Research Article Open Access
Investigation of Biodiesel Potential of Biomasses of Microalgaes Chlorella, Spirulina and Tetraselmis by NMR and GC-MS Techniques
Sarpal AS1*, Ingrid CR Costa1, Claudia MLL Teixeira2, Diego Filocomo2, Renata Candido2, Paulo RM Silva1, Valnei S. Cunha1 and Romeu J. Daroda11Instituto Nacional de Metrologia, Qualidade e Tecnologia– INMETRO. Avenida Nossa Senhora das Graças 50, Xerém, Duque de Caxias, RJ, Brazil
2Instituto Nacional de Tecnologia (INT), RJ, Brazil
- Corresponding Author:
- Sarpal AS
Instituto Nacional de Metrologia
Qualidade e Tecnologia– INMETRO
Avenida Nossa Senhora das Graças 50
Xerém, Duque de Caxias, RJ, Brazil
Tel: 0919899443336
E-mail: sarpal.as2@gmail.com
Received date: January 06, 2016; Accepted date: February 26, 2016; Published date: March 08, 2016
Citation: Sarpal AS, Costa ICR, Teixeira CMLL, Filocomo D, Candido R, et al. (2016) Investigation of Biodiesel Potential of Biomasses of Microalgaes Chlorella, Spirulina and Tetraselmis by NMR and GC-MS Techniques. J Biotechnol Biomater 6:220. doi:10.4172/2155-952X.1000220
Copyright: © 2016 Sarpal AS, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Biotechnology & Biomaterials
Abstract
The detailed component analyses of algal oils such as neutral (mono, di and triglycerides; free fatty acids) and polar (glyceroglyco/phospho) lipids, and their fatty acid profile including poly unsaturated fatty acids (PUFAs) (C18:3, C22:6) have been carried out by NMR (1H, 13C) and GC-MS techniques to explore their biodiesel potential. The algal oils were obtained by ultrasonic solvent extraction of microalgae biomasses cultivated on a lab scale. The results revealed that biomass and neutral lipids productivity as well as quality and composition of saturated and unsaturated fatty acid were influenced by media (modified RM6, F/2, WC), microalgae species/strains (Chlorella vulgaris, Spirulina platensi and Tetraselmis affchuii) and shape of the cultivated system (Erlenmeyer flask, polythene tubular or round container). The replacement of source of N and P with cheaper source of fertilizers (super phosphates + Chillean salt peter) in the cultivation media F/2 and WC has not affected the neutral lipid productivity, rather produced FAMEs with lower amount of Docosahexaenoic acid (DHA). The biomass cultivated in the modified RM6 media by Spirulina platensi is comprised of lower amount of unsaturated fatty esters without DHA (42.9% w/w) (PUFA as �?¤-linoleic) compared to other biomasses (61.2-69.8 %w/w), thus quite suitable for production of biodiesel with higher oxidation stability and higher cetane number. The algal oils generated from the biomasses in the cultivation system are promising feed stocks for biodiesel and value added products DHA and α, γ -linolenic acids. The developed fast and cost effective analytical strategy based on 1H NMR techniques will facilitate algae cultivators for screening of species and optimization of cultivation parameters to produce a choice of product, thus contribute partly in the overall reduction in the cost of production of biodiesel.
Keywords
Biodiesel; Microalgae biomass; NMR; GC-MS; Fatty acid profile; PUFA
Introduction
Microalgae are unicellular photosyntheticorganisms, generally of 2-50 μm in size, that require primarily three components to produce biomass, i.e., water, CO2, and sunlight with relatively higher photosynthetic efficiency of 3-8% against 0.5% for terrestrial plants. The microalgae grow in aquatic environment with diverse sources of water such as fresh, industrial waste, marine, brackish and open ponds. Microalgae grow best in waste water by utilizing the N, P and K of the industrial effluent, which are essential nutrients required for the cultivation of microalgae. It needs less water compared to regular terrestrial crops (20 L of water /1 L of biodiesel compared to 3000 L of water from vegetable oils). Algae can either be autotrophic or heterotrophic under natural growth conditions. There are three major advantages of cultivation of algae over regular vegetable crops, that attracts attention of cultivators, such as faster and high growth rate and productivity (200 times the regular oil crops, L oil/ha/year), no competition with cereal crops as algae is cultivated in the non-arid lands, sea, waste and industrial water and a higher CO2 sequestration capacity [1-7]. Moreover, microalgae cultivation process is environment friendly, mitigate CO2 and NOx from industrial flue gases by biofixation besides active agent of industrial wastewater bioremediation by removal of hazardous pollutants. In addition, algae biodiesel contains no sulfur and performs as well as petroleum based diesel, while reducing emissions of particulate matter (PM), CO, hydrocarbons, and SOx [6]. The higher biomass productivity (20-200 mg L-1day-1) and oil rich microalgae genera are Nannochloropsis, Botryococcus, Scenedesmus, Spirulina, Chlorella, Dunaliella, Phaeodactylum tricornutum, Isochrysis galbana, Monodus subterraneus, Tetraselmis and C. reinhardtii [4,8-33]. The microalgae Isochrysis, Phaeodactylum tricornutum, Pavlova and Thalassiosira can cultivate sufficient amount of omega-3 poly unsaturated fatty acids (PUFAs) in order to use as an alternative for fish oil [28,29]. The most important motivating factor of growing microalgae is due to its lipids content of 2-80 percent of total dry cell matter depending on the types of species and growth conditions employed for cultivation [5-7,13,14,32,34]. Like oilseed crops such as rapeseed and soybean oil, which have been extensively evaluated as sources of biodiesel, biodiesel from microalgae are regarded as a promising alternative environmentally sustainable renewable source of fuel for IC engines. Other potential utilization of microalgae is production of biofuels such as bio-hydrogen, jet fuel (hydrotreating), bio-ethanol and bio-butanol [7]. This makes microalgal oil as rich sources of biodiesel as well as value added co-products. Approximately 5,000-15,000 gal of biodiesel can be produced from algae biomass per acre per year, which reflects its potentiality [7]. The lipids of microalgae biomasses are comprised of neutral lipids (storage), polar lipids (structural), carbohydrates, hydrocarbons, and valuable co-products. Over 15,000 novel compounds originate from algae biomass [10]. The neutral lipids, triacyl glycerides (TAG) and free fatty acids (FFA), present in microalgae dry biomass, are the main potential components of interest to the biodiesel industry. Besides neutral lipids and polar lipids, various high-value co-products such as epoxy esters, pigments, antioxidants, β-carotenes, polysaccharides and vitamins can be extracted from microalgae. These co-products find extensive applications in pharmaceuticals, cosmetics, nutraceuticals, and functional foods industries [35,36].
Biodiesel potential of oleaginousmicroalgae biomass is associated with the biomass productivity, mainly content and quality of neutral lipids, achieved during cultivation process. The fatty acids profile of algal oil is comprised of C12-C24 saturated and unsaturated fatty acids, which is more or less similar to those present in vegetable and fish oils [16]. TAGs from microalgae biomass are converted into fatty acid methyl ester (FAME) (biodiesel) by usual chemical hydrolysis followed by esterification or enzymatic transesterification[26]. Biodiesel from microalgae can be used as B100 (neat biodiesel) or blended with conventional diesel at a level of 5% (B5) or 10% (B10) or 20% (B20). Most of microalgae biomasses are rich sources of n-3 ω3 and n-6 ω6 fatty acids including C18:3, C20:5 (Eicosapentaenoic acid, EPA) and C22:6 (Docosahexaenoic acid, DHA) as indicated by very high Iodine value of 90 to 140 of solvent extracts [16,28,29]. Thus biodiesel from algal oil is not an appropriate fuel for direct combustion in sensitive engines due to its high content of PUFAs (10-40% w/w) [16]. However, extraction of PUFA by solvent extraction methods or hydrotreating/ hydrocracking of algal oil are considered useful options for making biodiesel with oxidation stability similar to those inherited by biodiesel from oil crops [37].
Versatile techno economic aspects of cultivation of microalgae are that biochemical composition of the algal biomass can be mutated by selection of proper species / strains and varying cultivation parameters. This can significantly enhance the biomass productivity and oil yield, vary the chemical composition and fatty acid profile, and improve their quality aspects, and also provide the choice either in favor of biodiesel or value added products. The composition and nature of neutral lipids and their fatty acids profile, particularly unsaturated including PUFAs, are dependent on the cultivation parameters such as temperature, composition of medium, types of feed, light intensity etc. [6,16,38-42]. The different lipid induction techniques such as environment stress, nitrogen limitation or starvation, salinity factor, light irradiation, genetic modification etc. have been used to enhance the biomass and lipid productivity. Among these techniques, nitrogen starvation is most widely applied and studied in almost all the microalgae species that can be considered for the commercial production of biodiesel. Since algae biomass is potentially enriched with biodiesel components (neutral lipids) and food supplements (PUFAs), the cultivation parameters can be specified to conduct the desired activity. The occurrence and the extent to which TAGs are produced is species/strain-specific, and are ultimately controlled by the genetic make-up of individual organism. Under environment stress or lipid induction techniques, the TAG content can be increased to 20 to 50% of the dry cell weight [6,7]. The biomass and neutral lipid productivity with regard to the nature of triglycerides can be modulated by varying the composition of nutrient media, particularly concentration and source of nitrogen (sodium nitrate, urea, ammonium carbonate, potassium nitrate, ammonium nitrate) and phosphorus ( super phosphate fertilizer) as investigated in details by Sarpal et al [16] and Amin et al. [38]. Recently, a systematic studies was conducted on the use of cheaper sources of nitrogen (Chillean saltpeter; sodium nitrite), and phosphorus (superphosphates fertilizer) in order to study the effect on biomass productivity, lipid productivity and nature of fatty acid profile. It has been authenticated that by choice of an appropriate medium composition, biomass with desired characteristics can be produced. As indicated, the productivities of value added products PUFAs are enhanced by using a medium prepared with alternative sources of nitrogen. This provides an additional advantage of the lower cost of cultivation. However, it was achieved at the cost of neutral lipids whose productivities were found to be reduced immensely. The determination of fatty acid profile of FAMEs determined by both Nuclear Magnetic Resonance (NMR) and Gas chromatography-Mass Spectrometry (GC-MS) techniques has shown that cultivated microalgae biomasses are high potential sources of biodiesel. The nitrogen and phosphorus limitation in the cultivation media have been demonstrated to enhance the lipid content of 29-60 w/w % dry weights with higher content of saturated and mono unsaturated fatty acids [16]. The effect of UV irradiance for lipid induction has been shown to affect the fatty acid profile, particularly PUFAs with DHA and EPA chain [42].
The biomass productivity depends upon the cultivation system, photobioreactors [7,42-46]. Open pond raceways system with paddle wheel is cost effective and used for mass cultivation of algae biomass. The biomass concentration generally remains low between 0.1-0.15 g L-1 d-1. This is mainly because raceways are poorly mixed and optimum light intensity cannot be achieved. Closed photo bioreactor with different shapes and design such as tubular, helical, flat panel, horizontal and vertical, are most efficient photosynthetic mass cultivation system with biomass productivity achieved up to 0.215 g L-1 d-1 [7,42]. This is because high algal biomass concentrations can be achieved in closed PBR systems due to increase surface areas and sufficient sunlight penetration capability. However, it is at the cost of higher energy consumption at the cultivation stage compared to raceways pond systems [45].
In order to produce biodiesel by usual process of transesterification, lipids and fatty acids are extracted from the microalgae biomass. Lipids extraction efficiency is directly related to the overall process efficiency and considered important step in determining the overall cost of production of biodiesel. Chemical Solvent extraction methods in combination with milling (expression, expeller and bead milling/ beating) are applied to extract neutral lipids. Chemical methods involve extraction by solvents such as hexane/cyclohexane, chloroform, methanol, isopropanol and their mixtures by use of soxhlet, Accelerated Solvent Extraction (ASE), sonication and Supercritical Fluid Extraction (SFE) methods [47-54]. Soxhlet extraction process using hexane, and milling in combination with hexane are quite efficient in the extraction of neutral lipids (TAG, FFA),which are easily esterified into biodiesel [50]. Ultrasonication and microwave assisted extraction with appropriate solvents have many advantages such as reduced extraction time, reduced solvent consumption, and greater penetration of solvent into cellular materials causing high cell disruptive effects for quick release of oils [54,55]. Extractions using the subcritical or supercritical fluids (ethanol), mainly supercritical CO2, are applied to extract lipids and considered economically viable and green process as drying process is completely eliminated [52,53].
