl3.6H2O, 0.01 g L-1 CaCl2, and 0.01 g L-1 Mncl2.4H2O. Then, pH was adjusted to 7 ± 0.5. All the media were autoclaved at 121°C [49] for 15 minutes and were then added to the SSBR. |
| Preparing the solid mineral medium |
| In order to keep the used bacteria fresh in the bioreactor, the bacteria were cultured weekly on the mineral medium including dodecane as the only source of carbon. In order to create this medium, first 1 g L-1 yeast extract was added to the bottle. Afterwards, 15gr Agar- Agar was added and pH was adjusted at 7 ± 0.4. Then, the media were added to the plates and were kept to become solid. In order to provide the source of carbon, 20 μl dodecane was poured on the plates and it was distributed evenly by the pipette; such a way that a very thin layer of dodecane was located on the surface of the plates. The plates were then located in the incubator (37.5°C, 24 hours) in order to grow the bacteria. |
| Preparing and culturing the bacteria in the nutrient broth medium |
| In this study, a microbial consortium including three kinds of bacteria; i.e., Acinetobacter radioresistens, Bacillus subtilis, and Pseudomonas aeruginosahas, was used. These bacteria were isolated from the soil in another study [49]. In order to increase the number of bacteria and add them to the reactor, the bacteria which had been cultured in the Agar-Agar medium were cultured in the nutrient broth medium. Afterwards, they were located on the mixer in the incubator at 37.5°C for 24 hours in order to be grown. Thereafter, the nutrient broth medium including the grown bacteria was transferred to the test tubes in order to be isolated completely and was then centrifuged at 4000 rpm for 5 minutes. Finally, the optical density of the bacteria was measured at the wave length of 600 nm to ascertain the uniformity and equal distribution of the bacteria in all the bioreactors. It should be noted that the optical concentration of the bacteria was reached to one using normal saline. After all, the bacteria were added to the reactor [50]. |
| Measuring the number of active bacteria |
| To determine the number of the bacteria grown in the SSBR, samples were taken from the reactor at different times of operation and they were then cultured at three dilutions; i.e., 10-1, 10-2, and 10- 3, on the nutrient agar medium. After that, the samples were located in the incubator at 37.5°C for 24 hours. After being assured about their growth, the colonies were counted by the colony counter and the number of bacteria was reported based on CFU/ml. |
| Dodecane extraction and analysis |
| In order to analyze the residual of dodecane from the soil, dodecane was extracted from the soil through USEPA method 3550c [51]. Briefly, the sample was taken from the deposited sediment after the process of sedimentation and was dried at 37.5°C. Afterwards, 0.5gr of the dry soil was mixed with 0.5gr anhydrous sodium sulfate as the dehumidifier factor. The content of the Balon Joje was reached to 5ml with 4ml normal hexane and it was completely mixed in order to mix the soil with normal hexane. The Balon Joje was then put in the ultrasonic bath at 30°C for 2 minutes in order to extract dodecane. At the end of the extraction time, the upper liquid of the Balon Joje was transferred to a test tube. In order to extract dodecane more efficiently, this operation was repeated twice. After that, the test tube was located in the centrifuge system at 4000 rpm for 5 minutes to isolate the soil and upper liquid completely. Then, 1ml of the upper liquid was taken by the sampler and was moved to the vial. Afterwards, 10 μl of the internal standard (1,2,4-trichlorobenzene) was added to the vial by Hamilton syringe. Finally, 2 μl was taken from vial content by the injection syringe and was injected to the GC-FID system. The recovery percentage of dodecane was averagely obtained as 72% at different concentrations through the extraction method. |
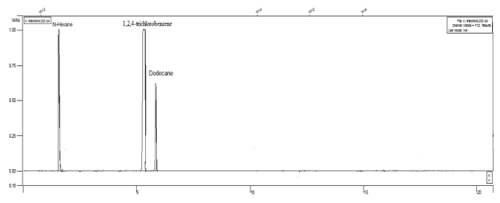
