Research Article Open Access
Investigation of the Diagnostic Performance of Dimethyl Arginine Derivate and Lisosomal Enzymes in Patients with Rheumatoid Arthritis
Spasovski D1*, Alabakovska S2, Jankulovski N3, Bozinovski G1, Bogdanovska S4, Grcevska L4, Sotirova T5, Genadieva-Stavric S5, Chaparovska D6, Gjorcev A7 and Sadicario S81Department of Rheumatology, University Clinical Centre, Skopje, Republic of Macedonia, Roman, Italy
2Instituts of Preclinical biochemistry, University Clinical Centre, Skopje, Republic of Macedonia, Roman, Italy
3Department of Digestive Churgery, University Clinical Centre, Skopje, Republic of Macedonia, Roman, Italy
4Department of Nephrology, University Clinical Centre, Skopje, Republic of Macedonia, Roman, Italy
5Department of Hematology, University Clinical Centre, Skopje, Republic of Macedonia, Roman, Italy
6Department of Toxicology, University Clinical Centre, Skopje, Republic of Macedonia, Roman, Italy
7Department of Pulmology, University Clinical Centre, Skopje, Republic of Macedonia, Roman, Italy
8Department of Cardiology, University Clinical Centre, Skopje, Republic of Macedonia, Roman, Italy
- *Corresponding Author:
- Dejan Spasovski
Department of Rheumatology, University Clinical Centre
Skopje, Republic of Macedonia, Rome, Italy
Tel: +389023147147
E-mail: drspasovski@yahoo.co.uk
Received date: September 29, 2014; Accepted date: October 20, 2014; Published date: October 22, 2014
Citation: Spasovski D, Alabakovska S, Jankulovski N, Bozinovski G, Bogdanovska S, et al. (2014) Investigation of the Diagnostic Performance of Dimethyl Arginine Derivate and Lisosomal Enzymes in Patients with Rheumatoid Arthritis. Interdiscip J Microinflammation 1:119. doi: 10.4172/2381-8727.1000119
Copyright: © 2014, Grazia RM et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at International Journal of Inflammation, Cancer and Integrative Therapy
Abstract
Introduction: Untreated Rheumatoid Arthritis (RA) has implications on renal tissue as one of the visceral manifestations of disease. Arthritis primarily damages the proximal renal tubules.
Aim: To compare the diagnostic values of laboratory variables; to find the predictive value of the positive and negative test and accuracy of the tests for Symmetric Dimethyl Arginine (SDMA), N-acetyl-β-D-glucosaminidase (NAG), microalbuminuria, rheumatoid factor (RF), C-reactive protein (CRP) andDisease Activity Score 28 index (DAS 28); and to detect the effect of untreated rheumatoid arthritis on glomerular and tubular function.
Patients and methods: Quantification of N-acetyl-β-D-glucosaminidase is used for colorimetric assay, ELISA method for detection of SDMA, immunoturbidimetry assay for microalbuminuria and agglutination test for RF. Serum and urine simples are examined in 70 participants (35 patients with untreated rheumatoid arthritis and 35 healthy individuals as control group).
Results: Of 35 examined patients with RA, we found presence of NAG enzymuria (sensitivity of the test; 37.14%) in 13, while microalbuminuria appeared in 4 patients (sensitivity of the test; 11.42%). SDMA was present in 26 patients (sensitivity of the test; 74.58%), while RF was detected in 17 patients (sensitivity of the test; 48.57%).
Conclusion: SDMA and NAG have higher sensitivity than microalbuminuria in the detection of asymptomatic renal impairment in untreated RA.
Keywords
Symmetric Dimethyl Arginine; N-acetyl-β-D-glucosaminidase; Microalbumin; Rheumatoid arthritis.
Introduction
Methylated derivative of the amino acid L-arginine is Symmetric dimethylarginine (SDMA). Body elimination of SDMA is exclusively performed by renal excretion. SDMA plasma concentration of SDMA is tightly related to renal function. SDMA concentration correlates with inulin clearance and is a reliable marker for renal function. In renal impairment elevated level of serum SDMA indicates future risk of cardiovascular attack and mortality correlates with the extent of renal impairment [1-3]. Most commonly used marker for renal excretory function-serum creatinine concentration, does not adequately reflect mild to moderate impairment of renal function.
The standard routine parameters used for assessment of glomerular filtration rate (GFR) have relatively low sensitivity, due to the large functional reserves of the kidney [4]. The kidney is an organ with great compensatory poptential and up to 50% of its functional capacity can be lost before any increase in degradational products of nitrogen metabolism and appearance of proteinuria, which force the patient to ask medical help. The renal function can be determined by many methods, such as immune, radiologic and cytologic analysis. Biochemical analyses as non-invasive methods have significant importance in the early detection of some pathological conditions that appear during therapy. Determination of the activity of enzymes and their isoenzymes in urine is such an example, because their activity in serum has little diagnostic value. Pathogenic mechanisms that lead to destruction of epithelial cells of proximal tubules and are responsible for the appearance of enzymuria are: immune mechanism, complement, lysosomal enzymes, and tubular obstruction by cell debris, protein cylinders, medicaments or proteinuria. Each of them, in a different degree contributes to the release of biochemical markers in urine, in a directly or indirectly.
