Research Article Open Access
Investigating a Model for Acute Ischemic Pain in Humans
| Freiberg FJ1, Abahji T2, Kreth S1, Irnich D1, Kuhlencordt PJ2, Crispin A3, Mussack T4, Hoffmann U2, Thiel M1,5 and Lang PM1* | |
| 1Department of Anaesthesiology, Ludwig Maximilians University, Munich, Germany | |
| 2Department of Angiology, Ludwig Maximilians University, Munich, Germany | |
| 3Department of Biometry, Epidemiology and Medical Informatics, Ludwig Maximilians University, Germany | |
| 4Department of Surgery, Ludwig Maximilians University, Munich, Germany | |
| 5Department of Anaesthesiology and Intensive Care Medicine, University Medical Centre Mannheim, Medical Faculty Mannheim, Ruprecht Karls University Heidelberg, Mannheim, Germany | |
| Corresponding Author : | Philip M. Lang Department of Anaesthesiology Ludwig Maximilians University Marchioninistr. 15, 81377 München, Germany Tel: +49 89 4400 73429 Fax: +49 89 4400 78886 E-mail: philip. lang@med.uni-muenchen.de |
| Received December 19, 2014; Accepted February 10, 2015; Published February 12, 2015 | |
| Citation: Freiberg FJ, Abahji T, Kreth S, Irnich D, Kuhlencordt PJ, et al. (2015) Investigating a Model for Acute Ischemic Pain in Humans. J Pain Relief 4:172. doi: 10.4172/2167-0846.1000172 | |
| Copyright: © 2015 Freiberg JF, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Journal of Pain & Relief
Abstract
Background:
The pathophysiology of acute ischemic pain is not well established. The aim of the present study was to investigate acute ischemic pain in humans with a view to establish a scientific model to perform future studies. We examined whether peripheral nerve damage and acute inflammation occur during short episodes of acute ischemia.
Methods: Eleven patients with unilateral peripheral arterial disease (PAD) performed treadmill exercise until intolerable ischemic pain urged them to stop. Blood samples were taken before, immediately after treadmill exercise and after a period of recovery. S100B-serum was detected via Elecsys® S100B Immunoassay and IL-6 levels determined via COBAS® IL6 Elecsys 2010.
Results: Treadmill exercise led to intolerable exercise-induced ischemic pain (Numeric Rating Scale) 9 ± 0.3 (0- 10, mean ± SEM)) in the PAD affected leg. Reduced ankle/brachial-index (ABI) (0.13 ± 0.05 mean ± SEM) with a mean ankle pressure of 26 ± 9 mmHg, pO2, pH and increased serum lactate indicated that severe ischemia occurred in the PAD affected leg. The inflammation marker IL-6 increased locally and systemically post exercise (n.s.).
Conclusion: We present an effective method to examine acute ischemic pain in humans. By focusing on changes in metabolic parameters in the affected limb, this model could potentially help to evaluate and detect changes during acute ischemic pain and thus contribute to the understanding of the underlying pathophysiology.