Analytical techniques, molecular, atomic and chromatographic, are being used extensively to cover wider range of applications to the broad spectrum of biofuels comprised of biodiesel, bio oils, biojet fuel and biohydrocarbons from edible and non-edible oils, oleaginous microalgae and yeast biomasses, and deoxygenated and hydrotreated biodiesels [16,55-65]. Analytical methods based on NMR techniques (1H, 13C, 31P etc.) are direct, rapid and convenient, and applied to provide detailed quantitative compositional analyses such as fat content, PUFA, unsaturated fatty acid profile, iodine value, monitoring cultivation, production and quality control of biodiesel obtained from different sources as discussed in details previously [16,55,56].
Chlorella, Spirulina and Tetraselmis species / strains have been widely investigated for their biodiesel, PUFA and co-product potential in fresh and waste water cultivation [16-29]. Systematic studies have been conducted in order to enhance the lipid content and improve quality of fatty acid profile by selection of appropriate media and species to meet the biodiesel specification (ASTM D6751 and EN 14214). In order to meet the similar objectives with the focused analytical strategies, the NMR spectroscopic (1H, 13C) techniques have been applied to explore the biodiesel potential of ultrasonic solvent extracts of biomasses (hereafter will be called algal oils) of microalgae Chlorella vulgaris (CHL), Spirulina platensis (SP) and Tetraselmis affchuii (TS) cultivated in different media composition on a lab scale. The quality parameters, which determines the quality of the lipids and corresponding FAMEs such as triglycerides (TG) and FFA content, iodine value (IV), PUFAs, and saturated and unsaturated fatty acids (SFA, UFA) have been determined after detailed 1H and 13C NMR spectral interpretation. The effect of the variation in the shape of the container on the lipid productivity and corresponding fatty acid profile has been studied.
Materials and Methods
Biomass production and productivity
The biomasses of microalgaes Chlorella vulgaris (CHL), Spirulina platensis (SP) and Tetraselmis affchuii (TS) were cultivated on a lab scale in the Erlenmeyer flasks. Microalgae CHL (CCMA-UFSCar 012) was kindly provided by Federal University of São Carlos. Their isolates are kept in the collection of microalgae cultures in the department of Botany, Federal University of São Carlos (CCMA-UFSCar, WDCM 835). Micoalgaes TS and SP were kindly provided by Prof. Gleyci Moser of University of State of Rio de Janeiro and by Prof. Sergio Lourenço from Fluminense Federal University, respectively. Microalgaes CHL, TS and SP were maintained in modified WC [16], F/2 [25] and Zarrouk media (RM6) [66] respectively in Erlenmeyer flasks with 300 mL, density of photonic flux around 135 μ E.m-2.s-1 of white fluorescent light, and temperature of 25 ± 2ºC. Cultivation was achieved with an appropriate light density, and finally inoculums were transferred to PET bottles. New cultures were prepared for each species using 10% v/v of inoculums for a total volume of 4 L, and in these cultivations the air was used as source of CO2 which was passed in the culture medium through air pumping at 3.0 L/min. The light source was fluorescent light except in the case of Tetraselmis sp (TSG1) cultivations where cool white LED IP65 5050 was used. The photonic flux density was around 120-150 μ E.m-2.s-1 in all the system. The temperature was maintained at 25 ± 2°C, except for Spirulina platensis, cultivation temperature was kept 32 ± 1°C. The cellular suspensions were centrifuged.
For TSG4F2SPS biomass cultivation, medium of Guillard F/2 was modified by replacement of the source of nitrate and phosphate with agricultural fertilizers in the same concentration of F/2 in terms of N and P [25]. The source of nitrate was replaced by Chilean saltpeter (sodium nitrite, designated as S) (Simple Mineral Fertilizer- VITAPLAN®) and phosphate source by simple superphosphate (designated SP) (Mineral Fertilizer- HERINGER®). For Spirulina platensis, RM6 modified by the addition of micro elements solution and FeSO4, which was quite different in composition from RM6 [66], was used as cultivation medium for the cultivation of biomass SPRM6. In a similar ways, the cultivations were carried out in polythene containers, P1 and P2 with different shapes, under identical conditions and media composition of F/2 using the microalgae Tetraselmis (G1 and G4). The biomasses were designated TSG1F2P1 and TSG4F2SPSP2 .The nomenclatures of biomasses are mentioned in the Table 1.
| Biomass/Algae oil | Species | Medium | Productivitymg/L/Day |
| CHLWCSPS/CHLWCSPSE | Chlorella vulgaris (CHL) | WC+superphosphates+ Chillean salt peter | 50.2 |
| TSG1F2/TSG1F2E | Tetraselmis aff chuii (TS) | F/2 | 35.8 |
| TSG4F2SPS/TSG4F2SPSE | Tetraselmis affchuii (TS) | F/2+ superphosphates | 26.2 |
| TSG1F2P1/TSG1F2P1E | Tetraselmis affchuii (TS) | F/2, container P1 | 58.4 |
| TSG4F2FP2/TSG4F2FP2E | Tetraselmis affchuii (TS) | F/2+ superphosphates,Container P2 | 46.0 |
| SPRM6/SPRM6E | Spirulina platensis (SP) | Modified RM6 | 30.2 |
Table 1: Nomenclature of ClMe (CHCl3-MeOH-H2O) extracts of algae biomasses cultured under different conditions/media and biomass productivity.
When the stationary phase was attained, the cultivation was interrupted and the cultures were centrifuged in a Rotixa 50RS centrifuge (Hettich) at 4ºC and 3500 rpm for 10 minutes. However, in the case of Spirulina, culture was filtered in filter screen (120 micron). The pellets (biomass) were collected and frozen. The frozen biomass was lyophilized in an Enterprise IID lyophilizer (Terroni). After lyophilization, the samples were kept in the refrigerator at -20ºC to protect from degradation until analysis. Comparisons among means of the biomass productivity attained with different cultivation media were made using Kruskal-Wallis test with confidence interval of 95%. Results of biomass productivities are given in the Table 1.
Extraction and analytical strategy
Single step ultrasonic extraction: The extraction of neutral (NL) (TAG + FFA) and polar lipids (PL) from the algae biomasses of Chlorella vulgaris (CHLSPS), Spirulina platensis (SPRM6) and Tetraselmis aff chuii (TSG1F2, TSG4F2SPS) were carried out by single step ultrasonic extraction methods using the solvent mixture of chloroform:methanol:water (2:1:0.3) (designated as ClMe) [16,55]. In order to enhance the extraction efficiency, the extractions were carried out in 50 mL measuring flask using 200 W (50 Hz) ultrasonic instruments. The amount of dry biomass taken was 100 to 120 mg in 20 ml of the solvent mixture and extraction carried out for 60 minutes at 30ºC. The suspensions in the flasks were alowed to settle for a period of 30 minutes. The clear superannuated layer (ClMe) was separated in a beaker and residue in the flask washed with 5 ml of methanol in order to extract the residual layer left in the flask. The methanol washed layer after settling was added in to the beaker containg ClMe layer.The extracted solution in the beaker was further filtered on a Whatman filter paper to remove suspended particles of the residue left over in the solution. The solvent in the extract was evaporated completely on a water bath. The extract was treated with 5mL of acetone and evaporated in order to remove traces of water in the extract. The dried extracts or algal oils were designated according to the strain followed by the cultivation medium and E(extract) such as CHLSPSE, SPRM6E, TSG1F2E, TSG4F2SPSE and SPRM6E, and stored in the refrigerator to avoid degradation. The residues in the respective beakers were transferred on a separate filter papers, dried and labeled accordingly. Each extraction was repeated 2 to 3 times and average weight of the extracts was taken, and termed as total lipid extracts (TL). The nomenclature of the algal oils are given in the Table 1.
Hydrolyses of algal oils for FAMEs conversion: The algal oils were converted into FAMEs by adopting the usual Methanol-KOH hydrolyses procedure. The hydrolyses procedure was optimized for right quantity of MeOH-KOH and BF3-MeOH to be taken for complete conversion into FAMEs. Depending upon the quantity of the algal oils available, 10 to 15 mg was taken in a 10 mL flask. Freshly prepared 2 to 3 mL of 1N MeOH-KOH solution (5.6 mg of KOH in 100 mL of MeOH) was added in a flask. The resulting solution was refluxed on a water bath for three hours at 70-75°C. The solution was cooled and added ~0.5 to 1 mL of BF3-MeOH and again refluxed for 30 minutes. The flask was cooled and added about 1 mL of saturated NaCl solution. The solution was transferred into the separating funnel and added 2 mL of water. The resulting solution was shaken with 5 mL of hexane. The superannuated layer was separated and hexane evaporated on a water bath. Small quantity of acetone was added during evaporation to remove traces of water. The final dried FAMEs samples were analyzed by GC-MS for fatty acid composition and NMR analyses for monitoring of FAMEs conversion. The conversion efficiency has been found to be around 60- 70% depending upon the amount of polar lipids present in the oils. A part of higher fatty acids such as C22:6 attached to polar lipids were not found to be hydrolyzed as indicated by the comparative NMR analyses of the algal oils and their corresponding FAMEs.
Instrumental analyses
GC-MS analyses: FAMEs were analyzed by GC-MS carried out on a Shimadzu Gas chromatograph equipped with Quadruple Mass spectrometer using electron impact ionization. A capillary polar PEG wax column (30 m length, 0.25 mm diameter, 0.25 μm film thickness) keeping injector and column temperature at 265°C and 250°C respectively was used for separation of FAMEs. Heptane solutions containing 3-5 mg of FAMEs were injected in split ratio of 10:1 applying the ramp: 70°C for three minutes, 10°C per minute from 70 to 240°C, hold time 13 minutes and finally 5°C per minute up to 250°C and held it for 10 minutes. The identification of all types of saturated and unsaturated components including DHA and EPA was carried by comparing with the profile of NIST (2772 SRM) and PUFE standards, and FAMEs of fish oil. Results are given in the Table 2.
| Sample | CHLSPS | SPRM6 | TSG4 F2 | TSG1 F2 | TSG4F2 P1 | TSG4F2 P2 | Soyastd | Fish | SFL | CN |
|---|---|---|---|---|---|---|---|---|---|---|
| C14:0 | 0.35 | 2.1 | ND | 0.26 | ND | ND | 7.28 | 66.2 | ||
| C16:0 | 31.82 | 39.29 | 26.12 | 27.54 | 27.48 | 36.2 | 11.19 | 17.91 | 6.62 | 74.5 |
| 9-C16:1 | 2.16 | 3.6 | 8.70 | 6.87 | 7.79 | 10.35 | 45.0 | |||
| 7-C16:1 | ND | 3.8 | 0.35 | 0.39 | 45.0 | |||||
| 7,10-C16:2 | 1.81 | ND | 0.59 | 0.37 | 1.30 | 25.0 | ||||
| 4,7,10-C16:3 | 2.13 | ND | 1.40 | 0.78 | 2.86 | 1.41 | ||||
| C16:3 | ND | ND | 0.39 | 1.00 | ||||||
| C18:0 | 1.5 | 4.24 | 0.64 | 0.96 | 1.1 | 2.1 | 3.58 | 3.91 | 6.37 | 86.9 |
| 9-C18:1 | 17.33 | 17.8 | 27.62 | 29.07 | 35.87 | 23.2 | 18.24 | 9.13 | 60.27 | 55.0 |
| 10-C18:1 | 9.74 | 1.28 | 3.45 | 4.38 | 3.71 | 2.0 | 3.69 | 55.0 | ||
| 9,12-C18:2 | 14.71 | 8.89 | 3.72 | 3.93 | 6.1 | 5.54 | 58.16 | 1.32 | 26.54 | 36.0 |
| 7,10-C18:2 | ND | ND | 0.58 | ND | 2.55 | 0.71 | 36.0 | |||
| 6,9,12-C18:3 | ND | 16.25 | 0.39 | 28.0 | ||||||
| 9,12,15- C18:3 | 4.2 | ND | 9.63 | 8.13 | 1.32 | 22.8 | 8.84 | 0.71 | 28.0 | |
| C20:0 | ND | 1.7 | 0.92 | 1.63 | --- | 0.8 | 100 | |||
| C20:1 | 1.81 | 82.0 | ||||||||
| C20:3 | 8.14 | ND | ND | ND | 3.4 | ND | 29.5 | |||
| 5,8,11,14-C20:4 | ND | 1.03 | 0.40 | 2.66 | 0.96 | |||||
| C20:5 | ND | ND | ND | ND | ND | ND | 19.1 | |||
| 4,7,10,13,16,19 -C22:6 | 1.74* | ND | 4.24* | 4.8* | 7.1* | 5.54* | 12.29 | 24.4 | ||
| UI | 5.27 | 1.08 | 10.64 | 9.10 | 2.21 | |||||
| SFA | 33.67 | 47.30 | 27.66 | 28.76 | 28.52 | 38.3 | 14.77 | 29.9 | 12.99 | |
| UFA | 61.06 | 51.62 | 61.70 | 62.14 | 72.48 | 61.7 | ||||
| Cetane no. | 51.23 | 57.62 | 48.07 | 50.29 | 54.47 | 53.35 | 44.89 | 42.55 | 53.18 | |
| Iodine value** | 98.9 | 81.3 | 117.6 | 96.2 | 92.1 | 97.4 | 141.1 | 199.0 | 109.5 |
CN = Cetane Number [69-71], *determined by NMR from corresponding FAMEs of algal oils, UI = Unidentified, SFL = Sunflower, ** by NMR analyses of FAMEs, std = standard
Table 2: Fatty acid composition (area %) of FAMEs of algal oils and vegetable oils by GC-MS.