| In order to measure the residual of dodecane in the study samples, the GC-FID system was used. CP-SILSCB (silica, USA) column (30 m length × 0.025 mm id × 0.25 μm film thickness) was used at a temperature program of 80°C for 1 min, increased to 125°C at 10°C min-1, held at 125°C for 5 min, increased to 270°C at 40°C min-1, and held at 270°C for 4 min. Moreover, nitrogen was used as a carrier gas at a constant flow of 2.7 ml min-1. Injector and detector temperatures were 210 and 250°C, respectively. In addition, the detection limit of the gas chromatography system was 8878 mg kg-1 for dodecane. The chromatogram of dodecane and internal standard (1,2,4-trichlorobenzene) has been shown in Figure 1 [50]. |
| Operation of SSBR |
| In a glass reactor with a working volume of 11 L containing polluted soil, the nutrients and bacteria were mixed with enough tap water to bring the total slurry volume to 5 L (Figure 2). Tap water was used for making the slurry of urban water. Each cycle of the reactor lasted for 74 hours, including 1 hour filling, 72 hours reaction and sedimentation, and 1 hour discharge. After the primary preparation of the reactor, urban water, bacterial inoculation liquid, 250 gr polluted soil, and the essential nutrients were added. The first sample was taken one hour after the beginning of the reactor’s working as the sample of the zero days. This one hour was considered for being assured about the uniformity of the materials in the reactor. In each cycle, the reaction process lasted for 21 hours. Then, the reactor was located in the depositing condition for 3 hours and the sample of the first day was taken. This was performed for three days (one cycle). At different times, 8 ml aliquots were sampled to determine the residual amounts of dodecane. Since the pressure of dodecane steam is 100 Pa at 105.3°C, the samples of the deposited sediment were located in the incubator at 37.5°C in order to be dried. A blank reactor was also operated along with the main reactor simultaneously. This reactor had all the conditions of the main reactor, except for the bacteria. To ascertain the lack of bacteria in the blank reactor, after extracting and injecting with chromatography system, the removal of dodecane was determined in the blank reactor at all the concentrations. The biodegradation rate of dodecane (%) was obtained by subtracting the total removal rate of dodecane (%) at all concentrations in the main reactor from the total removal rate of dodecane (%) in the blank reactor. Furthermore, to determine the total removal rate of dodecane (%) in the main and blank reactors, each reactor was operated for four times at 1, 4, 7, and 10 percent concentrations. During this time, other operation parameters, such as dissolved oxygen, pH, number of bacteria, and temperature were monitored. Dissolved oxygen was measured using DO meter (HACH-cat.no.58258-00), pH was measured using pH meter (pH lab-metrohm, Swiss 827), and temperature was measured using thermometer. The method used for measurement of the number of active bacteria was explained in section 2-6. The results showed that the highest biodegradation rate of dodecane occurred at the concentration of 1% dodecane. Thus, the reactor was prepared in similar conditions to the previous part and then 10 gr (2 percent) glucose was added to the reactor as the secondary source of carbon in order to compare the effect of adding an external source of carbon (glucose) on dodecane removal (%) in the conditions with and without co-metabolism at the concentration of 1% dodecane. A blank reactor was also considered for concentration of 1%, and samples were taken from the blank reactor on all the experiment days. After extracting the samples, injecting them to the chromatography system, and analyzing the results, the total removal percentage of dodecane in the main and blank reactors was determined at the concentration of 1% and adding of the external source of carbon (glucose). The biodegradation rate of dodecane (%) was obtained by subtracting the total removal rate of dodecane (%) at the concentration of 1% in the main reactor from the total removal rate of dodecane (%) in the blank reactor. Then, the obtained biodegradation rates were compared. |
| Statistical analysis |