| Keywords |
| Acute ischemia; Pain; Exercise; IL-6; S100B; PAD |
| Introduction |
| Atherosclerotic diseases, such as symptomatic peripheral arterial disease (PAD), are major causes of morbidity and mortality worldwide [1]. Atherosclerosis leads to narrowing (stenosis) and occlusion of the vessel potentially leading to reduced perfusion of the PAD affected leg. Intermittent claudication is the clinical presentation of exercise induced acute ischemic pain in PAD patients [2]. The aetiology, diagnosis and interventional and therapeutic options in PAD have been examined extensively [3-12], whereas the pathophysiology of acute ischemic pain in PAD has received considerably less attention. The classification, severity and diagnosis of PAD pain are influenced by the subjective reports of the patient [2]. The acute ischemia and its local impact cause the pain during exercise in PAD patients. A correlation between pain intensity and PAD stage has not been determined yet [2]. As such establishing a relationship between the metabolic processes during acute ischemia and the development of pain could facilitate the diagnosis and therapy of PAD and may help in the therapy of ischemic pain. In particular, a model to examine metabolic changes during acute ischemia directly in the affected limb in order to differentiate local from systemic changes might help to understand the pathophysiology of acute ischemic pain. |
| The pain associated with PAD is thought to result from chronic ischemia and comprises neuropathic and nociceptive pain component [13,14]. Whether peripheral nerve damage actually occurs during exercise induced acute ischemia is unknown. There is currently no diagnostic laboratory test for the detection of peripheral nerve damage. However, the neuroprotein S100B is reported as a biomarker for central nervous system damage, such as traumatic brain damage [15-18]. This study focused on whether S100B is released from peripheral nerves during exercise induced acute ischemic pain [18,19]. |
| An increase in the concentration of markers of systemic inflammation have been described in PAD [20-22]. IL-6 is a marker of acute inflammation and is used widely in clinical practice [23]. Although IL-6 is one of the best studied inflammation markers in PAD, reports of changes in IL-6 during acute ischemia vary considerably [22]. Signorelli et al. and Andreozzi et al. found increased levels of IL-6 after acute ischemia and acute treadmill exercise, whereas Fiotti et al. discovered lowered IL-6 levels after acute ischemia [20,22,24]. Therefore, it is of great interest to look at changes of IL-6 in PAD patients using the investigated model. |
| Materials and Methods |
| Patients |
| Eleven patients over 40 years with unilateral femoral-popliteal PAD, Fontaine grade II (peripheral arterial disease with claudication during exercise) were included in the study and gave their written informed consent one day before the procedure was performed. Patients were scheduled for catheter intervention the following day. Patients with pain at rest or other pain origin, diabetic polyneuropathy or deep vein thrombosis (as well in patient history) were excluded. The study followed the Declaration of Helsinki and was approved by the local ethics committee of the Ludwig-Maximilians-University Munich. Trial registration: Clinicaltrials.gov NCT02192242. Registered July 15th, 2014. |
| Study design |
| Intravenous catheters were placed into the cubital vein and the right and left femoral vein after local anaesthesia for the entire study. Blood samples were taken at baseline, immediately after exercise and after recovery from the PAD affected leg, the non-affected leg and the arm which served as a systemic control. Systemic blood pressure and the ankle-brachial index (ABI) were measured simultaneously during the blood sampling. Systolic blood pressure was determined using the Doppler method (logidop, Fa. Elcat, Wolfratshausen, Germany). |
| Following venous access, baseline blood sample and clinical examination, patients performed treadmill exercise (Fa. Woodway, Serie PPS 43med, Weil am Rhein, Germany) at a speed of 3.2 km/h up an incline slope of 12%. The time and distance until the first appearance of pain and time and distance until stopping the exercise because of maximum pain in the PAD affected leg were measured. Immediately after termination of the exercise and after recovery (20 minutes later) the above mentioned parameters were obtained again. The patients rated their pain at each point (baseline, immediately after exercise and after recovery) on a numerical rating scale with 0 indicating the absence of pain, while 10 represented the worst pain imaginable. Following the study completion, patients were monitored on the ward. |