NMR recordings: All the 1H NMR spectra were recorded on a Bruker 500 MHz NMR Spectrometerequipped with broad band probe (BB) and inverse probe (BBI). The solutions were prepared by dissolving approximately 5 to 10 mg of algal oils, FAMEs, vegetable / fish oils in 0.7-0.8 mL of CDCl3 containing internal reference TMS. Instrument parameters such as relaxation delay (D1) and receiver gain (RG) were optimized and 90° PW calibrated in order to sufficiently relax the nuclei to get the quantitative spectra as described in our previous work [56,57]. The 90° pulse width (PW) was calibrated for each sample. Relaxation delay (D1) = 10 s; P1 (90° PW) = 8.13-8.64 μs; NS (Number of Scans) = 16 or 32, Chemical shift range: 0-12 ppm. All the spectra were integrated thrice after proper phase and base line corrections and average integral areas were taken for quantitative analyses.
The 13C NMR spectra were recorded on a Bruker 500 MHz NMR spectrometer using BBI probe in CDCl3 solution of algal oils (~ 50 mg/0.8 mL) in the inverse gated mode. The overnight recordings (~ 10,000 scans) with a relaxation delay time of 5 s, PW of 15 μs and sweep width of 0-240 ppm were carried out. However, 25% weight by volume solutions of vegetable /fish oils were used for 13C NMR recordings with maximum number of scans of 2500-5000 under identical conditions [16,56].
Results
GC-MS analyses
Results of fatty acids profile of the FAMEs of algal and vegetable oils obtained by GC-MS are given in the Table 2. The fatty acid composition of algal oils were found to be in the range of C14 to C22 with higher amount of saturated fatty acids (SFA) compared to C16 to C18 present in vegetable oils.
1H NMR spectral features of algal oils
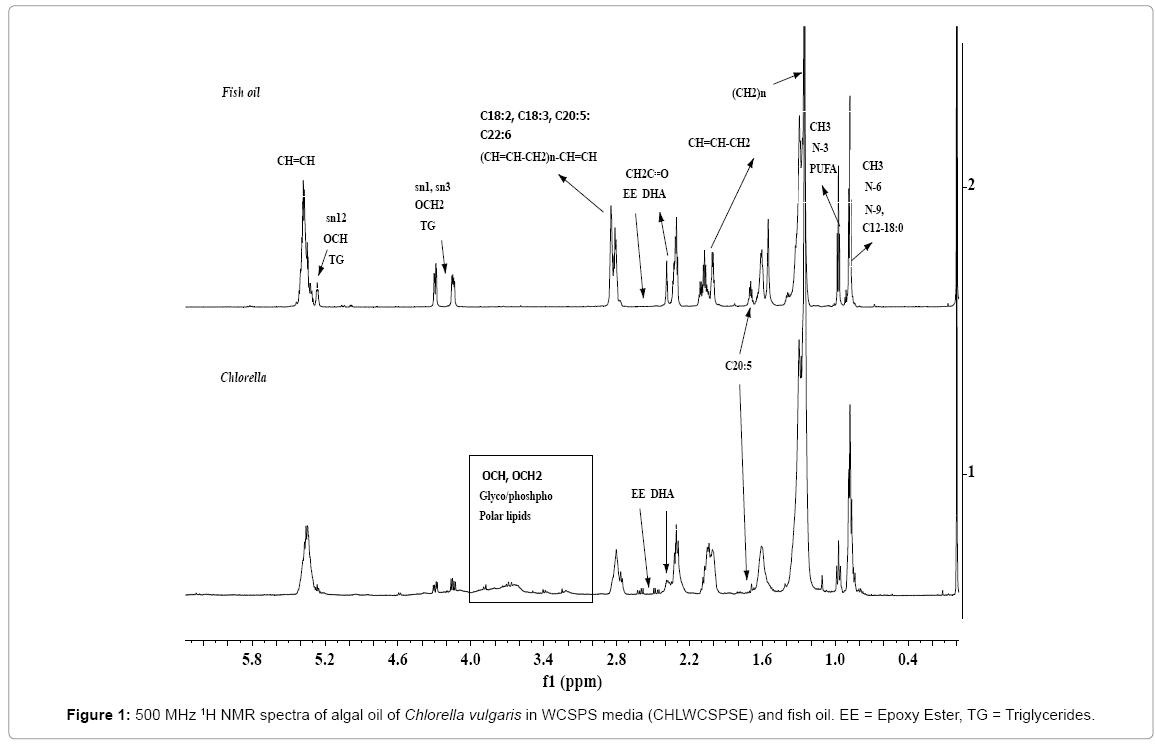
The spectral features of algal oils of microalgae biomasses and vegetable oils for the nature of neutral lipids (NL) (TAG, FFA) and polar lipids (PL) including their fatty acid profiles, and minor components such as sterols, hydrocarbons etc. have been described in details in our earlier work [16,55,56]. However, these are briefly presented in order to apprise the readers of the concept as well as connect with objectives of the work. The 1H NMR spectra of algal oils of Spirulina (SPRM6E), Tetraselmis (TSG4F2SPSE) and Chlorella (CHLWCSPSE) biomasses are given in the Figures 1-3. As shown in the Figure 1, the 1H NMR spectra of CHLWCSPSE, show signals characteristics of triglyerides (TG) comprising of saturated and unsaturated long alkyl chain fatty acids identical to those appeared in the spectrum of fish oil. Besides the signals due to TG, the signals corresponding to the functional groups of polar lipids are also prominently visible as marked in the Figure 1. The signals corresponding to functional groups of ester (OCH, sn2; OCH2, sn1 sn2), unsaturations (CH=CH), carbonyl of CH2C=O, bis allylic (-CH=CH-CH2)n, terminal CH3s of ω n-3 PUFAs (3 and more than 3 double bonds) and n-6/n-9 unsaturated fatty acids components, and (CH2)n of long fatty acids alkyl chain of both saturated and unsaturated fatty acids of TG and glyceroglyco / phospholipids (PL) are marked in the spectra for understanding their spectral features (Figures 1 and 2) [56]. The signals at 4.38 to 5.2 ppm, and 4.05 to 3.1 ppm were assigned to protons of OCH2 and OCH ester groups, and CHOH and CH2OH groups due to the glycerol part of PL as shown in the Figure 1. TG constitutes ~ 90% of the total mono, di and triglycerides as clearly depicted in the expanded view of the region 4-4.5 ppm (Figure 3) [16].
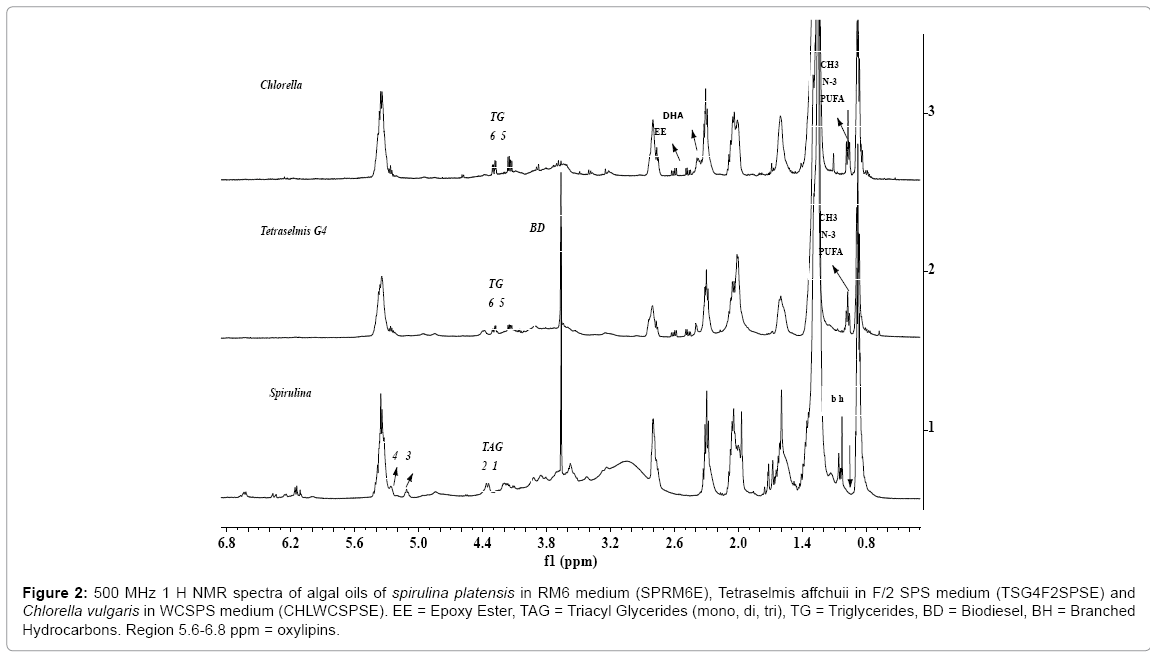
Figure 2: 500 MHz 1 H NMR spectra of algal oils of spirulina platensis in RM6 medium (SPRM6E), Tetraselmis affchuii in F/2 SPS medium (TSG4F2SPSE) and Chlorella vulgaris in WCSPS medium (CHLWCSPSE). EE = Epoxy Ester, TAG = Triacyl Glycerides (mono, di, tri), TG = Triglycerides, BD = Biodiesel, BH = Branched Hydrocarbons. Region 5.6-6.8 ppm = oxylipins.
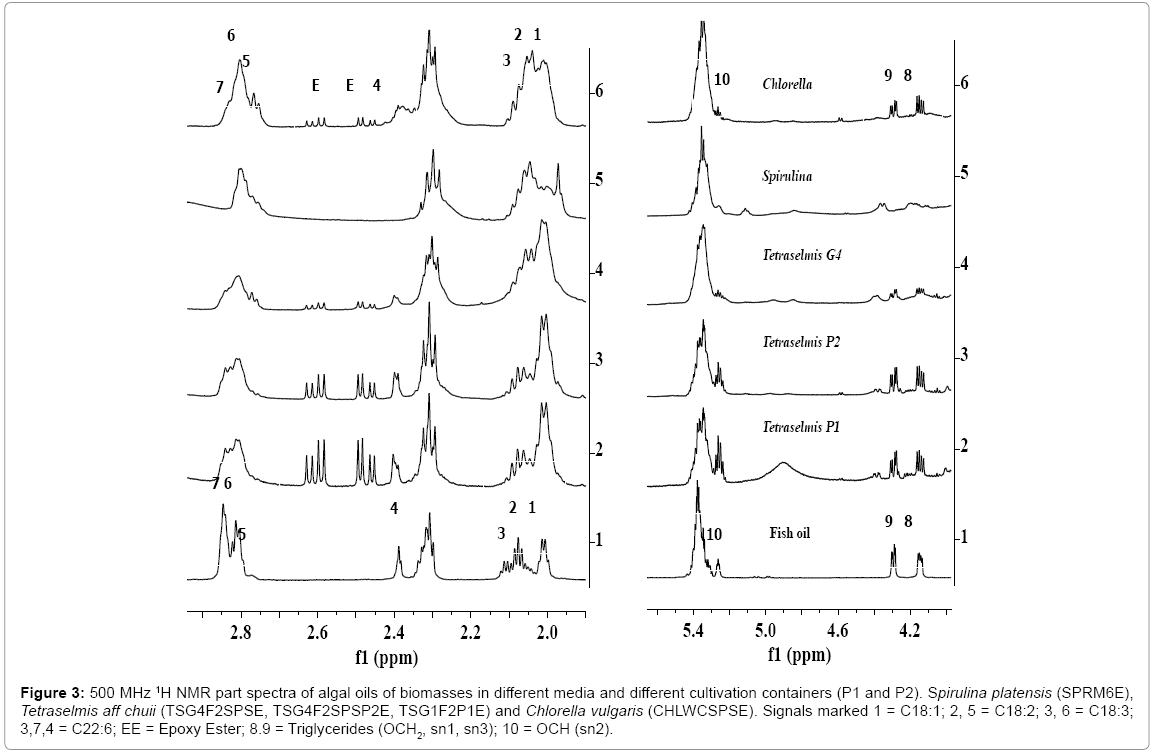
Figure 3: 500 MHz 1H NMR part spectra of algal oils of biomasses in different media and different cultivation containers (P1 and P2). Spirulina platensis (SPRM6E), Tetraselmis aff chuii (TSG4F2SPSE, TSG4F2SPSP2E, TSG1F2P1E) and Chlorella vulgaris (CHLWCSPSE). Signals marked 1 = C18:1; 2, 5 = C18:2; 3, 6 = C18:3; 3,7,4 = C22:6; EE = Epoxy Ester; 8.9 = Triglycerides (OCH2, sn1, sn3); 10 = OCH (sn2).