Renal markers for assessment of renal dysfunction
Some of the classes of urine proteins are used for assessment of asymptomatic renal dysfunction, i.e.:
1. Enzymes with high molecular weight, usually not filtered via glomeruli, but produced in the proximal tubular cells (N-acetyl-β-D-glucosaminidase; NAG);
2. Medium molecular weight proteins normally filtered via glomeruli in a very small quantity, while the vast amount is resorbed in the tubules (microalbumin) [5-10].
The most studied protein of all urine enzymes is urinary NAG (U-NAG). The enzyme belongs to hydrolase class, usually present in the lysosomes of proximal tubular cells [11]. In the human tissue and in biological liquids exists two main forms of the enzyme: A (Acid) and B (Basic) [12]. The isoform A (U-NAG-A) is the dominant form in normal urine [13]. At the end of cell maturation process it is found in the resolved form of cytosol. Its excretion is related to turnover and is noted as functional enzymuria. Iisoform B (U-NAG-B) depends on maturation and is more closely related to the bassment membrane, where it appears. Its presence in urine is correlated to cell lysis and, because of that is noted as lesion enzymuria [14]. NAG can be detected in circulation. Due to its large molecular weight (1,40,000 daltons) NAG cannot pass through an intact glomerular membrane. In healthy people, urine NAG is a representative of the total quantity released from the walls of the renal tubular cells [15], and is very sensitive marker for renal tubular damage.
Urine Albumin (molecular weight from 66 KDa) is quantitatively the most important protein in plasma and urine. It approximately composes up to 30% of urinary proteins, and appears to be a good indicator for assessment of glomerular permeability variations. These variations in glomerular permeability occur in diabetic and hypertensive nephropathy, nephritic syndrome, pre-eclampsia and glomerulonephritis. Urine albumin excretion has great individual variations and depends on physical activity or variations in food intake. From the pathophysiological aspect, microalbuminuria is caused by increased glomerular permeability for albumin, increased glomerular pressure and/or reduced tubular albumin reabsorption. Renal endothelium is closely involved in the regulation of these processes [16,17].
Aim
The aim of this study was to define the effect of untreated rheumatoid arthritis on glomerular and tubular function. The urine microalbumin was used as marker for glomerular function, while NAG was an indicator of proximal tubular damage.
Implication for health policy/practice/research/medical education:
Dimethyl arginine derivate, symmetric isoform has higher sensitivity than N-acetyl-β-D glucosaminidase. It is a relevant marker in the assessment of asymptomatic renal damages in untreated RA. Both forms can be used in routine clinical practice.
Patients and Methods
Diagnosis of disease in the group of patients examined in this study was established on the basis of revised diagnostic criteria for the classification of rheumatoid arthritis, suggested in 1987 by the American Association for Rheumatism (ARA) [18-21]. Patients had to fulfill at least four out of seven criteria. Criteria from one to four had to be present for at least six weeks. In the study were included 35 patients (28 women, 7 men) suffering from RA and 35 healthy individuals (18 women, 17 men) in the control group. The mean age was 56.68 (± 6.79) years (40-65 years) in RA group, and 46.2(±12.49) years (29–65 years) in control group. The mean disease duration was 43.97 (± 45.23) months (1–168 months). None of the patients had previous history of renal disease. Three patients had been previously treated with oral costeroids, while none of them used any non-steroidal anti-inflammatory drugs (NSAIDs). The rest of the patients refused to use any other medication before beginning of the study (Table 1).
| Ra group No of patients 35 value ( m ± sd ) |
Control healthy group No of subjects 35 value ( m ± sd ) |
|
|---|---|---|
| Male / female ratio | 7/28 | 17/18 |
| Mean age ( years) | 56,68 ( ± 6,79 ) ( 40-65 ) | 46,20 ( ± 12,49 ) ( 29-65 ) |
| Mean disease duration ( month ) | 43,97 ( ± 45,23 ) ( 6-168 ) | 0,00 ( ± 0,00 ) ( 0,00-0,00 ) |
Table 1: Patients’ data.
Inclusion criteria: The study involved newly diagnosed patients with untreated rheumatoid arthritis, aged 18-65 years.
Exclusion criteria: Patients with disease or condition that could directly or indirectly influence the results:
1. Patients with previous medical history for diseases of the spleen, thyroid gland, lung and hepatal damage, renal, cardiovascular, neurologic and hematologic diseases.
2. Patients with diabetes mellitus, acute infections, malignant neoplasms and febrile conditions.
3. Patients treated with antibiotics and salicylate six months prior to the beginning of the study.
4. Patients with hypertension, gouty arthritis, urinary infections, and various autoimmune diseases such as mixed connective tissue disorders and vaculitis.