| Blood gas analysis (BGA) |
| BGA was analysed via an automatic blood gas analysis tool ABL 700 (Brønshøj, Dänemark). The pO2, pCO2, pH, venous oxygen saturation, base excess, HCO3 and lactate were analysed in venous blood. |
| Ankle-brachial index (ABI) |
| ABI was used to document the presence of PAD, defined by an ABI ≤0.90. The ABI was measured via conventional Doppler method and calculated as the highest detectable systolic Doppler measurement of the anterior and posterior tibial artery and the highest systolic pressure in the arms. |
| Analysis of S100B |
| S-100B-serum levels were detected via Elecsys® S-100B Immunoassay (Roche Diagnostics, Penzberg Deutschland). The total duration of the assay was 18 minutes at 37°C. The test works via a sandwich principle. In the first incubation step the antigen in the sample formed a sandwich complex with a biotinylated monoclonal S100-specific antibody and a monoclonal S100-specific antibody labeled with a ruthenium complex. In the second step streptavidin-coated microparticles were added and the complex bound to the solid phase via interaction of biotin and streptavidin. The electrochemiluminescence measuring cell was constructed as a flow chamber and had three main tasks: |
| 1. The separation of unbound and bound substances. The streptavidincoated microparticles loaded with immune complexes were drawn to the surface of the electrode with the help of a magnet and held there temporarily. Unbound reagent components and excess sample material were then removed from the measuring cell with ProCell system buffer. |
| 2. The Generation of electrochemiluminescence. Application of a defined voltage induces the electrochemiluminescent reaction and the resulting light emission was measured directly by the photomultiplier. |
| 3. At the end of the electrochemiluminescent reaction the microparticles were removed with the measuring cell cleaning solution. The measuring cell was ready for the next measurement. |
| 4. The electrochemiluminescent label was a ruthenium complex. A photomultiplier measured the signal intensity in Relative Light Units (RLU) [25]. |
| Analysis of IL-6 |
| IL-6 levels were detected via COBAS® IL6 Elecsys 2010 (GNR 511, 512) (Roche Diagnostics Mannheim, Deutschland). The method employs the sandwich principle described for S100B [26]. |
| Statistical analysis |
| A mixed effect model was performed to determine the significance of changes in IL-6, S100B, BGA and lactate across the time points baseline, directly after exercise and recovery on the affected and nonaffected leg and arm. The significance level was set at p<0.05. Data are given as means ± SEM. |
| Results |
| Basic patient characteristics and risk factors are summarized in Table 1A. |
| Treadmill exercise and pain |
| To induce acute ischemic pain, patients performed treadmill exercise until intolerable pain developed. Treadmill exercise results are summarized in Table 1B. At baseline the mean reported pain was 0. Directly after treadmill exercise, pain was rated at 9.0 ± 0.3. After recovery reported pain was 0.1 ± 0.1. |
| Ischemia |
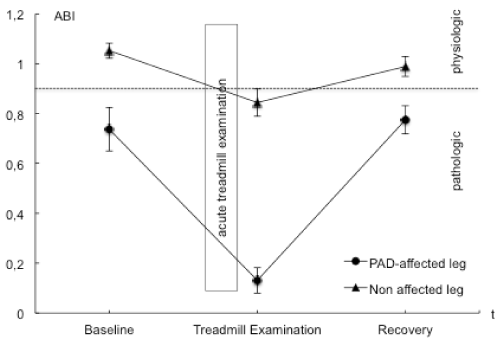
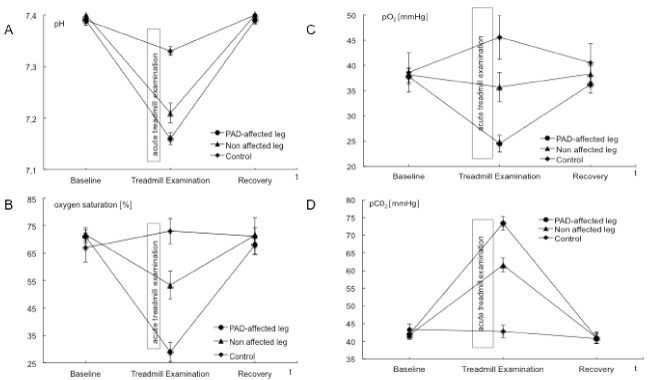
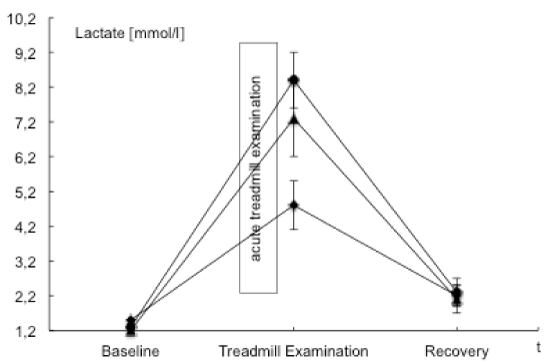
| BGA, ABI values and the post exercise decrease of ankle pressures were evaluated to examine and validate acute ischemia. ABI values in the PAD affected leg were significantly lower than those in the nonaffected leg. A mean perfusion pressure of 26 ± 9 mmHg was measured post exercise in the ankle arteries of the PAD affected leg. Venous BGA values in the PAD affected leg showed typical ischemic changes with an increase in venous pCO2 and base excess and a decrease in pH, HCO3, venous oxygen saturation and pO2. These changes were not observed in the non-affected leg nor in blood samples from the arm that served as the systemic control. The interaction of points of time and measure points showed statistically significant changes in pO2, pCO2, pH, lactate and venous oxygen saturation. Ischemic changes are summarized in Tables 2 and 4 as well as Figures 1-3. |