The presence of PUFAs (three and more than three double bonds, ω n-3 types) is confirmed by the appearance of a triplet at 0.96-0.98 ppm due to terminal CH3, which is clearly separated from the terminal CH3 at 0.88 ppm due to saturated (C18:0, C16:0, C14 etc.) or n-6/n-9 types of unsaturated (C18:1, C18:2) components. The spectra also indicate less intense signals at 2.39 and 2.42 ppm (CH2C=O), and 2.84 ppm due to bis allylic (-CH=CH-CH2)n compared to signals at 2.24-2.37 ppm (CH2C=O ) corresponding to long alkyl chain fatty acids components. These signals were assigned to C22:6.
The signals corresponding to unsaturated fatty esters (C18:1, C18:2, C18:3, C22:6) in the algal extracts are compared with those present in the NMR spectra of fish oil as depicted in the expanded view of spectra in the chemical shift region of 1.9 to 2.9 ppm (Figure 3). These are marked as C18:1 (signal 1), C18:2 (signals 2, 5), C18:3 and C20:3 (signals 2, 6), and C22:6 (signals 3, 4, 7). The signals marked as EE in the chemical shift regions of 2.42-2.66 ppm in the Figures 1-2, and visible more clearly in the expanded part of the spectra presented in Figure 3, were assigned to epoxy groups of linolenic or DHA or EPA types of components. The identities of epoxy types of fatty esters, were further confirmed by 13CNMR and 2DNMR (HSQC-TOCSY) analyses as described previously [56]. As shown in the Figure 2, the 1H NMR spectra of algal extracts clearly shows very weak intensity signals in the region of 5.6-7.0 ppm corresponding to conjugated/nonconjugated unsaturated protons of oxylipins (lipoxygenase-derived oxygenated acids products) of polyunsaturated fatty acids/esters such as DHA and EPA [56]. These signals are quite intense in case of SPRM6E compared to signals of TSG4F2SPSE and CHLWCSPSE, thereby indicate their higher amount in the extracts. The signals due to CH3s of sterols were not observed in the regions of 0.5-0.6 ppm, as reported earlier in similar studies on microalgae extracts [56].
The spectrum of SPRM6E algal oil show features quite different from TSG4F2SPSE and CHLWCSPSE. The signals due to OCH2 (4.28 ppm and 4.14 ppm; sn1, sn3) and OCH (4.26 ppm, sn2) triglycerides (TG) are not observed in the spectrum of SPRM6E (Figures 2 and 3). The signals characteristics of mono and di glycerides in the region of 4.16-4.39 ppm with different multiplicity are clearly visible in comparison to the spectral features of TSG4F2SPSE and CHLWCSPSE (Figure 2) [67]. The spectrum also indicates a sharp signal at 1.03 ppm (marked bh), which was probably assigned to C-(CH3)3 types of signals. Such types of signals generally appear in the spectra of isoprene types of hydrocarbons [cross ref. in ref. 56]. The spectra of SPRM6E clearly show characteristic signals at 2.81 ppm due to vinylic groups and absence of terminal CH3 at 0.98 ppm, thereby indicate the presence of ω n-6 linolenic ester. This is in contrast to signals observed at 0.98 ppm and 2.81 ppm for α- linolenic ester appeared in the spectra of TSG4F2SPSE and CHLWCSPSE. The confirmation of mono and di glycerides, and ω n-6 linolenic ester in SPRM6E has been described in details in the succeeding section.
The 1H NMR spectra of SPRM6E and TSG4F2SPSE indicate sharp and intense signals at 3.67 ppm, which have been assigned to OCH3 group of FAMEs or biodiesel (BD). The 13C NMR analyses have also confirmed the presence of FAMES in these oils. The FAMEs could either be present due to naturally occurring or might have been formed by the reaction of CH3OH with the glycerides present in the algal oils during ultrasonic extraction of lipids from biomasses [30]. It has been proved that the algal oil under studies contained both naturally occurring biodiesel as well as biodiesel formed during ultrasonic extraction in a similar way to those formed by transesterification process. The work is in progress to further confirm these observations.
Effect of shape of cultivation system (container) on composition of lipids
In order to study the effect of shape of container on the cultivation process and lipid composition including fatty acid profiles, the algae biomasses were generated in the containers with different shapes, P1(tubular) and P2(round), under identical media composition as used in the cultivation of biomasses TSG1F2 and TSG4F2SPS.The ClMe extracts or algal oils of these biomasses were designated as TSG1F2P1E and TSG4F2SPSP2E. The 1H NMR spectral features of algal oils of these biomasses as shown in the Figure 3, show variation in the content of TG (signals 8, 9, 10) and fatty acid composition; particularly DHA (signal 4), C18:N=1-3 (signals 1, 2, 3, 5, 6 7) and epoxy esters (signals EE) compared to those appeared in the spectra of TSG1F2E and TSG4F2SPSE cultivated in other containers. Since signals due to epoxy esters (EE) and TG are quite intense, this indicates their presence at quite high concentration compared to other oils investigated in the present studies.
13C NMR analyses of algal oils
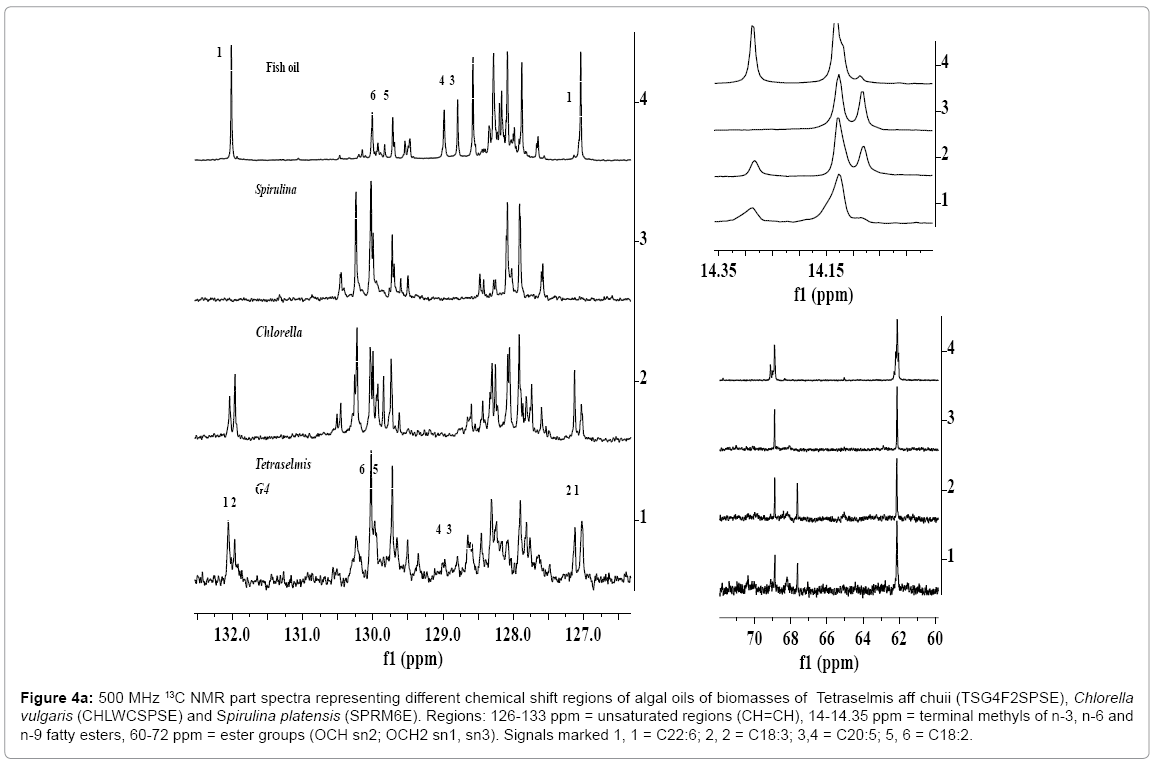
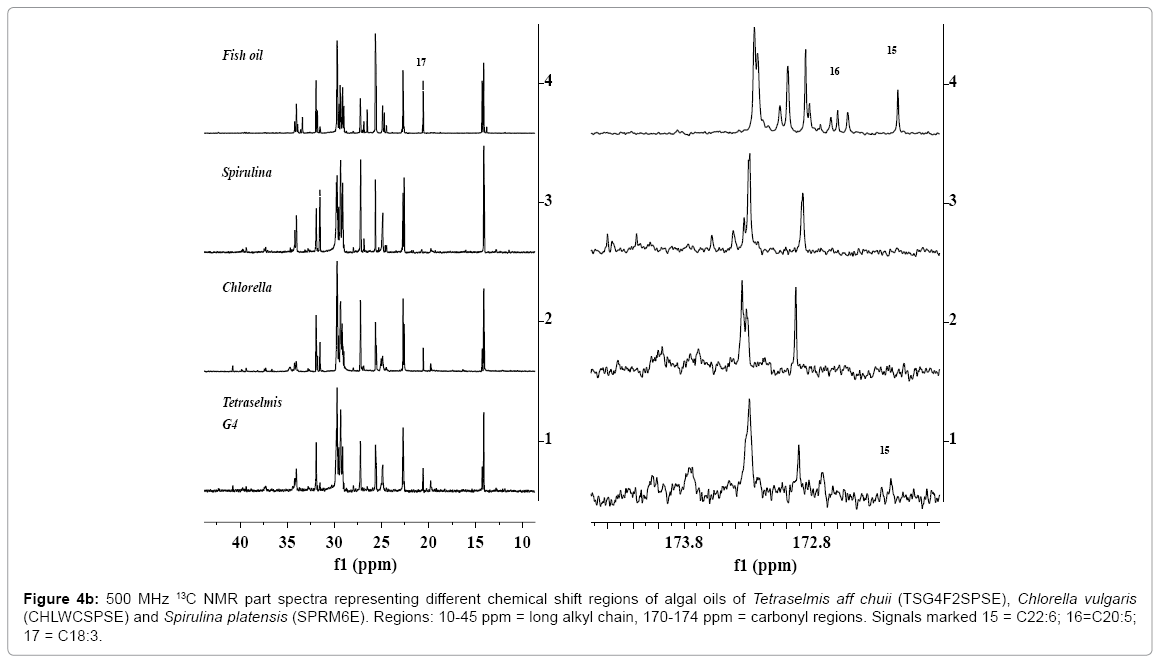
As discussed in the preceding section, 1H NMR spectral analyses of algal extracts provide detailed information about the presence of neutral and polar lipids and types of saturated and unsaturated fatty acid components, particularly identification of DHA and α or ?-linolenic components. The 13C NMR analyses were carried out to divulge more authentic information about the presence of unsaturated fatty acids/ester and epoxy esters, particularly the presence of α and ?-linolenic, and mono, di and tri glycerides. The 13C NMR spectra of vegetable and algal oils offer several advantages relative to 1H NMR spectra due to larger chemical shift range of 20 times higher, which display well resolved, distinct and sharp signals. This enables to determine oil profiling and their fatty acid composition more precisely, provided quantitative spectra are recoded [16,56]. The 13C NMR spectra of algal oils, CHLWCSPSE, TSG4F2SPSE and SPRM6E as shown in the expanded views of five chemical shift regions in Figures 4a and 4b, show well resolved and sharp signals characteristic of triglycerides and their corresponding fatty acid chain. The spectrum of fish oil is given for comparison with the spectral features of algal oils. The spectra display carbonyl group of saturated and unsaturated fatty esters between 172- 174.6 ppm (Figure 4a); unsaturated carbons (CH=CH) of C18:N (N=1- 3) and higher carbon numbers between 126-132 ppm (Figure 4b); OCH2 (sn-1, 3) and OCH (sn-2) of TG at 62.05 and 68.87 ppm (Figure 4b); CH2C=O at 33.8-34.0 ppm, and long alkyl saturated and unsaturated fatty chain at 31.93 ppm (?- CH2), 22.7 ppm (β-CH2), 14.1-14.3 ppm (α-CH3), and 29-30 ppm (CH2)n and 23-27 ppm (CH2s) (Figure 4b). The spectra show clearly and well resolved carbonyl signal due of DHA at 172.20 ppm (sn2) (Figure 4b, signal 15). The presence of PUFAs was also confirmed from the appearance of characteristics signals at 127.12 ppm and 132.06 due to C18:3, and 127.02 ppm and 132.0 ppm due to C22:6. The carbonyls signals due to FFA were clearly visible at 178-179 ppm (not shown) in the spectrum of CHLWCSPSE, but practically found absent in the other oils. The spectral features of algal oils were compared with the spectral features of fish oil containing 12.29 % of C22:6, 19.1 % of 20:5 and 0.71% of C18:3 (Table 2). The spectra of TSG4F2SPS showed weak intensity signals (3, 4 and 16) of features similar to C20:5 of fish oil (128.0, 129.0 ppm) (signals 3, 4) (Figure 4a). However, its presence was ruled out by 2D NMR analyses, and fatty acid profile by GC-MS (Table 2) [56]. The signals at 40.93 ppm, 67.60 ppm and 169.17 ppm were assigned to epoxy esters of higher carbon number unsaturated fatty acid/esters as discussed in details in the 1H NMR and 2D NMR spectral analyses of algals [56].