5. Patients treated with antihypertensive, cardiac and antidiabetic therapy.
6. Patients who received blood transfusions and overweight patients.
7. Hypersensitivity to some medications or their components.
8. Excluded were patients whose results show that in 0 spot increased levels of degradation products: serum and urine creatinine, serum urea and disorders in hematologic or enzymatic status.
All patients provided informed consent.
Clinical evaluation of disease activity
A subspecialist in this field performed clinical evaluation. The disease activity was evaluated by Disease Activity Score 28 index (DAS 28) [22-25]. The index is a mathematical formula that allows us to get a uniquely composed quantitative score, which constits of palpation of painful sensitive joints (max number 28), swollen joints (max number 28), Westergren ESR, and the patient’s global assessment of the activity of the disease (0–100 mm Visual Analogous Scale, VAS) and the morning stiffness (minutes). DAS 28 index is ranked from 0 to 10 and a score under 3.2 ranks the disease as low-activity. The assessment of glomerular filtration rate (GFR) was calculated by the Cocroft-Gault formula [26].
Laboratory assessment
For a clinical assessment of the basic disease, the following laboratory variables had to be measured: CBC, acute phase reactants, anti CCP2, C-reactive protein (CRP), Rheumatic Factor (RF), Alkaline Phosphatase (AP), Aspartate Aminotransferase (AST), Alanine Aminotransferase (ALT), Creatinine Kinase (CK), Lactate Dehydrogenase (LDH), serum urea and creatinine. Urine samples were taken not only for routine urine analysis, but alsp for detection of SDMA, NAG, urine creatinine and microalbuminuria.
ElISA technology of Dld-Diagnostika-Gmbh for Detection of Symmetric Dimethyl Arginine (SDMA)
Principle of the assay: The competitive SDMA-ELISA uses the microliter plate format. SDMA is bound to the solid phase of the microtiter plate. SDMA in the samples is acylated and competes with solid phase bound SDMA for a fixed number of rabbit anti-SDMA antiserum binding sites. When the system is in equilibrium, free antigen and free antigen-antiserum complexes are removed by washing. The antibody bound to the solid phase SDMA is detected by anti-rabbit/peroxidase. The substrate TMB/peroxidase reaction is monitored at 450 nm. The amount of antibody bound to the solid phase SDMA is inversely proportional to the SDMA concentration in the sample.
Reference value: 0.3-0.7 µmol/L
Immunoturbidimetric assay for the determination of urinary albumin (Randox laboratories limited)
Principle of the assay: Immunoturbidimetric assay for determination of urin albumin: for random urin albumin measurement an early morning mid-stream specimen should be taken. Centrifuge cloudy samples before use and analyze the clear supernatant. Reference values are 2.0-20.0 mg/L. An undiluted sample is added to the buffer containing the antibody specific for human serum albumin. The absorbance (340 nm) of the resulting turbid solution is proportional to the concentration of albumin in the urine sample. By constructing a standard curve from the absorbance of standards, the albumin concentration in the sample could be determined. The assay could be carried out manually (at room temperature) or with an automated analyzer using DAKO tests [27]. Sample collection and storage: for random urin albumin measurement use an early morning mid-stream sample. Centrifuge cloudy samples before use and analyze clear supernatant in the assay.
Reference value: 2.0-20.0 mg/L.
Colorimetric assay for the determination of N-acetyl-β-d glucosaminidase (NAG) in urine (Roche)
Principle of the assay: Colorimetric assay for the determination of Acetyl-β-D-glucosaminidase in urine: 3-Cresolsulfonphthaleinyl- N-Acetyl-β-D-glucosaminidase, sodium salt, is hydrolyzed by N-Acetyl-β-D glucosaminidase (NAG) with release of 3-cresol-sulfonphthalein, sodium salt (3-cresol purple), which is measured photometrically at 580 nm using a ROCHE test. Turbid urines should be centrifuged and the supernatant decant.
Reference values are: NAG urine 0.27–1.18 U/mmol creatinine.
Serum urea: Was detected by the method of Kassire. Reference value is 3–7.8 mmol/L.
Serum and urine creatinine: Were detected by the Jaffe method. Reference values for serum and urine creatinine are 45–109 µmol/L and 7–17 µmol/L, respectively.
C-reactive protein (CRP): Was detected using the test of agglutination (Latex CRP test); (BioSystems S.A. Reagents & Instruments Costa Brava 30, Barcelona, Spain). Reference value is under 6 mg/L CRP in serum.
Rheumatoid factor (RF) was detected using the test of agglutination (Latex RF test) (BioSystems S.A. Reagents and Instruments Costa Brava 30, Barcelona, Spain). Reference value is under 8 mg/L RF in serum. For the specification of erythrocyte sedimentation rate (ESR) we used quantitative - Westergren method, and normal values are: 7–8 mm for men, 11–16 mm for women.