| Data of BGA and lactate in the PAD affected leg demonstrated acute ischemia after acute treadmill exercise (Table 2 and Figures 1-3). Comparing baseline and acute treadmill exercise, values derived from the BGA showed the greatest changes in the PAD affected leg (Table 2). Lactate, pCO2, pH, HCO3, venous oxygen saturation and pO2 showed statistically significant differences in comparing locations and points of time (Tables 2 and 4). |
| S100B |
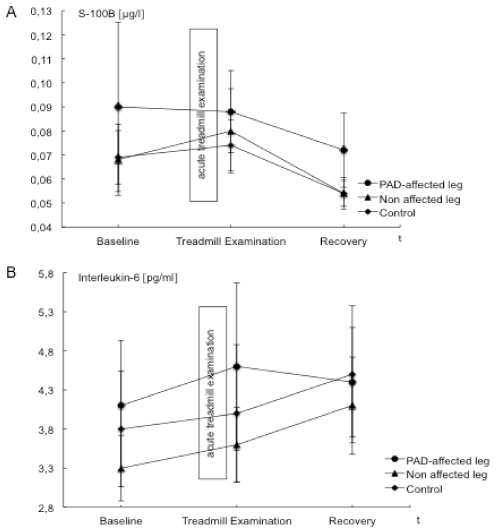
| S100B levels in the PAD affected leg were highest at baseline and decreased at the end of acute treadmill exercise and further after a period of recovery. S100B values determined from the control leg and the systemic control increased minimally after treadmill exercise and decreased during recovery. Data are illustrated in Table 3 and Figure 4. |
| IL-6 |
| The baseline level of IL-6 was higher in the affected leg compared to the control leg. After treadmill exercise although IL-6 levels increased in the affected and control leg as well as for the systemic control, the increase in the affected leg were higher than that in the control leg (Table 3 and Figure 4). |
| Discussion |
| The aim of this study was to establish a model to examine acute ischemic pain in humans. During treadmill exercise, acute ischemia and the associated pain were evident in the PAD affected leg but not in non-affected leg nor in the parameters monitoring the systemic response. As an example of use, we evaluated whether S100B levels and Il-6 levels changed during acute ischemic pain. |
| Model to examine acute ischemia |
| In order to detect local metabolic changes in metabolites during acute ischemia it was of great importance that ischemia developed in the PAD affected leg and not in the non-affected leg or the systemic control. Additionally, these metabolic changes had to be immediately measured. Blood samples were taken simultaneously at all three locations which was achieved by placement of peripheral venous catheters in the cubital vein and the right and left femoral vein. Catheters were installed at study baseline and remained in situ for the entire study. This way allowed immediate blood sampling following acute treadmill exercise. Blood samples from the cubital vein served as systemic controls, while blood samples from the femoral vein in the PAD affected leg reflected acute ischemia. Whereas blood samples from the femoral vein of the non-affected leg served as exercise controls. The outcome measures ABI, BGA and lactate were evaluated at baseline, immediately after treadmill exercise to detect acute ischemia and after recovery. |
| At baseline, ABI values were elevated in the PAD affected leg indicating an arterial stenosis. The control leg presented physiological values. In PAD affected limbs, acute treadmill exercise reduced ABI values as well as absolute perfusion pressures, representing an inadequate blood supply to the poststenotic area and an inadequate perfusion [3]. Even though ABI values in the non-affected leg also decreased to pathological values (0.84 ± 0.05, mean ± SEM) (normal values 0.9-1.3), these changes were minor in comparison to the PAD affected leg (0.13 ± 0.05, mean ± SEM) [3]. The absolute values of ankle pressure were 133 ± 10 mmHg indicating absence of ischemia. Therefore, ischemia with an inadequate poststenotic perfusion, defined as an ankle occlusive pressure below 50 mmHg was documented for the affected leg during acute treadmill exercise, can be assumed. |
| The changes in BGA and lactate are in accordance to earlier described changes during acute ischemia [2,27]. These results support the idea that in the PAD affected leg acute ischemia results in an inadequate perfusion in the poststenotic tissue which represents a deficit in oxygen and substrate supply and a deficient elimination of metabolic products [2]. These findings were less expressed in the nonaffected leg and could not observe in the systemic control. |
| A previous study from Neumann et al. used a similar approach [28]. They investigated 17 patients with PAD, performed venous and arterial cannulation and looked at activation and deformability of neutrophils. Claudication was achieved by consecutive toe stands instead of the treadmill testing in the present study. Ischemia was determined by ABI and lactate concentration, further parameters were not documented. Changes in the arterial system were either less pronounced than those in the venous system (neutrophils) or not affected (lactate). These findings support the determination of metabolic changes in the venous system as a method to document ischemia. In the current study, the ischemic state was more rigorously documented by performing a blood gas analysis to assess changes in pO2 and pH. |