Figure 4a: 500 MHz 13C NMR part spectra representing different chemical shift regions of algal oils of biomasses of Tetraselmis aff chuii (TSG4F2SPSE), Chlorella vulgaris (CHLWCSPSE) and Spirulina platensis (SPRM6E). Regions: 126-133 ppm = unsaturated regions (CH=CH), 14-14.35 ppm = terminal methyls of n-3, n-6 and n-9 fatty esters, 60-72 ppm = ester groups (OCH sn2; OCH2 sn1, sn3). Signals marked 1, 1 = C22:6; 2, 2 = C18:3; 3,4 = C20:5; 5, 6 = C18:2.
Figure 4b: 500 MHz 13C NMR part spectra representing different chemical shift regions of algal oils of Tetraselmis aff chuii (TSG4F2SPSE), Chlorella vulgaris (CHLWCSPSE) and Spirulina platensis (SPRM6E). Regions: 10-45 ppm = long alkyl chain, 170-174 ppm = carbonyl regions. Signals marked 15 = C22:6; 16=C20:5; 17 = C18:3.
The signals of very weak intensity between the intense signals, particularly between 60-72 ppm, are due to CH2O, CH2OH, CHO and CHOH groups of glyceroglyco lipids and may also be due to mono and diglycerides; which were also indicated by the 1H NMR spectral analyses of the algal oils These types of signals were seen more prominently in the spectra of SPRM6E, which have also showed the presence of mono and diglycerides, and polar group functionality as discussed in details in the succeeding section (Figures 5 and 6).
The expanded views of the region 13-15 ppm (terminal methyls) as indicated in the 13C NMR spectra of different algal oils cultivated under different media show variation in the intensities corresponding to signals at 14.09 ppm (C18:2), 14.13 ppm (C18:1 + CS:0) and 14.29 ppm (PUFAs, C18:3 + C22:6) (Figure 4a). The spectra of fish oil and algal oil TSG4F2SPSE indicate very low intensity signals due to C18:2 compared to signals of CHLWCSPSE and SPRM6E. This reveals very low concentration of C18:2 in these oils, which were also, supported by 1H NMR and GC-MS analyses of their corresponding FAMEs (Table 2). Since the spectra of SPRM6E did not show signals at 14.29, 20.56, 127.12, 127.02, 132.06 and 132.0 ppm, this indicated the absence of ω n-3 C18:3 and C22:6. However, the spectra of SPRM6E showed the presence of ω n-6 18:3 (?- linolenic acid) as discussed in details in the succeeding section.
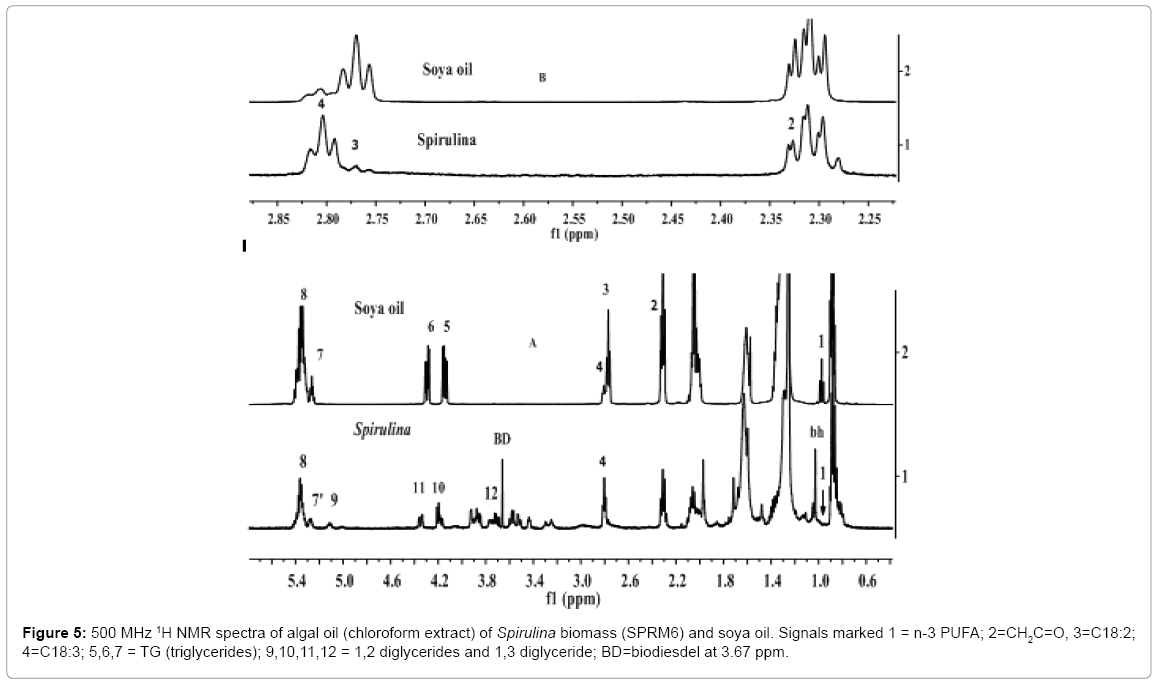
Confirmation of ?- linolenic acid and mono, di glycerides in microalgae Spirulina platensis by 1H and 13C NMR analyses
As discussed in the preceding section, the spectrum of SPRM6E showed the absence of TG and α-linolenic acid (n-3) as the characteristic signals at 4.28 ppm and 4.14 ppm (symmetrical multiplet of OCH2: sn1, sn3) due to TG, and 0.98 ppm (n-3 CH3) due to α-linolenic acid / ester were found to be completely absent. However, the signals characteristic of ?- linolenic (n-6), and mono and di glycerides have been found clearly at 2.81 ppm, and 4.2 ppm and 4.33 ppm respectively. In order to confirm the presence of these components, the algae biomass was extracted with chloroform to pre concentrate their quantity in the sample. The 1H NMR spectral features of the chloroform extract was compared with those of soya oil as given in Figure 5. Soya oil is comprised of n-3 α-C18:3 (7.4% w/w) and other saturated and unsaturated fatty acid as determined by GC-MS analyses (Table 2). The signals marked 1 and 4 in the spectrum of soya oil are due to hydrogens of n-3 CH3 (0.98 ppm) and bis allylic (-CH=CH-CH2)3 (2.81 ppm) functional groups, respectively of C18:3 fatty acid chain. The absence of signal due to n-3 CH3 (marked 1with arrow) in the spectrum of algal oil of Spirulina confirmed the absence of n-3 C18:3. However, the appearance of signal at 2.81 ppm due to bis allylic (-CH=CH-CH2)3 as marked in the spectrum clearly shows the presence of n-6 ?-linolenic. The signal marked 3 in both the spectra are due to bis allylic (-CH=CH-CH2)2 hydrogens of C18:2 fatty acid chain. The relative higher intensities of signals 4 due to ?-linolenic acid in the spectra of algal oil compared to signal of α-linolenic acid in the spectra of soya oil as shown in the expanded part of the spectra (Figure 5B) show higher amount in the oil from Spirulina. The presence of ?-linolenic fatty acid in the Spirulina extract was also confirmed by GC-MS analyses (Table 2).
The signals due to mono and di glycerides are more prominent in the 1H NMR spectrum of chloroform extract compared to those present in the ClMe extract of Spirulina (Figures 2 and 5A). The signals specific to different isomers of mono (MG) and di glycerides (DG) were assigned after comparison with the spectral features of standards of mono (1MG, 2 MG), di (1,2 DG; 1,3 DG) and tri glycerides (TG) as discussed by Kumar et al. [58] and Nieva-Echevarría et al. [66]. The signals due to 1, 2 DG are quite prominent and intense (signals 7, 9, 10, 11) compared to 1, 3 DG (signal 10). The characteristic signals due to 2 MG (4.95 ppm) did not appear in the spectrum. The presence of 1MG could not be ascertained due to overlapping with signals of glyceroglyco lipids in the regions of 3-4ppm.
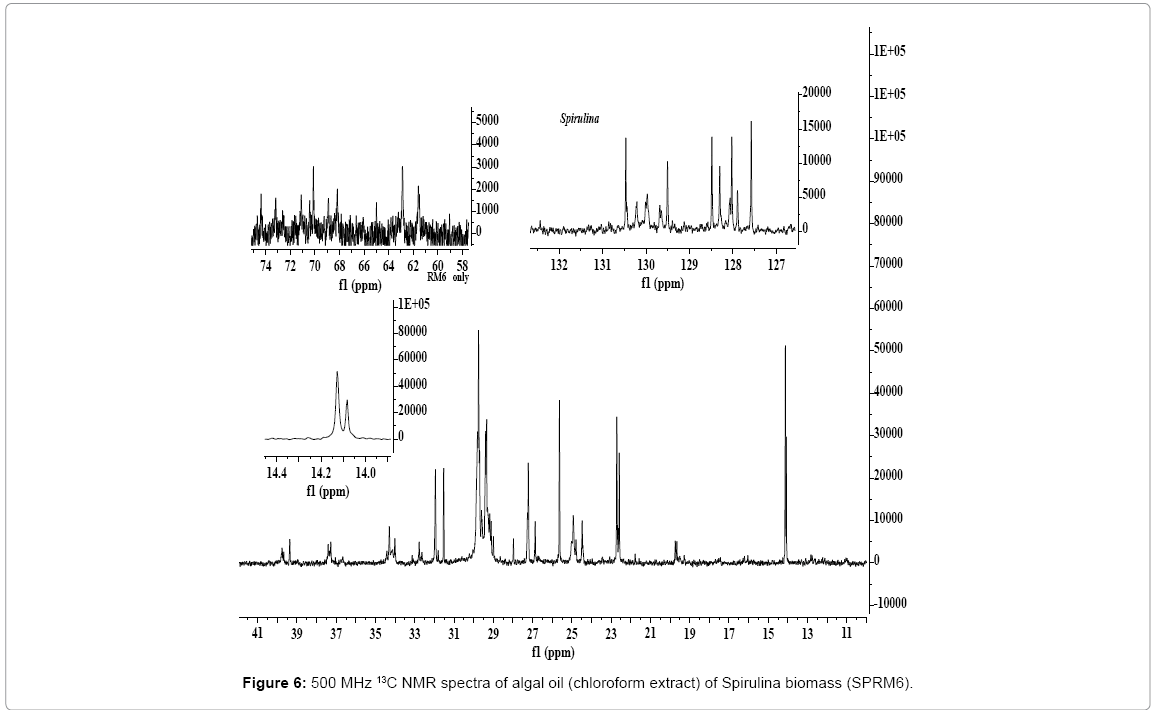
The 13CNMR analysis has also provided conclusive evidence of the presence of ? - linolenic acid, and mono and di glycerides in the chloroform extract of Spirulina. As shown in the Figure 6, large numbers of signals are observed in the chemical shift region of 60-75 ppm due to triacylgycerides (TAG) and polar lipids (PL) as compared to three intense signals at 62.07 ppm and 68.87 ppm due to TG and 67.59 ppm due to epoxy esters appeared in the 13C NMR spectra of Chlorella, Tetraselmis and fish oil (Figures 4a and 4b). The signals due 1, 2-DG at 61.53, 62.8 and 72.05 ppm and signals due to 1,3-DG at 64.89 and 68.16 ppm are clearly visible [58]. The characteristic signals due to TG at 62.07 and 68.87 ppm was not observed. The presence of 1 MG and 2 MG could not be confirmed as signals due to these components might be overlapped with the signals due to polar lipids at 68.88 ppm, 70.11 ppm, 70.39 ppm, 71.0 ppm and 74.3 ppm.