Ethical issues
The research followed the tenets of the Declaration of Helsinki. Informed consents were obtained. All patients took part in this study voluntary. The research was approved by ethical committee of University Clinical Center of Skopje, Republic of Macedonia.
Statistical analysis
For testing the importance of the difference between two arithmetic means, with respect to a proportion, we used the Student’s t-test that compares the mean values of certain numerical parameters between two groups and a Wilcoxon-matched test for independent samples. Sensitivity and specificity for positive and negative test of examined variables were defined by the test of sensitivity and specificity. P value between 0.05 and 0.1 was taken as statistically significant. Data processing was done by statistical package - Statistica 7.0.
Results
We found NAG enzymuria in 13 patients (37.14%) patients with RA in our group, while microalbuminuria was detected in 4 patients (11.42%). RF was detected in 17 patients (48.57%). Four patients were NAG and RF positive (11.42%), while 3 patients were microalbuminuria and RF positive (8.57%). Among 18 RF negative patients, 9 patients (25.71%) were NAG positive, while in 1 patient (2.85%) microalbuminuria was detected. In 13 patients (37.14%) NAG enzymuria was not detected; they were RF positive. In 14 patients (40%), microalbuminuria was not detected; they were RF positive. Among 18 RF negative patients, NAG enzymuria was detected in 9 patients (50%), while microalbuminuria was detected in 1 patient (5.55%).
Among 22 patients without NAG enzymuria, 13 patients (59.09%) were RF positive. However, among the 31 patients without microalbuminuria, 14 patients (45.16%) were RF positive. Among 35 RA patients NAG sensitivity was 37.14%, microalbuminuria sensitivity was 11.42%, while RF sensitivity was 48.57%. Among 17 RF positive RA patients, presence of NAG was found in 4 patients and its sensitivity was 30.76%, while the presence of microalbuminuria was detected in 3 patients and its sensitivity was 17.34%. Among 18 RF negative patients, NAG enzymuria appeared in 9 patients and its sensitivity was 50%. Microalbuminuria appeared in 1 patient and its sensitivity was 5.55%.
In the healthy control group, 8 patients (22.85%) were NAG positive, 2 patients (5.71%) were microalbuminuria positive and RF was present in 2 patients (5.71%). Table 2, summarizes the data. Diagnostic value of Symmetric Dimethyl Arginine (SDMA), N-Acetyl-β-D Glucosaminidase (NAG) and microalbuminuria in urine in patients with rheumatoid arthritis (RA) For NAG, microalbumin and for other laboratory variables in rheumatoid arthritis, sensitivity, specificity, predictive value of the positive or negative test and their precision are shown in Table 3. NAG has better diagnostic performances than microalbuminuria in term of sensitivity (sensitivity 37.14% vs. 11.42%), but lower specificity (specificity 77.14% vs. 94.28%) in the detection of renal tubular damage in untreated RA. SDMA, NAG, microalbuminuria and DAS 28 index of the intensity of disease Among 35 patients with RA, DAS 28>3.2 was replaced in 28 patients (80%). In 17 seropositive RF patients, replacement of DAS 28>3.2 was detected in 15 patients (88.23%). Of these 15 patients, 3 were NAG positive (20%) and their Mean±SD [1.86 (±1.06)] was extended (1.2–3.1), while microalbuminuria was positive in 2 patients (13.13%) and their Mean ± SD [21.35(± 0.21)] was extended (21.2–21.5).
| ra untreated group NO of patients 35 value (m ± sd) | ra group sero- NO of patients 18 value (m ± sd) |
ra group sero+ NO of patients 17 value (m ± sd) |
control healthy group NO of subjects 35 value (m ± sd) |
|
|---|---|---|---|---|
| Positive / Negative | Positive / Negative | Positive / Negative | Positive / Negative | |
| nag + >1,18 (u/mmol/crea) |
13/221,096 (± 0,68) (0,25-3,1) |
9/91,12 (± 0,58) (0,32-2,1) |
4/131,07 (± 0,79) (0,25-3,1) |
8/271,00 (± 0,50) (0,26-1,91) |
| microalbuminuria + >20(mg/dl) |
4/3115,16 (± 5,55) (5,50-35,2) |
1/1715,03 (± 6,24) ( 8,30-35,2) |
3/1415,30 (± 4,90 ) ( 5,50-25,7) |
2/3315,56 (± 12,46 )( 1,75-56,4) |
| serum creatinine < 49-109 >mol/L |
3/3267,55 (± 14,76) (41-108) |
1/1768,24 (± 14,16)(44-108) | 2/1566,82 (± 15,77 )(41-99) | 2/3374,95 (± 19,72 )(44-135) |