| Ischemic pain |
| Pain was rated subjectively by the patient. In all patients, treadmill exercise was terminated only because of maximum pain development in the PAD affected leg and not because of other reasons i.e. distress or chest pain. In all patients the maximum tolerable pain in the PAD affected leg could be observed during acute treadmill exercise and an ankle occlusion pressure below 50 mmHg was reached. |
| S100B |
| Using S100B levels as an index for peripheral nerve damage, shortterm acute ischemia did not induce nerve damage. Whether S100B can serve as a surrogate marker for peripheral nerve damage remains to be determined. In general, S100B levels<0.100 μg/l are considered physiological, indicating minor probability of cerebrovascular change or central nerve system damage [29]. Previous studies indicated that physiological S100B levels are lower than the commonly used reference value of <0.100 μg/l [29,30]. At baseline only 2 out of 11 patients and after acute treadmill examination only 3 out of 11 patients in our study showed S100B levels above 0.1 μg/l. Hence, the reference value for S100B levels is not sensitive for the detection of changes in the peripheral nervous system with a lower absolute nerve fibre density than the CNS [29,30]. Reference values for S100B after peripheral nerve damage do not exist. In our PAD patients peripheral nerve damage was not detectable using S100B (Table 3 and Figure 4). Additional difficulties with the detection of S100B following acute ischemia may be related to the time of analysis following ischemia. Biberthaler et al. reported increased S100B levels after severe brain injury. In contrary to our study, blood withdrawal was 116 ± 18.8 minutes after trauma [29] and not immediately after the assumed trauma (like in the presented study). Anderson et al. measured elevated S100B levels intraoperatively and immediately postoperatively during coronary artery bypass [29,30] and observed a rapid increase in S100B from damaged tissues. Nevertheless it remains difficult to compare the severity of a coronary artery bypass or severe brain injury to exercise induced acute ischemia possibly affecting peripheral nerves. In the periphery, S100B is not only released by Schwann cells but also from muscle and adipose cells [30,31]. An increase in S100B proteins could result from peripheral nerve damage, but also from damages to other tissues. Further studies to establish S100B reference values for peripheral nerve damage and guidelines for the time of sampling would be beneficial. |
| IL-6 |
| IL-6 levels served as a marker for inflammation. Previous reports of changes in IL-6 during acute ischemia are somewhat varied with some groups reporting increases and others decreased levels of IL-6 after acute ischemia and acute treadmill exercise [20,22,24]. Even though the IL-6 level changes were not significant in this study, an increase after acute treadmill exercise as described by Andreozzi et al. und Signorelli et al. was noted [22,24]. Although the experimental setup in this model was similar, significant changes could not be found in our analysis. Earlier studies looked at patients with more severe PAD, thus it might be possible that IL-6 levels progressively increase with advancing stages of the disease. |
| In summary, exercise in PAD patients led to intolerable exerciseinduced ischemic pain and this was associated with acute local ischemia (changes in ABI, ankle occlusive pressure, pO2, pH and serum lactate) and an increase in S100B was also observed although the absolute S100B level was below that normally used to evaluate nerve damage in the central nervous system. |
| We present an effective model to examine acute ischemia and acute ischemic pain in humans. It offers the opportunity to look at metabolites during acute treadmill exercise and compare the local effect of exercise and acute ischemia against a systemic control. This research might be useful in evaluating different pathophysiological states, such as chronic compartment syndrome or further more. |
References
- Andras A, Ferket B (2014) Screening for peripheral arterial disease.Cochrane Database Syst Rev 4: CD010835.