As discussed above, it would be difficult to make clear distinction between α and ?-linolenic components when both are present in a sample. It will be further difficult to assign signals in a complex matrices of algal oils comprising of fatty acid components of both neutral and polar lipids. However, the identities of each can be realized when observed separately as shown in the 1H NMR spectral analyses. The 13C NMR spectral analyses provide unambiguously assignment characteristic of α and ?-linolenic acid or esters [58,63]. The signals characteristic of n-3α isomer were found to appear at 20.56 and 14.29 ppm (C1 carbon) in the spectra of Chlorella and Tetraselmis, but found absent in the spectrum of Spirulina (Figure 4b) [63]. The other characteristic signals due to ?-linolenic acid were observed at 22.56 ppm, 25.65 ppm, 31.53 ppm, 127.56 ppm, 128.0 ppm and 130.42 ppm in comparison to those signals assigned to α-linolenic acid at 14.29 ppm, 20.56 ppm, 22.70 ppm and 31.93 ppm (Figure 6).
IH NMR analyses of FAMEs of algal oils
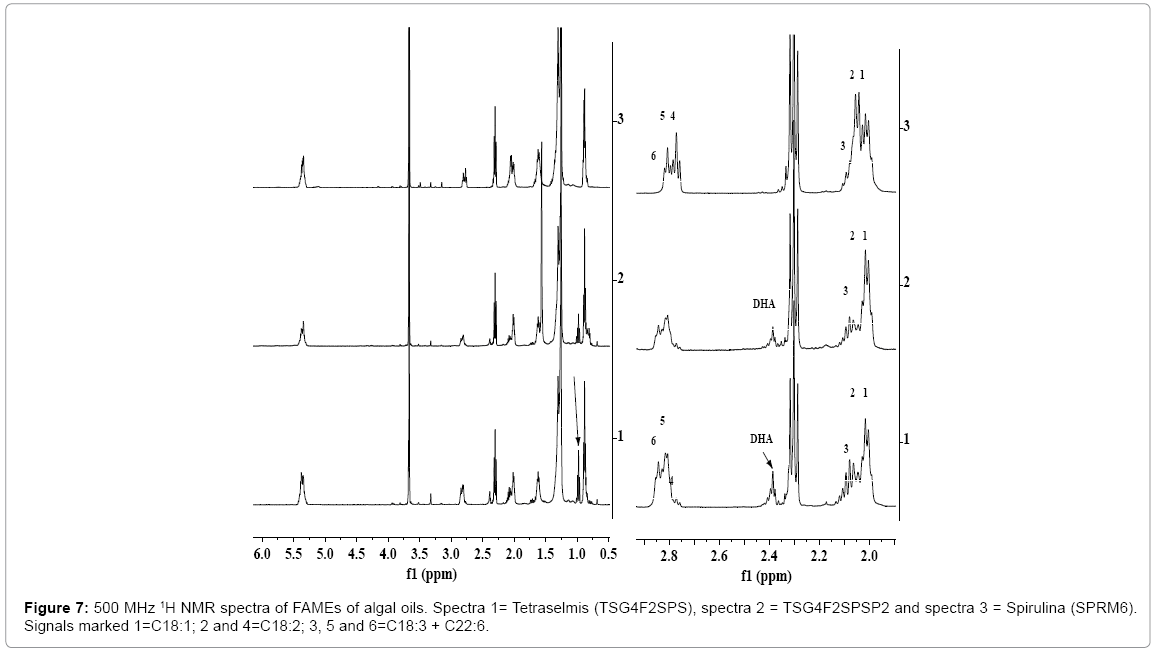
The 1H NMR spectra of FAMEs of algal oils show all the characteristics signals such as ester group (OCH3) at 3.67 ppm and protons of unsaturated (n-3, n-6 and n-9; C18:N=0-3, C22:6 etc.) and saturated fatty esters (C14:0, C16:0, C18:0, etc.) (Figure 7). The nature of fatty acid profile was found to be similar to those observed in the 1H NMR spectra of their respective algal oils, the same was also confirmed by NMR and GC-MS analyses (Table 2). There is a variation observed in the content of each fatty acid in FAMEs compared to those observed in the spectra respective algal oils (Figures 1-3), particularly for PUFAs (C22:6 and C18:3). This has been ascribed to the partial hydrolyses of PUFAs attached to polar fractions (glycerol / phospho lipids) under the applied conditions of trans-esterifications [16,56]. The chemical shift regions corresponding to different fatty esters were assigned and marked in the spectra. The relative intensities of the signals represent their relative amount in the FAMEs.
The 1H and 13C NMR chemical shift regions assigned to different functional groups of respective components of algal oils, which have been used for identification and quantification, are compiled in the Table 3.
| QualityP | TGITG | FFAIFFA | C18:1 | C18:2 | C18:3n-3ILA | C18:3n-6γ | C20:5 | C22:6IDHA | PUFAIPF | UFAIUFA | IVIUS |
| 1HNMRppm | (4. 25-4.34) | 2.32-2.38 | 2.0 | 2.77 | 2.810.98(0.925-1.02) | 2.80-2.81 | 1.68 | 2.39-2.40(2.37-2.42) | 0.98(0.925-1.02) | 1.86-2.18 | 5.05-5.65 |
| 13CNMRppm | 62.07-62.1368.89 | 179-180 | 14.13 | 14.09 | 14.29, 20.56 22.7 31.93 127.12 132.06 |
22.56 25.63 31.53 127.56 |
128.0 128.98 |
14.29 20.60 127.02 132.0 172.25 172.7 |
14.29 |
P = Parameter, TG = Triglycerides, FFA = Free Fatty Acid, PUFA = Polyunsaturated Fatty Acids, UFA = Total Unsaturated Fatty Acids, IV = Iodine Value, the values inthe bracket are the chemical shift regions used for integrated intensities of a particular quality parameter, ITG, IFFA etc. are the integrated intensities of TG, FFA, etc.parameters in the respective chemical shift regions.
Table 3: 1H and 13C NMR characteristic chemical shift regions of different components of algal oils.
Determination of composition and quality parameters of algal oils
The neutral lipids (NL) (TG, FFA, BD) and polar lipids (PL) content, and quality parameters such as n-3/n-6 linolenic (α,γ), PUFA and iodine values of algal oils were determined by methods based on 1H NMR techniques. The methods for derivation of these equations used for determination of parameters have been described in our previous published work [16,55-58]. The following equations were used:
TG = 26.06x2?ITG - 0.62 Eq 1
FFA = 23.57xIFFA -7.84 Eq 2
BD(biodiesel )= 12.33?IOCH3 Eq 3
PUFA = 10.75xIPF Eq 4
C18:3(α or γ) = 10.75xILA Eq 5
UFA (O+L+LA+DHA etc.) = 8.52xIUFA + 4.14 Eq 6
DHA(C22:6) = 16.33xIDHA Eq 7
Iodine value(IV) = 15.78xIUS Eq 8
The ITG, IFFA, etc. are the integrated intensities in the chemical shift regions of functional groups corresponding to a particular parameter in the 1H NMR spectra of FAMES or algal oils as explained in the Table 3. The quality parameters of algal oils determined by the applications of the Eq 1-8 are given in the Table 4.
| Sample | TG | TG* | BD* | FFA* | NL* | PL* | TL* | PUF* | DHA* | DHA | LALAa | PUF | UFA | SFA | IV |
| CHLSPS | 33.4 | 8.8 | ND | 4.3 | 13.1 | 13.3 | 26.4 | 4.6 | 1.63 | 6.2 | 11.1 | 17.3 | 61.2 | 38.8 | 104.4 |
| SPRM6 | 33.7 | 2.4 | 4.0 | ND | 6.4 | 5.6 | 12.0 | 2.0 | ND | ND | 16.3a | 16.3a | 42.9 | 57.1 | 78.4 |
| TSG4F2F | 30.6 | 8.1 | 8.1 | ND | 16.2 | 10.3 | 26.5 | 4.3 | 1.48 | 5.6 | 10.5 | 16.1 | 65.4 | 34.6 | 111.2 |
| TSG1F2 | 57.8 | 17.5 | ND | ND | 17.5 | 12.8 | 30.3 | 8.3 | 3.24 | 10.7 | 16.6 | 27.3 | 68.8 | 31.2 | 124.6 |
| TSG1F2P1 | 70.8 | 18.1 | ND | ND | 18.1 | 7.4 | 25.5 | 5.7 | 2.26 | 8.9 | 13.6 | 22.5 | 63.7 | 36.3 | 123.3 |
| TSG4F2FP2 | 61.9 | 16.8 | ND | ND | 16.8 | 10.4 | 27.2 | 5.8 | 2.31 | 8.5 | 13.0 | 21.5 | 69.8 | 30.2 | 130.8 |
| Soya oil | 99.4 | 99.4 | ND | ND | 99.4 | --- | 99.4 | --- | --- | --- | 7.8 | 7.8 | 85.2 | 14.8 | 141.1 |
* = amount present in the biomass, without * = present in the algal oil, TG = Triglycerides, BD = Biodiesel, FFA = Free Fatty Acid, NL = Neutral Lipids, PL = Polar Lipids, TL = Total Lipids Content (NL + PL), LA = α-C18:3 (ω n-3), LAa = ɤ-C18:3 (ω n-6), PUF = PUFA = Poly Unsaturated Fatty Acids (DHA + LA), UFA = Unsaturated Fatty Acids, SFA = Saturated Fatty Acids, IV = Iodine Values, F = SPS (source of N and P) = Chillean Salt Peter (S) + Superphosphates (SP), TG in SPRM6 is comprised of mono and di-glycerides. ND = Not Detectable, ---- = zero %
Table 4: Quality parameters (% w/w) of algal oils of microalgae biomasses cultivated by different strains and media by NMR methods.
Discussion
Comparison of composition of algal oils from biomasses and biodiesel potential
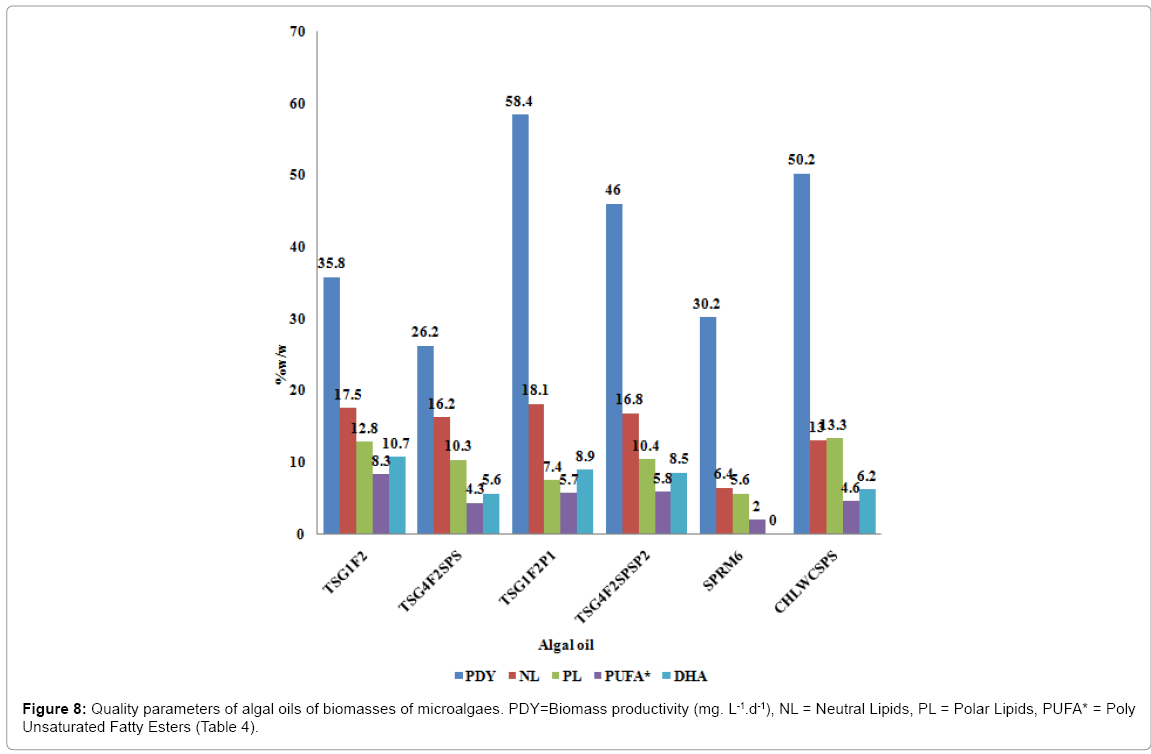
In order to discuss the biodiesel and PUFA potential of different biomasses generated under different culture conditions, media and species, the bar chart graphs depicting the variation in the quality parameters were plotted (Figure 8 and Table 4). The biomass productivities (mg. L-1.d-1) were the most affected by the media, species and types of cultivation system (container) used for cultivation. The highest biomass productivity of 58.4 mg.L-1.d-1 (TSG1F2P1) and 46.0 mg. L-1.d-1 (TSG4F2SPSP2) were archived by cultivation in containers with different shapes (P1 and P2) by microalgae Tetraselmis affchuii compared to corresponding TSG1F2 (35.8 mg. L-1.d-1) and TSG4F2SPS (26.2 mg. L-1.d-1 ), and other biomasses cultivated in the Erlenmeyer flasks under identical conditions. The extracted algal oil i.e. the total lipids (TL), is also the highest in case of TSG1F2P1 (30.3% w/w) compared to other algae biomasses found in the range of 25.5-27.2 5% w/w. However in case of SPRM6E, the TL were the lowest (12.0% w/w). The most important parameters associated with the biodiesel potential is the amount and quality of generated neutral lipids (NL), notwithstanding the biomass productivity or TL achieved in a cultivation process. The importance of these parameters has already been discussed previously [16].The highest amount of NL (16.2-18.1% w/w) were generated by biomasses from microalgae Tetraselmis affchuii (TSG1F2,TSG1F2P1 ,TSG4F2SPS,TSG4F2SPSP2) compared to biomasses from Chlorella vulgaris (CHLWCSPS) (13.1% w/w) and Spirulina platensis (SPRM6) (6.4% w/w). It is important to note that the replacement of source of N and P in F/2 media by cheaper source of fertilizers (Superphosphate and Chillean salt peter) (SPS) has produced equivalent results in terms of TL and NL (TSG1F2,TSG4F2SPS) (Table 4). However, there is 50% reduction in the PUFAs* (*means in the biomass) content in case of TSG4F2SPS (4.3% w/w) compared to TSG1F2 (8.35% w/w). Thus, biomass TSG4F2SPS would be quite suitable for the production of biodiesel with enhanced oxidation stability. The SPRM6 biomass has produced the lowest amount of NL (6.4% w/w) besides the comparable biomass productivity of 30.2 mg. L-1.d-1. Thus, optimization of all cultivation parameters, media, species and types of cultivation systems, are important for generation of higher amount of NL suitable for biodiesel production.