| urine creatinine < 7-17>mol/L |
9/2610,41 (± 4,71) (3,1-25,4) |
6/129,26 (± 4,54) (3,1-18) |
3/1411,62 (± 4,72 ) (5,8-25,4) |
5/309,15 (± 4,22)(1,8-20,4) |
| serum urea + >7,8 mmol/L |
4/315,66 (± 1,46) (3,00-8,60) |
0/185,52 (± 1,33) (3,00-7,5) |
4/135,82 (± 1,62 ) (3,80-8,6) |
1/344,94 (± 1,28)(2,50-7,2) |
| Gfr+ >90 ml/min | 14/2199,19 (± 24,46) (56,08-157,30) |
7/1199,19 (± 24,46)(64,67-142,59) | 7/1099,19 (± 25,22 )(56,08-157,30) | 4/31113,80 (± 30,86)(69,98-177,74) |
| das 28+ > 3,2 | 28/74,79 (± 1,56) (1,85-7,03) |
13/54,56 (± 1,76) (1,85-7,03) |
15/25,04 (± 1,33) (2,47-6,83) |
0/350,00 (± 0,00)(0,00-0,00) |
| Sdma+>0,7 mmol/L | 26/943,20 (± 65,13) (0-300) |
14/457,50 (±81,40)(0-300) | 12/528,05 (±38,72) (0-120) |
0/350,00 (± 0,00)(0,00-0,00) |
| Rf+30 >iu/ml | 17/18346,15 (± 625,22) (0,00-1920) |
0/180,00 (± 0,00) (0,00-0,00) |
17/0712,67 (± 743,72)(30-1920) | 2/3313,71 (± 38,73 )(0,00-120) |
| Crp +12 > mg/l | 14/2146,86 (± 79,19) (0,00-384) |
3/158,66 (± 24,62) (0,00-96) |
13/487,31 (± 96,44)(0,00-384) | 4/315,48 (±12,80) (0,00-48) |
| sedimentation +> 16 mm/h |
27/848,62 (± 39,81) (2,0-120) |
13/543,94 (± 39,82)(2,0-120) | 14/353,58 (± 40,39)(5,0-120) | 4/319,42 (± 8,21)(2,0-44) |
| acpa antibody > 1,26 u/ml |
23/121,71 (± 0,69) (0,92-3,0) |
11/71,56 (± 0,59) (0,93-2,6) |
12/51,87 (± 0,77) (0,92-3,0) |
1/340,95 (± 0,10)(0,90-1,38) |
Table 2: SDMA (μmol/l), NAG (u/mmol/crea), microalbuminuria (mg/dl) and other labaratory variables in RA and healthy control group.
| nag ra untreated group NO of patients 35 |
nag ra group sero- NO of patients 18 |
nag ra group sero+ NO of patients 17 |
micro albuminuriara untreated group NO of patients 35 |
micro albuminuriaragrousero- NO of patients 18 |
micro albuminuria ragroupsero+ NO of patients 17 |
serum creatinine ra untreated group NO of patients 35 |
serum creatinine ra group sero- NO of patients 18 |
serum creatinine ragroupsero+ NO of patients 17 o 17 |
|
|---|---|---|---|---|---|---|---|---|---|
| Sensitivity% | 37,14 | 50 | 30,76 | 11,42 | 5,55 | 17,64 | 8,57 | 5,55 | 11,76 |
| Specificity % | 77,14 | 77,14 | 77,14 | 94,28 | 94,28 | 94,28 | 94,28 | 94,28 | 94,28 |
| predictive values for positive test% |
61,90 | 52,94 | 33,33 | 66,66 | 33,33 | 60 | 60 | 33,33 | 50 |
| predictive values for negative test% |
44,89 | 25 | 32,5 | 48,43 | 34 | 29,78 | 49,23 | 34 | 31,25 |
| Accuracy % | 57,14 | 67,92 | 59,61 | 52,85 | 64,15 | 69,29 | 51,42 | 64,15 | 67,30 |
| urine creatinine ra untreated group NO of patients 35 |
urine creatinine ra group sero- NO of patients 18 |
urine creatinine ragroupsero+ NO of patients 17 |
serum urea ra untreated group NO of patients 35 |
serum urea ragroupsero- NO of patients 18 | serum urea ragroupsero+ NO of patients 17 | gfr ra untreated group NO of patients 35 |
gfr ragroupsero- NO of patients 18 |
gfr ragroupsero+ NO of patients 17 |
|
| Sensitivity % | 25,71 | 33,33 | 17,64 | 11,42 | 0 | 23,52 | 40 | 38,88 | 41,17 |
| Specificity % | 85,71 | 85,71 | 85,71 | 97,14 | 97,14 | 97,14 | 88,57 | 88,57 | 88,57 |
| predictive values for positive test % | 64,28 | 54,54 | 37,5 | 80 | 0 | 80 | 77,77 | 63,63 | 63,63 |
| predictive values for negative test% | 46,42 | 28,57 | 31,88 | 47,69 | 34,61 | 27,65 | 40,38 | 26,19 | 24,39 |
| Accuracy % | 55,71 | 67,92 | 63,46 | 54,28 | 64,15 | 73,07 | 64,28 | 71,69 | 73,03 |
| RF Ra untreated group NO of patients 35 |
RF ra group sero- NO of patients 18 |
RF ragroupsero+ NO of patients 17 |
CRP ra untreated group NO of patients 35 |
CRP ra group sero- NO of patients 18 |
CRP ragroupsero+ NO of patients 17 |
SER ra untreated group NO of patients 35 |
SER ra group sero- NO of patients 18 |
SER ragroupsero+ NO of patients 17 |
|
| Sensitivity% | 48,57 | 0 | 100 | 66,66 | 16,66 | 76,47 | 77,14 | 72,22 | 82,35 |
| Specificity % | 94,28 | 94,28 | 94,28 | 88,57 | 88,57 | 88,57 | 88,57 | 88,57 | 88,57 |
| 89,47 | 0 | 89,47 | 77,77 | 42,85 | 76,47 | 87,09 | 76,47 | 77,77 | |
| predictive values for negative test% | 35,29 | 35,29 | 0 | 40,38 | 36,60 | 11,42 | 20,51 | 13,88 | 8,82 |
| Accuracy % | 71,42 | 62,26 | 96,15 | 64,28 | 64,15 | 84,61 | 82,85 | 83,01 | 86,53 |
Table 3: Diagnostic performance of SDMA, NAG, microalbuminuria and other laboratory variables in rheumatoid arthritis.