| Various statistical methods are available for optimizing removal conditions. In this study, One Factor at a Time approach was used [52]. All the samples were taken in duplicates and the line in the graphs represents the average value. All the data obtained in the study were subjected to statistical analysis of way correlation coefficient with SPSS Version 19.0. Significance was considered to be at the p<0.05 probability level. |
| Results |
| The water added to the reactor was tap water whose specifications were measured using the polarography system as follows: (Based on the mgL-1) nickel: 1-10, cobalt: 30-60, solfate: 120-160, nitrate: 25-37, nitrite: 2-8, chloride:120-140, bromide: 1-2, floride: 0.4-1, total iron: 0.07, iron: 0.04, calcium: 60, and magnesium: 80-120. The soil used in the study was also analyzed using a physicochemical method and the results have been shown in Table 1. |
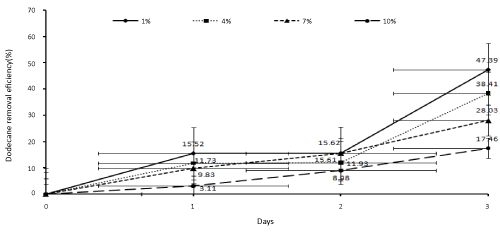
| In order to determine the biodegradation percentage of dodecane in the SSBR at the concentrations of 1, 4, 7, and 10 percent, the samples were collected on the zero (one hour after the reactor’s beginning of working), first, second, and third days. After extraction, the samples were injected to the gas chromatography system. Then, the results were statistically analyzed and the biodegradation percentage of dodecane was determined. The results have been presented in Figure 3. |
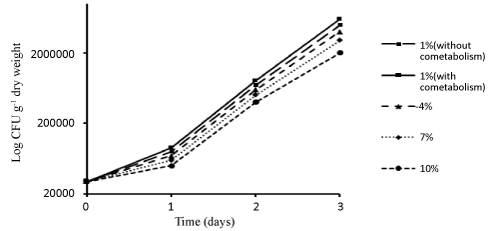
| In order to determine the growth rate of bacteria in different concentrations during different days, some samples were taken from the reactor. The results related to the number of bacteria (CFU/ml) in different experimental conditions during different days have been shown in Figure 4. |
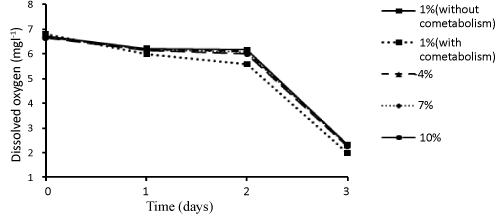
| Samples were also collected to determine the amount of dissolved oxygen in different concentrations of dodecane during different days. The results related to the dissolved oxygen (as mgl-1) in different experimental conditions during different days have been shown in Figure 5. Accordingly, a negative, strong linear relationship was observed between dodecane concentration and amount of dissolved oxygen on the zero, first, second, and third days (r= -0.98, P ≤ 0.05); such a way that increase of concentration led to a decrease in the amount of dissolved oxygen. |
| In order to determine the pH, some samples were taken from the reactor on the zero (one hour after the reactor’s beginning of working) first, second, and third days. pH was measured using the pH-meter system. The results showed that in concentrations of 1, 4, 7, and 10 percent of dodecane, pH respectively decreased to 1.07, 1.08, 1.09, and 1.1 after three days. Moreover, a significant strong, negative, linear relationship was found between dodecane concentration and pH on the zero, first, second, and third days (r= -0.96, P≤0.05); such a way that pH decreased following the increase in the concentration. |
| Also, samples were collected from the reactor on the zero (one hour after the reactor’s beginning of working), first, second, and third days in order to determine the temperature. Based on the results, in the concentrations of 1, 4, 7, and 10 percent of dodecane, temperature respectively increased to 4.9, 5.7, 5.8, and 6.1 after three days. Furthermore, a significant, strong, positive, linear relationship was found between the concentration and temperature on the zero, first, second, and third days (r=0.95, P ≤ 0.05); such a way that increase of concentration resulted in an increase in the temperature. |
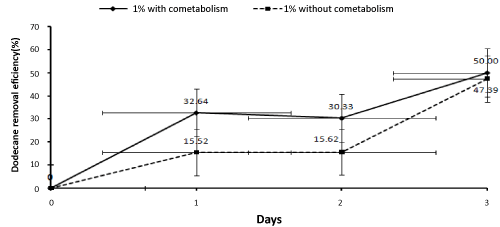
| Since the highest percentage of dodecane biodegradation was related to the concentration of 1 percent, this concentration was used to investigate the effect of co-metabolism. Samples were taken from the reactor in order to determine the effect of adding an external carbon source (glucose) on the biodegradation percentage of dodecane at the concentration of 1% and compare the results to the condition without co-metabolism on the zero (one hour after the reactor’s beginning of working ), first, second, and third days. After extraction, the samples were injected to the gas chromatography system. Then, the results were statistically analyzed and the biodegradation percentage of dodecane was determined in the condition with the effect of co- metabolism. Afterwards, the results were compared to the condition without cometabolism and the findings have been presented in Figure 6. |