The PUFAs (3 and more than 3 double bonds) contents of algal oils were also found to be dependent on the types of media and species employed for the cultivation of biomass. The PUFAs content was determined in the range of 16.1-27.3 % w/w depending upon the media and species involved in the cultivation (Table 4). This was also reflected by the higher range of iodine values determined in the range of 104.4-130.8 g I2 / 100 g. The highest amount of PUFAs of 8.3% w/w is produced by TSG1F2, which was cultivated without SPS as source of N and P, compared to other biomasses in the range of 2.0 to5.8% w/w. The lowest amount of 2.0% w/w (γ-C18:3) was generated in biomass SPRM6 cultivated in the modified RM6 media. The amount of DHA produced is also the highest in TSG1F2 (3.2% w/w) compared to other biomasses. The saturated fatty acid esters (SFA) is in the range of 30.2 to 57.1% w/w compared to regular crops oils from sunflower (12.99% w/w) and soya (14.77% w/w) oils. Thus, biomasses generated in the present investigated cultivation system are promising feed stocks for both biodiesel and PUFA potentials.
Composition and calculated cetane number of FAMEs of algal oils
The quality of FAMEs or biodiesel from algal oils is directly related to the nature of saturated and unsaturated fatty acids including PUFA generated by a microalgae cultivation process [14,16,68]. The biodiesel produced by transesterification of biomasses directly or from algal oils has to meet the specification laid down in ASTM D6751 and EN14214, which critically restrict the level of C18:3 (max 12%), PUFAs (= or < less than 1%) and iodine value (max 120 g of I2/100 g) in order to use as a fuel in the combustion engines. As shown in the Table 2, the fatty acid profile determined by GC-MS is composed of saturated and unsaturated fatty acids in the range of C12-C22:6, prominent being C16:0 (26.12- 39.29 5% w/w), C16:1 (2.16-9.05% w/w), C18:1 (19.08-39.58% w/w), C18:2 (3.93-14.71% w/w), C18:3 (4.2-22.8% w/w) and C22:6 (1.74-7.1% w/w). The γ-C18:3 have been confirmed in SPRM6E by GC-MS as well as by NMR analyses. Generally, these types of fatty acids are found in FAMEs of algal oils [6,9,13-17,36,37,42,62,68]. The cetane numbers of FAMEs of algal, soya, sunflower and fish oils were calculated based on the standard or published cetane numbers of individual FAMEs (Table 2) [68-70]. Results presented in the Table 2 show calculated cetane numbers in the range of 48.07-57.62 compared to vegetable oils in the range of 44.89-53.18. The highest value of cetane number of 57.62 of SPRM6 is due to the higher amount of saturated fatty acids esters (47.3% w/w) and absence of C22:6. Though the FAMEs under studies meet the limits of iodine values and cetane numbers specified in the specification ASTM D6571 and EN 14214, the presence of PUFAs (= or > 4 double bonds) and C18:3 content beyond the limit makes unsuitable to be used as biodiesel. The PUFAs including C22:6 are extracted by SFC methods such as supercritical CO2 extraction to limit the PUFAs content in algal fuels [24,28,49,52,53]. PUFAs including γ-linolenic acids are used as food supplements in various formulations as a replacement of fish oil products [71-73]. Other practical options used are the deoxygenation and catalytic conversion to remove PUFAs and produced biodiesel with improved properties [64,65].
Analytical strategies integrated with environmental pollution control and co-product development - a cost effective options for biodiesel production.
The biodiesel production from microalgae is not cost effective due to energy and infrastructure intensive steps involved in the cultivation, harvesting and oil extraction processes. The commercialization of algae biofuels is also dependent on the economics of the process [6,9,42,45]. Higher cost of biodiesel production from microalgae can be more environmentally sustainable, cost-effective, and profitable if combined with processes such as wastewater and flue gas treatment, and pollution control including biological fixation of GHG (CO2 and NOx) as advocated by consolidated studies by Slade and Bauen [45] and Zhang et al. [3]. The cost can further be reduced by successful development and commercialization of high-value co-products, polymers or pigments, proteins, food supplements EPA/DHA rich oils, and other useful nutraceuticals. Dual use of Chlorella, Scenedesmus and B. braunii species have been conducted for remediation or nutrient removal of N and P from waste water as well as production of lipids for biodiesel production. Thus biodiesel production coupled with these potentials of microalgae is an attractive option in terms of reducing the overall energy requirement and making biodiesel commercialization a cost effective sustainable sources of biofuels.
Analytical strategies based on NMR developed methods are direct, rapid, cost effective, requires minimum quantity of sample (5 mg of algal oil), and involves concept of green chemistry as analyses is carried out without subjecting to transesterification of microalgae biomasses as discussed in the preceding sections and also previously [16]. The detailed composition of neutral and polar lipids including fatty acid profiles and PUFAs can be obtained from a single 1H NMR spectral analyses. The newly developed NMR methods can also be standardized for serial analyses of number of lipid extracts from large number of algae biomasses by the use of auto sampler in the same way as practiced by GC-FID or GC-MS methods, thus contributing immensely in reducing the cost of analyses compared to other techniques. The analyses by NMR methods offer sufficient scope and advantages in situations when large number of samples are generated from various cultivationprocesses such as kinetic studies, effect of variation in the composition of nutrient media on the lipid productivities, altering the biosynthesis pathways through genetic modification, selection of species or strains for enhancement in the neutral lipid productivities and quality aspects for meeting biodiesel specification or achieving objectives for enhancement of yield of n-3 PUFAs products. The developed fast and cost effective analytical strategy based on 1H NMR techniques will facilitate algae cultivators for screening of species and optimization of cultivation parameters to produce choice of product, thus contribute partly in the overall reduction in the cost of production of biodiesel [16].
Conclusion
It is quite evident that NMR spectroscopic techniques are reliable and versatile in unraveling the complex chemistry of algal oils obtained from biomasses cultivated in different media by different microalgae species. The detailed fatty acid composition analyses by NMR and GCMS have indicated that algal oils are suitable feed stocks for biodiesel as well as PUFAs potential. The biomass and neutral lipids productivity, and nature of their corresponding fatty acid profile are both media and species specific. PUFAs in the algal oil are rich in n-3/n-6 α, γ-C18:3 and n-3 C22:6, and thus offer alternative sources of food supplement in place of fish oil. The study showed that proper selection of media, species and cultivation system (container) was important to cultivate biomasses with desired characteristics either suitable for biodiesel or PUFAs potential. This has been amply demonstrated in case of Tetraselmis aff chuii cultivated in modified F/2 medium (modified with cheaper source of N and P; SPS), where biomass with lower amount of DHA was produced. The biomass cultivated in the modified RM6 media by Spirulina platensi is comprised of lower amount of unsaturated fatty esters without DHA (42.9% w/w) (PUFA as ?-linolenic) compared to other biomasses (61.2- 69.8% w/w), thus quite suitable for production of biodiesel with higher oxidation stability and higher cetane number. The shape of cultivation system i.e. container has profound effect on the biomass and neutral lipid productivity, and fatty acid composition, particularly PUFAs (C18:3, C22:6), as proved in case of change of container from Erlenmeyer flasks to P1 and P2 due to enhancement of photosynthetic efficiency. Analytical strategies developed in the present studies, if integrated with the pollution control and development of co-products, can contribute in reduction in the overall cost of production of biodiesel.
Acknowledgement
We sincerely thank National Institute of Metrology, Standardization and Industrial Quality (INMETRO), RJ, Brazil for carrying out this research work which is of national importance to Brazil in view of the development of fuels from sustainable resources, and National Institute of Technology (INT), RJ, Brazil for collaborating with us in our mission for search for sustainable oil resources for biodiesel, specially the technical assistance of Gustavo Melo Lima. We also express our thanks and gratitude to National Council for Scientific and Technological Development (CNPq) for grant of fellowship.
References
- Singh J, Gu S (2010) Commercialization potential of microalgae for biofuels production. Renewable and Sustainable Energy Reviews 14: 2596-2610.
- Marc Veillette, MostafaChamoumi, JosianeNikiema, Nathalie Faucheux, Michèle Heitz (2012) Production of Biodiesel from Microalgae, Advances in Chemical Engineering.
- Zhang X, Rong J, Chen H, He C, Wang Q (2014) Current status and outlook in the application of microalgae in biodiesel production and environmental protection- review. Front Energy Res 32: 1-15.
- Zhou X, Ge H, Xia L, Zhang D, Hu C (2013) Evaluation of oil-producing algae as potential biodiesel feedstock. BioresourTechnol 134: 24-29.
- Chisti Y (2007) Biodiesel from microalgae. Biotechnol Adv 25: 294-306.
- Mata TM, Martins AA, Caetano NS (2010) Microalgae for biodiesel production and other applications: A review. Renewable and Sustainable Energy Reviews 14: 217-232.
- Behera S, Singh R, Arora R, Sharma NK, Shukla M, et al. (2015) Scope of algae as third generation biofuels. Front Bioeng Biotechnol 2: 90.
- Richmond A, Hu Q (2013) Handbook of Microalgal Culture: Applied Phycology and Biotechnology. Wiley & Sons.
- Sheehan J, Dunahay T, Benemann J,Roessler P (1998) A look back at the U.S. Department of Energy’s Aquatic Species Program biodiesel from algae. National Renewable Energy Laboratory (NREL).
- Cardozo KH, Guaratini T, Barros MP, Falcão VR, Tonon AP, et al. (2007) Metabolites from algae with economical impact. CompBiochemPhysiol C Toxicol Pharmacol 146: 60-78.
- Lee HW, Roh SW, Cho K, Kim KN, Cha IT, et al. (2015) Phylogenetic analysis of microalgae based on highly abundant proteins using mass spectrometry. Talanta 132: 630-634.
- Araujo GS, Matos LJBL, Gonçalves LRB, Fernandes FAN, Farias WRL (2011) Bioprospecting for oil producing microalgal strains: Evaluation of oil and biomass production for ten microalgal strains. Bioresource Technology 102: 5248-5250.
- Fakhry EM, Maghraby DMEL (2013) Fatty Acids Composition and Biodiesel Characterization of Dunaliellasalina. Journal of Water Resource and Protection 5: 894-899.
- Nascimento IA, Marques SSI, Cabanelas ITD, Pereira SA, Druzian JI, et al. (2013) Screening microalgae strains for biodiesel production: lipid productivity and estimation of fuel quality based on fatty acids profiles as selective criteria. Bioenerg Res 6: 1-13.
- Lang I, Hodac L, Friedl T, Feussner I (2011) Fatty acid profiles and their distribution patterns in microalgae: a comprehensive analysis of more than 2000 strains from the SAG culture collection. BMC Plant Biol 11: 124.