| SDMA ra untreated group NO of patients 35 |
SDMA ra group sero- NO of patients 18 |
SDMA ragroupsero+ NO of patients 17 |
DAS 28 raunreated group NO of patients 35 |
DAS 28 ra group sero- NO of patients 18 |
DAS 28 ragroupsero+ NO of patients 17 |
|
|---|---|---|---|---|---|---|
| Sensitivity % | 74,28 | 77,77 | 70,58 | 80 | 72,22 | 88,23 |
| Specificity % | 100 | 100 | 100 | 100 | 100 | 100 |
| predictive values for positive test % | 100 | 100 | 100 | 100 | 100 | 100 |
| predictive values for negative test % | 20,45 | 10,25 | 12,5 | 16,16 | 12,5 | 5,40 |
| accuracy% | 87,14 | 92,48 | 90,38 | 90 | 90,56 | 96,15 |
Table 4: NAG has better diagnostic performances than microalbuminuria in term of sensitivity.
Of these 13 patients, 8 were NAG positive (61.53%) and their M ± SD [1.54 (± 0.40)] was extended (1.19–2.1), while microalbuminuria was not present in any of these patients. Seronegative RF patients have greater titer of NAG than RF positive patients [1.12 (± 0.58)] (0.32–2.1) vs. [1.07 (± 0.79)] (0.25–3.1)), and greater DAS 28>3.2 index [5.04 (± 1.33)] (2.47–6.83) vs. [4.56 (± 1.76)] (1.85–7.03)). There is no statistical relation between these two groups of NAG (p=0.65).
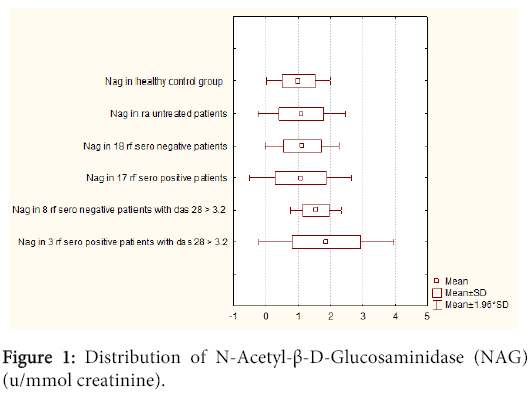
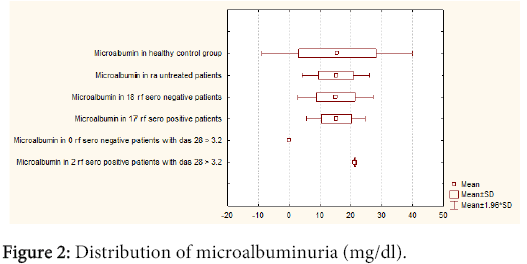
However, positive RF patients with DAS 28>3.2 have greater NAG induction of circumference than seronegative RF with DAS 28>3.2 [1.86 (± 1.06)] (1.2–3.1) vs. [1.54 (± 0.40)] (1.19– 2.1). There is no statistical corelation between these two groups of NAG (p=0.59) (Figure 1).