| Discussion |
| Various physical, chemical and biological contaminants have been used to clean oil contaminations. In the field of biological cleanup, Shields et al. [6], Kim and Hao [16], Lee and Gibson [19] and Lee et al. [18] reported the positive effect of the presence of Pseudomonas bacteria in removing organic pollutants [6,16,18,19]. Wackett et al. [7] reported the positive effect of bacillus on decomposing organic pollutant compounds. Bossert and Bartha [8] reported the effects of bacteria like pseudomonas, arthrobacter, Corynebacterium, Flavobacterium, Achromobacter, Micrococcus, Nocardia, and Mycobacterium as the most active bacterial species in decomposition of hydrocarbons in the soil. Das and Mukherjee [11] used Bacillus and Pseudomonas to reduce TPH in soil in north-eastern India. The results demonstrated the ability of the bacteria to reduce TPH. Also, acinetobacter radioresistens was used for removal of parathion [53] and long-chain alkanes; Acinetobacter calcoaceticus was used for removal of long-chain alkanes [54] and acinetobacter for removing Chloroaniline [55]. Therefore, taking into account the studies on the ability of acinetobacter, bacillus and pseudomonas in analysis of petroleum hydrocarbons, in this study, a microbial consortium consisting of three types of bacteria, i.e. Acinetobacter radioresistens, Bacillus subtilis and Pseudomonas aeruginosa, is used for Dodecane removal in slurry sequencing batch reactor |
| The results show that biological removal of Dodecane was high on the first day and reduced in the second day. The third day, a rise was again observed. The Dodecane removal rate at 1% concentration was 15.52% on the first day, 0.1% on the second day and 31.77% on the third day (Figure 6). Fluctuation of biological removal rate can be due to the fact that on the first day there was enough carbon and nutrients that were used by microorganisms. But, on the second day, these sources of carbon and nutrients were finished leading to competition between bacteria which in turn led to reduced rate of Dodecane biological removal. On the third day, with the increase in the consortium compatibility with the reactor and the number of bacteria, the bacterial consortium began using the rest of carbon sources and nutrients. As a result, the biological Dodecane removal increased. Since the present study, because the experiments were carried out in different seasons, the reactor encountered inappropriate temperature conditions which can disturb the reactor and affect the biological removal of Dodecane. Another factor affecting the biological removal of Dodecane is the possibility that reactor is not fully disinfected during the evacuation and preparation for the next cycle and thus degradation factors enter the reactor from the outdoors. Also, Dodecane may be evaporated due to low solubility in water. This fact has been noted in other studies [9]. |
| In the present study, the growth of bacteria increased over time (Figure 4). That can be because bacterial consortium adapted to the conditions of the reactor. The growth rate of bacterial consortium in lower Dodecane concentrations was higher than in higher Dodecane concentrations. The reason can be that with the increase in Dodecane concentrations, bacteria were trapped in the oil layers and failed to have the functionality needed to remove Dodecane. In Boopathy's study [47] on bioremediation of tetryl-contaminated soil using SSBR, within 30 days of the reactor operation, count of bacteria under both aerobic and anaerobic conditions was high in reactor with molasses as the growth substrate. This shows the presence of aerobic and anoxic bacteria in the contaminated soil. In the reactor operated without molasses, the number of bacteria was significantly lower. This indicates that Tetryl was the sole carbon source not used for growth. |