- Rieger H, Scheffler A (1999) [Pain in chronic peripheral arterial occlusive disease. Pathogenesis and therapy].Internist (Berl) 40: 133-139.
- Abul-Khoudoud O (2006) Diagnosis and risk assessment of lower extremity peripheral arterial disease.J Endovasc Ther 2: II10-18.
- Bhatt DL, Steg PG, Ohman EM, Hirsch AT, Ikeda Yet al. (2006) International prevalence, recognition, and treatment of cardiovascular risk factors in outpatients with atherothrombosis.JAMA 295: 180-189.
- Garcia LA (2006) Epidemiology and pathophysiology of lower extremity peripheral arterial disease.J Endovasc Ther 13 Suppl 2: II3-9.
- Gottsäter A (2006) Managing risk factors for atherosclerosis in critical limb ischaemia.Eur J Vasc Endovasc Surg 32: 478-483.
- Hiatt WR, Goto S, Comerota AJ (2005) Cilostazol and the Management of Vascular Disease. Atherosclerosis supplements 6.
- Jensen SA, Vatten LJ, Myhre HO (2006) The prevalence of chronic critical lower limb ischaemia in a population of 20,000 subjects 40-69 years of age.Eur J Vasc Endovasc Surg 32: 60-65.
- Lumsden AB, Rice TW (2006) Medical management of peripheral arterial disease: a therapeutic algorithm.J Endovasc Ther 13 Suppl 2: II19-29.
- Muntner P, Wildman RP, Reynolds K, Desalvo KB, Chen J, et al. (2005) Relationship between HbA1c level and peripheral arterial disease.Diabetes Care 28: 1981-1987.
- Mussack T1, Klauss V, Ruppert V, Gippner-Steppert C, Biberthaler P, et al. (2006) Rapid measurement of S-100B serum protein levels by Elecsys S100 immunoassay in patients undergoing carotid artery stenting or endarterectomy.Clin Biochem 39: 349-356.
- Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, et al. (2007) Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). J VascSurg 45 Suppl S: S5-67.
- Rüger LJ, Irnich D, Abahji TN, Crispin A, Hoffmann U, et al. (2008) Characteristics of chronic ischemic pain in patients with peripheral arterial disease.Pain 139: 201-208.
- Lang PM, Schober GM, Rolke R, Wagner S, Hilge R, et al. (2006) Sensory neuropathy and signs of central sensitization in patients with peripheral arterial disease.Pain 124: 190-200.
- Beaudeux J, Dequen L, Foglietti M (1999) [Pathophysiologic aspects of S-100beta protein: a new biological marker of brain pathology].Ann Biol Clin (Paris) 57: 261-272.
- Mori T, Tan J, Arendash GW, Koyama N, Nojima Y, et al. (2008) Overexpression of human S100B exacerbates brain damage and periinfarct gliosis after permanent focal ischemia. Stroke 39: 2114-2121.
- Tanaka Y, Koizumi C, Marumo T, Omura T, Yoshida S (2007) Serum S100B is a useful surrogate marker for long-term outcomes in photochemically-induced thrombotic stroke rat models.Life Sci 81: 657-663.