The difference in microalbuminuria in seronegative RF patients was neglectable when compared to seropositive RF patients [15.03 (± 6.24)] (8.30-35.2) vs. [15.30 (± 4.90)] (5.50-25.7)). Between these two groups with microalbuminuria no statistical corelation was found (p=0.71) (Figure 2). Statistical corelation was found between DAS 28 index in RF positive and negative patients (p=0.37), while statistical corelation was not found between two groups DAS 28>3.2, NAG positive. There was not also between RF positive and negative patients (p=0.28). Statistical corelation was not found using Wilcoxon-matched test between NAG in RA and healthy control group for p<0.05 (p= 0.55), microalbuminuria in RA and healthy control group (p=0.28). There was statistical correlation between NAG and microalbuminuria in the RA group for p<0.05. A statistical correlation was found using a Wilcoxon-matched test between: NAG in RA and age, duration of disease in months, DAS 28 index, RF, CRP, ESR, SDMA, serum and urine creatinine and serum urea in the same group for p<0.05 : NAG vs. age (p<0.001); NAG vs. duration of disease in months (p<0.001); NAG vs. DAS 28 p=( p<0.001); NAG vs. RF (p=0.01); NAG vs. CRP (p=0.04); NAG vs. ESR (p<0.001); NAG vs. serum creatinine (p<0.001); NAG vs urine creatinine (p<0.001); NAG vs. serum urea (p<0.001). Statistical correlation was found using Wilcoxon-matched test between microalbuminuria in RA and age, duration of disease in months, DAS 28 index, RF, ESR, serum and urine creatinine and serum urea in the same group for p<0.05: microalbuminuria vs. age (p<0.001); microalbuminuria vs. duration of disease in months (p<0.001); microalbuminuria vs. DAS 28 (p<0.001); microalbuminuria vs. RF (p=0.04); microalbuminuria vs. ESR (p<0.001), microalbuminuria vs. serum creatinine (p<0.001); microalbuminuria vs. urine creatinine (p<0.001); microalbumin vs. serum urea (p<0.001). There was no statistical correlation using Wilcoxon-matched test between microalbuminuria in RA with CRP and SDMA in the same group: microalbuminuria vs. CRP (p=0.09); microalbuminuria vs. SDMA (p=0.11).
Discussion
Our study shows that NAG is the most relevant marker for assessment of asymptomatic renal dysfunction. NAG sensitivity is higher in comparison with the microalbuminuria sensitivity (37.14% vs. 11.42%). It is close to the GFR sensitivity calculated with creatinine clearance by Cocroft-Gault (40%), as the mathematical score is composed of serum creatinine, age and body weight. NAG is an isolated laboratory variable dominant in the diagnosis of asymptomatic renal tubular dysfunction [19-27]. The other standard analyses used for assessment of renal function have shown low sensitivity: serum and urine creatinine, serum urea (8.57% vs. 25.71% vs. 11.42%). Seropositivity influences the appearance of NAG induction, as was shown in our example - serpositive RF patients with DAS 28>3.2 have much higher NAG induction than seronegative RF with DAS 28>3.2. Statistical corelation between disease duration in months and NAG enzymuria (p<0.001) shows that untreated RA have implications on renal issue as one of the visceral manifestations of disease. Untreated RA primarily damages the tubules, but in a very small amount also the glomerular apparatus. SDMA, in our study shows very high diagnostic value.
Conclusion
In conclusion, SDMA has higher sensitivity than NAG and microalbuminuria. It is a relevant marker in the assessment of asymptomatic renal impairment in untreated RA. These renal markers could be used in routine clinical practice.
References
- Bode-Böger SM, Scalera F, Kielstein JT, Martens-Lobenhoffer J, Breithardt G, Fobker M, et al. (2006) Symmetrical Dimethylarginine: A new combined parameter for renal function and extent of coronary artery disease. J Am SocNephrol 17: 1128-1134.
- Kielstein JT, Salpeter SR, Bode-Böger SM, Cooke JP, Fliser D, et al. (2006) Symmetric dimethylarginine (SDMA) as endogenous marker of renal function-a meta-analysis Nephrol. Dial. Transplant 21: 2446 -2451.
- Wanby P1, Teerlink T, Brudin L, Brattström L, Nilsson I, et al. (2006) Asymmetric dimethylarginine (ADMA) as a risk marker for stroke and TIA in a Swedish population. Atherosclerosis 185: 271-277.
- Chiu JSP (1994) Models used to asses renal function. Drug Devel Res32: 247-55.
- Mueller PW (1993) Detecting the renal effects of cadmium toxicity. ClinChem 39: 743-745.
- Maruhn D, Paar D, Bock KD (1979) Lysosomal and brush border membrane enzymes in urine of patients with renal artery stenosis and with essential hypertension. ClinBiochem 12: 228-220.
- Vanderlinde RE (1981) Urinary enzyme measurements in the diagnosis of renal disorders. Ann Clin Lab Sci 11: 189-201.
- Price RG (1982) Urinary enzymes, nephrotoxicity and renal disease. Toxicology 23: 99-134.
- Johnston ID, Jones NF, Scoble JE, Yuen CT, Price RG (1983) The diagnostic value of urinary enzyme measurements in hypertension. ClinChimActa 133: 317-325.
- Sandberg T, Bergmark J, Hultberg B, Jagenburg R, Trollfors B (1986) Diagnostic potential of urinary enzymes and beta 2-microglobulin in acute urinary tract infection. Acta Med Scand 219: 489-495.