| In this study the amount of dissolved oxygen was decreased with time (Figure 5). This could be due to higher activity of microorganisms and their higher growth and oxygen uptake rate (OUR) over time. On the other hand, given the direct relationship between temperature and dissolved oxygen, this reduction of dissolved oxygen can be because of increased temperature over time. Juneson et al. [10] used a bacterial consortium including Brevibacterium iodinum, Rhodococcus luteus and Bacillus brevis for studying biodegradation of bis (2-ethylhexyl) phthalate in a soil slurry-sequencing batch reactor. The Dissolved oxygen levels decreased with time. In other words, the activity of microorganisms and the rate of oxygen uptake increased. Also, Venkata et al. [48] studied bioslurry phase degradation of di-ethyl phthalate (DEP) contaminated soil in periodic discontinuous mode operation as well as the effect of bioaugmentation using ETP microflora on the degradation with a total cycle period of 48 h. The rate of oxygen consumption by the microflora was increased over time in all modes [48]. |
| Physical and chemical agents such as soil properties, nutrients, temperature, pH, amounts of contaminants, humidity, water-soil ratio and micro-organisms affect soil bioremediation [56]. In this study, after three days, the temperature values at Dodecane concentrations of 1, 4, 7 and 10 percent increased 7.9, 5.4, 5.8 and 6.1°C respectively. Temperature affects microbial activity and change bioremediation [12]. Usually, the optimum growth of different bacterial species occurs in a limited temperature range (25-45°C) [57]. Biochemical reaction rate increases with increasing temperature. Moreover, increasing the temperature increases the solubility of contaminant and reduces its absorption on the soil. In general, temperatures above 40 ° C reduce biodegradation due to their destruction of enzymes and proteins. If the temperature is reduced to zero degrees Celsius, biodegradation stops [58]. |
| Environmental pH affects cell metabolism and function of enzymes. In this study, during soil remediation, the pH that was adjusted at the beginning at 7, gradually moved towards acidification so that the actual pH values after three days at Dodecane concentrations of 1, 4, 7 and 10%, were respectively 1.07, 1.08, 1.09 and 1.1. The decrease in pH can be due to production of material with acidic nature by the microbial consortium and the acidic nature of the materials broken. In a study by Juneson et al. [10] on biodegradation of bis(2-ethylhexyl) phthalate in an SSBR, the drop of pH was reported to be due to ammonium uptake during biomass growth. Tang et al. [5] reported that hydrocarbon composition changes during Bioremediation led to changes in the chemical properties of the soil and in the pH. They also attributed changes in soil pH to microbial activity rather than to changes in the TPH concentrations. This is consistent findings of the present study, because the decrease in pH was observed only in the reactor having bacteria (main reactor) and the pH in samples without bacteria (control reactor) did not change significantly. Verstraete et al. [59] reported that adjusting the pH to acidic conditions (pH=5.4) to near neutral (pH=7.4) would result in a doubling of the rate of contaminated soil biodegradation [59]. Therefore, in case of constant pH adjustment during bioremediation, Dodecane biodegradation rate may be higher than current levels. However, further studies are needed to investigate this hypothesis. Partovinia et al. [1] concluded that with decreasing pH, the total amount of organic carbon is reduced. Also, the ability of bacteria to produce biosurfactants was the highest at pH=7. The reason was the structure of produced biosurfactants which was micellar. This structure plays an important role in the emulsion process and usually is formed at pH>6.8. As a result, production of biosurfactants facilitates the use of hydrophobic non-miscible materials. |