- Tanaka Y, Marumo T, Omura T, Yoshida S (2008) Relationship between cerebrospinal and peripheral S100B levels after focal cerebral ischemia in rats.Neurosci Lett 436: 40-43.
- Mielck F, Ziarkowski A, Hanekop G, Armstrong VW, Hilgers R, et al. (2005) Cerebral inflammatory response during and after cardiac surgery.Eur J Anaesthesiol 22: 347-352.
- Fiotti N, Giansante C, Ponte E, Delbello C, Calabrese S, et al. (1999) Atherosclerosis and inflammation. Patterns of cytokine regulation in patients with peripheral arterial disease.Atherosclerosis 145: 51-60.
- Signorelli SS, Mazzarino MC, Di Pino L, Malaponte G, Porto C, et al. (2003) High circulating levels of cytokines (IL-6 and TNFalpha), adhesion molecules (VCAM-1 and ICAM-1) and selectins in patients with peripheral arterial disease at rest and after a treadmill test.Vasc Med 8: 15-19.
- Signorelli SS, Mazzarino MC, Spandidos DA, Malaponte G (2007) Proinflammatory circulating molecules in peripheral arterial disease.Int J Mol Med 20: 279-286.
- Van Snick J (1990) Interleukin-6: an overview.Annu Rev Immunol 8: 253-278.
- Andreozzi GM, Martini R, Cordova R, D'Eri A, Salmistraro G, et al. (2007) Circulating levels of cytokines (IL-6 and IL-1beta) in patients with intermittent claudication, at rest, after maximal exercise treadmill test and during restore phase. Could they be progression markers of the disease? Int Angiol 26: 245-252.
- Product Information, Elecsys® S100, Elecsys 1010/2010 Systems, Modular Analytics E170, Roche Diagnostics GmbH, Februar 2005.
- Product Information COBAS® IL6 Elecsys 2010 sRoche Diagnostics GmbH, 2012.
- Pipinos II, Judge AR, Selsby JT, Zhen Z, Swanson SA, et al. (2008) Basic science review: the myopathy of peripheral arterial occlusive disease: part 2. Oxidative stress, neuropathy, and shift in muscle fiber type. VascEndovascular Surg 42: 101-112.
- Neumann FJ, Waas W, Diehm C, Weiss T, Haupt HM, et al. (1990) Activation and decreased deformability of neutrophils after intermittent claudication.Circulation 82: 922-929.
- Biberthaler P, Mussack T, Wiedemann E, Kanz KG, Koelsch M, et al. (2001) Evaluation of S-100b as a specific marker for neuronal damage due to minor head trauma.World J Surg 25: 93-97.
- Anderson RE, Hansson LO, Nilsson O, Liska J, Settergren G, et al. (2001) Increase in serum S100A1-B and S100BB during cardiac surgery arises from extracerebral sources.Ann Thorac Surg 71: 1512-1517.
- Pham N, Fazio V, Cucullo L, Teng Q, Biberthaler P, et al. (2010) Extracranial sources of S100B do not affect serum levels.PLoS One 5.
Tables and Figures at a glance
| Table 1 | Table 2 | Table 3 | Table 4 |
Figures at a glance
 |
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 | Figure 4 |
Relevant Topics
- Acupuncture
- Acute Pain
- Analgesics
- Anesthesia
- Arthroscopy
- Chronic Back Pain
- Chronic Pain
- Hypnosis
- Low Back Pain
- Meditation
- Musculoskeletal pain
- Natural Pain Relievers
- Nociceptive Pain
- Opioid
- Orthopedics
- Pain and Mental Health
- Pain killer drugs
- Pain Mechanisms and Pathophysiology
- Pain Medication
- Pain Medicine
- Pain Relief and Traditional Medicine
- Pain Sensation
- Pain Tolerance
- Post-Operative Pain
- Reaction to Pain
Recommended Journals
Article Tools
Article Usage
- Total views: 13515
- [From(publication date):
March-2015 - Apr 04, 2025] - Breakdown by view type
- HTML page views : 9032
- PDF downloads : 4483