- Kuni CM, Chesney RW, Craig WA, Albert A, England MD,De Angelis C, et al. (1978) Enzymuria as a marker of renal injury and disease. Studies of N-acetyl-ß-D-glucosaminidase in the general population and in patients with renal disease. Pediatrics 62: 751-760.
- Neufeld EF (1989) Natural history and inherited disorders of a lysosomal enzyme, beta-hexosaminidase. J BiolChem 264: 10927-10930.
- Gibey R, Dupond JL, Henry JC (1984) Urinary N-acetyl-beta-D-glucosaminidase (NAG) isoenzyme profiles: a tool for evaluating nephrotoxicity of aminoglycosides and cephalosporins. ClinChimActa 137: 1-11.
- Bourbouze R, Bernard M, Baumann FC, Pérez-González N, Martín-Barrientos J, Cabezas JA, eta l. (1978) Subcellular distribution of N-acetyl-beta-D-glucosaminidaseisoenzymes in the rabbit kidney cortex. Cell MolBiol30: 67-74.
- Burton CJ, Walls J (1994) Proximal tubular cell, proteinuria and tubulo-interstitial scarring. Nephron 68: 287-293.
- Mogensen CE, Chachati A, Christensen CK, Close CF, Deckert T, et al. (1985) Microalbuminuria: an early marker of renal involvement in diabetes. Uremia Invest 9: 85-95.
- Rowe DJ1, Dawnay A, Watts GF (1990) Microalbuminuria in diabetes mellitus: review and recommendations for the measurement of albumin in urine. Ann ClinBiochem 27 : 297-312.
- Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. (1988) The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 31: 315-24.
- Wiland P1, Wiela-Hojenska A, Glowska A, Chlebicki A, Hurkacz M, et al. (2004) Renal function in rheumatoid arthritis patients treated with methotrexate and infliximab. ClinExpRheumatol 22: 469-472.
- Tracy TS1, Krohn K, Jones DR, Bradley JD, Hall SD, et al. (1992) The effects of a salicylate, ibuprofen, and naproxen on the disposition of methotrexate in patients with rheumatoid arthritis. Eur J ClinPharmacol 42: 121-125.
- Wiland P, Swierkot J, Szechinski J (1997) N-acetyl-beta-D-glucosaminidase urinary excretion as an early indicator of kidney dysfunction in rheumatoid arthritis patients on low-dose methotrexate treatment. Br J Rheumatol36: 59-63.
- Saito M1, Uechi Y, Nakabayashi K, Kitamoto K, Nagasawa T (1993) [Clinical significance of microalbuminuria in patients with rheumatoid arthritis]. Nihon JinzoGakkai Shi 35: 815-821.
- Iqbal MP1, Ali AA, Waqar MA, Mehboobali N (1998) Urinary N-acetyl-beta-D-glucosaminidase in rheumatoid arthritis. ExpMol Med 30: 165-169.
- Svendsen KB, Ellingsen T, Bech JN, Pfeiffer-Jensen M, Stengaard-Pedersen K, Pedersen EB, et al. (2005) Urinary excretion of alpha-GST and albumin in rheumatoid arthritis patients treated with methotrexate or other DMARDs alone or in combination with NSAIDs. Scand J Rheumatol34: 34-9.
- Wiland P1, Szechiński J (1994) N-acetyl-beta-D-glucosaminidaseenzymuria as an indicator in monitoring the therapy of some rheumatic diseases with potentially nephrotoxic drugs. Arch ImmunolTherExp (Warsz) 42: 331-336.
- Wiland P1, Wiela-Hojeńska A, Swierkot J, Hurkacz M, Orzechowska-Juzwenko K, et al. (2003) [Renal tubular dysfunction in patients with rheumatoid arthritis starting with low dose of methotrexate]. Pol Arch Med Wewn 110: 855-862.
- Zafirovska KG1, Bogdanovska SV, Marina N, Gruev T, Lozance L (1993) Urinary excretion of three specific renal tubular enzymes in patients treated with nonsteroidal anti-inflammatory drugs (NSAID). Ren Fail 15: 51-54.
Relevant Topics
Recommended Journals
- Journal of Lung Cancer Diagnosis & Treatment
- Advances in Cancer Prevention
- Breast Cancer: Current Research
- Cancer Surgery
- Immunology: Current Research
- Current Trend in Gynecologic Oncology
- Journal of Cancer Diagnosis
- Journal of Gastrointestinal Cancer and Stromal Tumors
- Cervical Cancer: Open Access
- Journal of Mucosal Immunology Research
- Journal of Oncology Research and Treatment
- Journal of Orthopedic Oncology
- Journal of Prostate Cancer
- Research and Reviews on Pathogens
Article Tools
Article Usage
- Total views: 14167
- [From(publication date):
December-2014 - Nov 21, 2024] - Breakdown by view type
- HTML page views : 9807
- PDF downloads : 4360