| In this study, Dodecane removal at concentration of 1% reduced over time by adding cometabolism (glucose) During the first to third days, the levels of biological Dodecane removal reduced 3, 15 and 17 percent respectively (Figure 3). That can be because of glucose consumption by bacteria and depletion of the external carbon source (glucose). In Boopathy [40] study on biodegradation of the tetrylcontaminated soils in slurry sequencing batch reactor, during 30 days of operation with molasses as co-substrate, the tetryl concentration under aerobic conditions increased from 4200 up to 3800 mg/kg. While under anaerobic conditions it reduced from 4200 to 0.05 mg kg-1. In other words, removing tetryl in anaerobic conditions was significantly higher than in aerobic conditions. The results of Boopathy [40] on the use of anaerobic soil slurry reactors for the removal of total petroleum hydrocarbons (TPH) under anaerobic conditions, showed that the rate of diesel degradation under mixed electron acceptor conditions followed by sulfate-, nitrate-reducing, methanogenic and control (passive bioremediation) conditions were 80, 55, 50, 40 and 27 respectively. Thus it can be concluded that higher percentage of Dodecane removal with glucose as co-substrate can be achieved under anaerobic conditions, though further studies are needed to investigate this hypothesis. |
| Conclusion |
| In this study, SSBR was used by application of a bacterial consortium, including Acinetobacter radioresistens, Bacillus subtilis, and Pseudomonas aeruginosa, to remove dodecane from soil at different initial concentrations (1-10%). According to the study findings, increase in the initial dodecane concentration in the soil decreased dodecane removal. Besides, the best condition for total biodegradation was at the concentration of 1% (10000 mg dodecane/kg dry soil). In this study, the removal rate of dodecane reached 45.95% (mg dodecane/kg dry soil) on the third day. The biodegradation rate of dodecane was respectively 47.39%, 38.41%, 28.03%, and 17.46% at the concentrations of 1, 4, 7, and 10 percent at the end of the third day. Furthermore, the dissolved oxygen rate decreased following the increase in the microbial activity. In addition, the medium changed much more through the acidic condition, while the conditions never became anaerobic or anoxic. Also, pH did not intolerably decrease by the bacteria. Moreover, adding an external carbon source (glucose) in the first and second day had a significant effect on Dodecane removal, but this effect reduced on the third day. Overall, the findings of the current study showed that SSBR could be used as an effective method for the soils contaminated with oil products. Also, the secondary carbon source should gradually be added to the reactor in order to increase its efficiency. |
| Acknowledgements |
| The authors would like to appreciate the Research Vice-chancellor of Shiraz University of Medical Sciences for financially supporting the research (proposal No. 92-6794). They are also grateful for Ms. A. Keivanshekouh at the Research Improvement Center of Shiraz University of Medical Sciences for improving the use of English in the manuscript. |
References
|
Tables and Figures at a glance
| Table 1 |
Figures at a glance
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 |
 |
 |
 |
| Figure 4 | Figure 5 | Figure 6 |
Relevant Topics
- Anaerobic Biodegradation
- Biodegradable Balloons
- Biodegradable Confetti
- Biodegradable Diapers
- Biodegradable Plastics
- Biodegradable Sunscreen
- Biodegradation
- Bioremediation Bacteria
- Bioremediation Oil Spills
- Bioremediation Plants
- Bioremediation Products
- Ex Situ Bioremediation
- Heavy Metal Bioremediation
- In Situ Bioremediation
- Mycoremediation
- Non Biodegradable
- Phytoremediation
- Sewage Water Treatment
- Soil Bioremediation
- Types of Upwelling
- Waste Degredation
- Xenobiotics
Recommended Journals
Article Tools
Article Usage
- Total views: 14656
- [From(publication date):
December-2014 - Apr 03, 2025] - Breakdown by view type
- HTML page views : 10072
- PDF downloads : 4584