- Sarpal AS, Teixeira CMLL, Silva PRM, Monteiro TV, Itacolomy J, et al. (2015) NMR techniques for determination of lipid content in microalgal biomass and their use in monitoring the cultivation with biodiesel potential. ApplMicrobiolBiotechnol 100: 2471-2485.
- Al-lwayzy SH, Yusaf T, Al-Juboori, RA (2014) Biofuels from the Fresh Water Microalgae Chlorella vulgaris (FWM-CV) for Diesel Engines. Energies 7: 1829-1851.
- Widjaja A, Chien CC, Ju YH (2019) Study of increasing lipid production from fresh water microalgae Chlorella vulgaris. J Taiwan Inst Chem Eng 40: 13-20.
- Yadavalli R, Rao R, Rao CS (2012) Lipid accumulation studies in Chlorella pyrenoidosa using customized photobioreactor-effect of nitrogen source, light intensity and mode of operation. International Journal of Engineering Research and Applications 2: 2446-2453.
- Lv JM, Cheng LH, Xu XH, Zhang L, Chen HL (2010) Enhanced lipid production of Chlorella vulgaris by adjustment of cultivation conditions. Bioresour Technol 101: 6797-6804.
- Blair MF, Kokabian B, Gude VG (2014) Light and growth medium effect on Chlorella vulgaris biomass production. Journal of environmental chemical engineering 2: 665-674.
- Frumento D, Casazza AA, Arni SA, Converti A (2013) Cultivation of Chlorella vulgaris in tubular photobioreactors: A lipid source for biodiesel production. Biochemical engineering journal 81: 120-125.
- Converti A, Casazza AA, Ortiz EY, Perego P, del Borghi M (2009) Effect of temperature and nitrogen concentration on the growth and lipid content of Nannochloropsisoculata and Chlorella vulgaris for biodiesel production. Chem Eng Process Process Intensif 48: 1146-1151.
- Couto RM, Simões PC, Reis A, Da Silva TL, Martins VH, et al. (2010) Supercritical fluid extraction of lipids from the heterotrophic microalga Crypthecodiniumcohnii. Engineering in Life Sciences 10: 158-164.
- Coêlho AAC, Barros MUC, Bezerra JHC, Silva JWA, Moreira RL, et al. (2013) Growth of the microalgae Tetraselmistetrathele and nitrate depletion in culture medium Guillard f/2 and Conway. Acta Scientiarum Biological Sciences Maringá 35: 163-168.
- Jamaluddin H, MohdZain NA, Idris A (2014) Biodiesel production via lipase catalysedtransesterification of microalgae lipids from Tetraselmis sp. Renewable Energy 68: 1-5.
- Kumaran P, Saifuddin N, Janarthanan S (2014) Potential of Microalgae TetraselmisChuii as Feedstock for Biodiesel Application. International Review of Mechanical Engineering 8:136.
- Winwooda RJ (2013) Recent developments in the commercial production of DHA and EPA rich oils from micro-algae. OCL 20: D604.
- Martins DA, Custódio L, Barreira L, Pereira H, Ben-Hamadou R, et al. (2013) Alternative sources of n-3 long-chain polyunsaturated fatty acids in marine microalgae. Mar Drugs 11: 2259-2281.
- Aurora V, Valencia H, Us-Vázquez RA, Larqué-Saavedra, FA, Barahona-Pérez LF (2012) Naturally occurring fatty acid methyl esters and ethyl esters in the green microalga Chlamydomonasreinhardtii. Annals of Microbiology 62: 865.
- Wu L, Xu L, Hu C (2015) Screening and Characterization of Oleaginous Microalgal Species from Northern Xinjiang. J MicrobiolBiotechnol 25: 910-917.
- El-Shimi HI, Attia NK, El-Sheltawy ST, El-Diwani GI (2013) Biodiesel Production from Spirulina-Platensis Microalgae by In-Situ Transesterification Process. Journal of Sustainable Bioenergy Systems 3: 224-233.
- Griffiths M, Harrison S (2009) Lipid productivity as a key characteristic for choosing algal species for biodiesel production. J Appl Phycol 21: 493-507.
- Singh N, Dhar D (2011) Microalgae as second generation biofuel- A review. Agronomy SustDevelopm 31: 605-629.
- Bishop WM, Zubeck HM (2012) Evaluation of microalgae for use as nutraceuticals and nutritional supplements. J Nutr Food Sci, 2: 147.
- Zhou X, Ge H, Xia L, Zhang D, Hu C (2013) Evaluation of oil-producing algae as potential biodiesel feedstock. Bioresour Technol 134: 24-29.
- Tang S, Qin C, Wang H, Li S, Tian S (2011) Study on supercritical extraction of lipids and enrichment of DHA from oil-rich microalgae. J Supercrit Fluids 57: 44-49.
- Amin NF, Khalafallah MA, Ali MA, Abou-Sdera SA, Matter IA (2013) Effect of some nitrogen sources on growth and lipid of microalgae Chlorella sp. for biodiesel production. Journal of Applied Sciences Research 9: 4845-4855.
- Mata TM, Almeida R, Caetano NS (2013) Effect of the culture nutrients on the biomass and lipid productivities of microalgae Dunaliellatertiolecta. Chemical Engineering Transaction 32: 973-978.
- Sharma KK, Schuhmann H, Schenk PM (2012) High Lipid Induction in Microalgae for Biodiesel Production. Energies 5: 1532-1553.
- Courchesne NM, Parisien A, Wang B, Lan CQ (2009) Enhancement of lipid production using biochemical, genetic and transcription factor engineering approaches. J Biotechnol 141: 31-41.
- Schenk PM, Thomas-Hall SR, Stephens E, Marx U, Mussgnug J, et al. (2008) Second generation biofuels: High-efficiency microalgae for biodiesel production. Bioenergy Res 1: 20-43.
- Borowitzka MA (1999) Commercial production of microalgae: ponds, tanks, tubes and fermenters. Journal of Biotechnology 70: 313-321.
- Carvalho AP, Meireles LA, Malcata FX (2006) Microalgal reactors: a review of enclosed system designs and performances. Biotechnol Prog 22: 1490-1506.
- Slade R, Bauen A (2013) Micro-algae cultivation for biofuels: Cost, energy balance, environmental impacts and future prospects. Biomass and Bioenergy 53: 29 -38.
- Sun A, Davis R, Starbuck M, Ben-Amotz, A Pate R, et al. (2011) Comparative cost analysis of algal oil production for biofuels. Energy 36: 5169-5179.
- Anthony R, Stuart B (2015) Solvent extraction and characterization of neutral lipids in Oocystis sp. Frontiers in Energy Research 64: 1-5.
- Lee JY, Yoo C, Jun SY, Ahn CY, Oh HM (2010) Comparison of several methods for effective lipid extraction from microalgae. BioresourTechnol 101 Suppl 1: S75-77.
- Li Y, Naghdi FG, Garg S, Adarme-Vega TC, Thurecht KJ, et al. (2014) A comparative study: the impact of different lipid extraction methods on current microalgal lipid research. Microbial Cell Factories 13:14.
- Kanda H, Li P, Yoshimura T, Okada S (2013) Wet extraction of hydrocarbons from Botryococcusbraunii by dimethyl ether as compared with dry extraction by hexane. Fuel 105: 535-539.
- Chen M, Liu T, Chen X, Chen L, Zhang W, et al. (2012) Subcritical co-solvents extraction of lipid from wet microalgae pastes of Nannochloropsis sp. Eur J Lipid SciTechnol 114: 205-212.
- Mouahid A, Crampon C, Toudji SAA, Badens E (2013) Supercritical CO2 extraction of neutral lipids from microalgae: experiments and modelling. JSupercrit. Fluids 77: 7-16.
- Martínez J, de Aguiar AC (2014) Extraction of triacylglycerols and fatty acids using Supercritical Fluids – Review. Current Analytical Chemistry 10: 67-77.
- Cravotto G, Boffa L, Mantegna S, Perego P, Avogadro M, et al. (2008) Improved extraction of vegetable oils under high-intensity ultrasound and/or microwaves. Ultrason Sonochem 15: 898-902.
- Sarpal AS, Silva PRM, Pinto RF, Cunha VS, Daroda RJ, et al. (2014) Biodiesel potential of oleaginous yeast biomass by NMR spectroscopic techniques. Energy Fuels 28: 3766-3777.
- Sarpal AS, Teixeira CM, Silva PR, Lima GM, Silva SR, et al. (2015) Determination of lipid content of oleaginous microalgal biomass by NMR spectroscopic and GC-MS techniques. Anal Bioanal Chem 407: 3799-3816.
- Sarpal AS, Silva PRM, Silva SR, Monteiro TV, Itacolomy J, et al. (2015) A Direct Method for the determination of iodine value of biodiesel by Quantitative Nuclear Magnetic Resonance (q1H-NMR) Spectroscopy. Energy Fuels 29: 7956-7968.
- Kumar R, Bansal V, Patel MB, Sarpal AS (2014) Compositional analysis of algal biomass in a Nuclear Magnetic Resonance (NMR) tube. Journal of Algal Biomass Utilization 5: 36-45.
- Han Y, Wen Q, Chen Z, Pengfei L (2011) Review of methods used for microalgal lipid content analysis. Energy Procidia 12: 944-950.
- Laurens LML, Dempster TA, Jones HDT, Wolfrum EJ, Van Wychen S, et al. (2012) Algal biomass constituent analysis: method uncertainties and investigation of the underlying measuring chemistries. Analytical Chemistry 84: 1879-1887.
- Barbano D, Diaz R, Zhang L, Sandrin T, Gerken H, et al. (2015) Rapid Characterization of Microalgae and Microalgae Mixtures Using Matrix-Assisted Laser Desorption Ionization Time-Of-Flight Mass Spectrometry (MALDI-TOF MS). PLOS ONE 1-13.
- Gouk SW, Cheng SW, Hock Ong AS, Chuah CH (2012) Rapid and direct quantitative analysis of positional fatty acids in triacylglycerols using 13C NMR Eur. J Lipid SciTechnol 114: 510-519.
- Gunstone FD (1990) The 13C-NMR spectra of oils containing?-linolenic acid. Chemistry and physics of lipids 56: 201-207.
- Lixiong Li, Edward Coppola, Jeffrey Rine, Miller JL, Walker D (2010) Catalytic Hydrothermal Conversion of Triglycerides to Non-ester Biofuels. Energy Fuels 24: 1305-1315.
- Zhao C, Brück T, Lercher JA (2013) Catalytic deoxygenation of microalgae oil to green hydrocarbons. Green Chemistry 15: 1720-1739.
- Guillard RR, Lorenzen CJ (1972) Yellow-green algae with chlorophyllide. Jr of Phycology 8:10-14.
- Nieva-Echevarría B, Goicoechea E, Manzanos MJ, Guillén MD (2014) A method based on 1H NMR spectral data useful to evaluate the hydrolysis level in complex lipid mixtures. Food Research International 66: 379-387.
- Raoof B, Kaushik BD, Prasanna R (2006) Formulation of a low-cost medium for mass production of Spirulina. Biomass and Bioenergy 30: 537-542.
- Knothe G (2012) Fuel Properties of Highly Polyunsaturated Fatty Acid Methyl Esters. Prediction of Fuel Properties of Algal Biodiesel. Energy Fuels 26: 5265-5273.
- Gopinath A, Puhan S, Nagarajan G (2010) Effect of biodiesel structural configuration on its ignition quality. IJEE 1: 295-306.
- Ramírez-Verduzco LF, Rodríguez-Rodríguez JE, Alicia delRayo Jaramillo-Jacob ADR (2012) Predicting cetane number, kinematic viscosity, density and higher heating value of biodiesel from its fatty acid methyl ester composition. Fuel 91: 102-111.
- Ryckebosch E, Bruneel C, Termote-Verhalle R, Goiris K, Muylaert K, et al. (2014) Nutritional evaluation of microalgae oils rich in omega-3 long chain polyunsaturated fatty acids as an alternative for fish oil. Food Chem 160: 393-400.
- Surette ME, Stull D, Lindemann J (2008) The impact of a medical food containing gammalinolenic and eicosapentaenoic acids on asthma management and the quality of life of adult asthma patients. Curr Med Res Opin 24: 559-567.
Relevant Topics
- Agricultural biotechnology
- Animal biotechnology
- Applied Biotechnology
- Biocatalysis
- Biofabrication
- Biomaterial implants
- Biomaterial-Based Drug Delivery Systems
- Bioprinting of Tissue Constructs
- Biotechnology applications
- Cardiovascular biomaterials
- CRISPR-Cas9 in Biotechnology
- Nano biotechnology
- Smart Biomaterials
- White/industrial biotechnology
Recommended Journals
Article Tools
Article Usage
- Total views: 17658
- [From(publication date):
March-2016 - Jul 12, 2025] - Breakdown by view type
- HTML page views : 16244
- PDF downloads : 1414