Invasive Lobular Carcinoma of the Breast: The Use of Radiological Appearance to Classify Tumor Subtypes for Better Prediction of Long-Term Outcome
Received: 31-Mar-2014 / Accepted Date: 04-Jul-2014 / Published Date: 06-Jul-2014 DOI: 10.4172/2161-0681.1000179
Abstract
We compared the survival of women diagnosed with invasive lobular carcinoma subtypes prior to and during the mammography screening era to determine the impact of early detection and treatment in an early phase upon survival according to tumor subtypes. Using mammographic tumor features, we classified 428 consecutive invasive lobular carcinoma cases of the breast into four subgroups according to their mammographic tumor features.
Life-table-based survival curves and adjusted survival curves with the accelerated failure time method were plotted to demonstrate the 14-year follow-up survival outcome with adjustment for tumor size, node status, histologic malignancy grade, tumor focality and treatment. Survival of invasive lobular carcinoma subtypes was substantially improved as a result of early detection through mammography screening and treatment in an early phase, except for the subtype presenting as architectural distortion on the mammogram, even with the use of modern therapeutic methods. There is a clear need to further develop diagnostic and management strategies to decrease mortality from the diffusely infiltrating special subtype of invasive lobular carcinoma.
Keywords: Breast neoplasms; Carcinoma; Lobular
314633Introduction
Breast cancer is an extremely heterogeneous disease. There are often several histologic subtypes a) within the same tumor mass (intratumoral heterogeneity), b) among separate tumor foci (intertumoral heterogeneity), c) but also within specific histologic categories, including the many subtypes of in situ carcinoma and four subtypes of invasive lobular carcinoma (ILC). The histologic classification of in situ and invasive breast cancers as ductal, lobular, and other types has been complemented using biomarkers (ER/PR receptors, EGFR, E-cadherin, Ki67 proliferating index, HER-2/neu expression, etc.) and molecular classification such as basal like, luminal A, luminal B subtypes, etc., in order to add prognostic information to the histologic tumor types [1-3].
The mammographic image is a black and white representation of the low power large section histology image of the breast tissue. Correlation of the radiologic tumor features with large section histology and long-term patient outcome has demonstrated that the mammographic appearance of breast cancers is a strong prognostic factor [4-8]. Thus, breast imaging, especially mammography, simplifies the histologic complexity of invasive breast cancers, since different histologic subtypes may have identical mammographic presentation. For example, invasive “ductal carcinomas”, tubular cancers and tubulo-lobular cancers may all present as stellate tumors on the mammogram. Likewise, poorly differentiated invasive “ductal” cancer, medullary cancer, mucinous, invasive micropapillary and papillary breast cancers, invasive cribriform carcinoma, invasive apocrine carcinoma, the solid form of invasive lobular carcinoma, etc. may all have a mammographic presentation of circular/oval-shaped tumors. Architectural distortion on the mammogram can represent either a subtype of invasive lobular carcinoma which is difficult to detect despite its large extent, or a duct forming invasive breast cancer with or without associated microcalcifications. In particular, the prognostic importance of the apparent site of origin of each breast cancer subtype (developing from either the epithelial cells within the ducts themselves or from the epithelial cells lining the acini of the terminal ductal lobular units) could provide the foundation for an improved classification. The important prognostic distinction between the cancers of ductal and acinar origin can be made on the basis of mammographic appearance [5,8].
The heterogeneous nature of invasive lobular carcinoma, including the diverse outcome of the individual subtypes, is well known. Subclassification has been attempted using histological features and, more recently, using immunohistochemistry [9-11]. Neither of these approaches correlates reliably with tumor structure at the subgross level, nor with long-term outcome. For example, a 10x10 mm solitary stellate tumor with excellent, >95% 25-year survival can be described histologically as “classic invasive lobular carcinoma” based on cellular appearance and immunohistochemistry, but the same histologic diagnosis could also be given to a 70 mm diffuse tumor with architectural distortion and with only 60% long-term survival. In this article we present a new subclassification of ILC based on mammographic tumor features which reflects the structural changes in the breast tissue, concurring with the apparent site of tumor origin and long-term patient outcome.
Materials and Methods
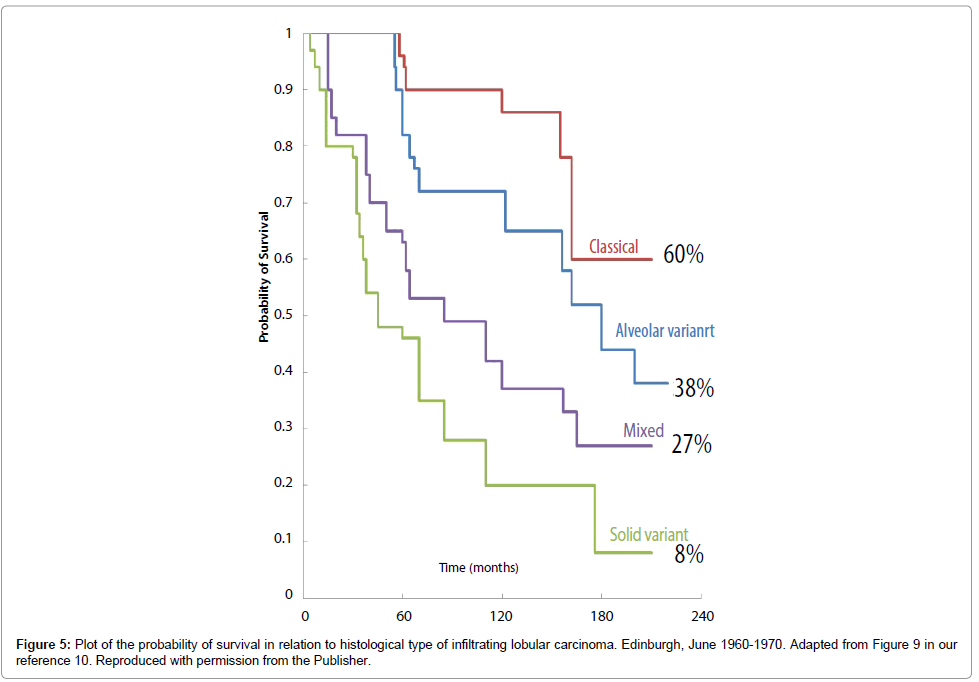
We have collected data, including mammographic presentation, on 428 consecutive cases of invasive lobular carcinoma of the breast, histologically confirmed in women aged 30-92 at the Falun Central Hospital, Sweden during the calendar years from 1996 through 2010. We have compared the outcome of these ILC cases with the outcome of ILC cases diagnosed and treated 25-30 years earlier. In 1982, Dixon et al classified 103 invasive lobular carcinomas, diagnosed in Edinburgh between 1960 and 1970, by histological appearance as classical, solid variant, alveolar variant, or mixed [10]. We classified the invasive lobular carcinomas in our series and made an approximate correlation with this histologic classification [10], which is similar to the comparison between the mammographic patterns of the ILC subtypes and histopathology published by Mendelson et al. [12].
Using mammographic tumor features, we classified the invasive lobular carcinomas in our series and made an approximate correlation with this histologic classification [10]:
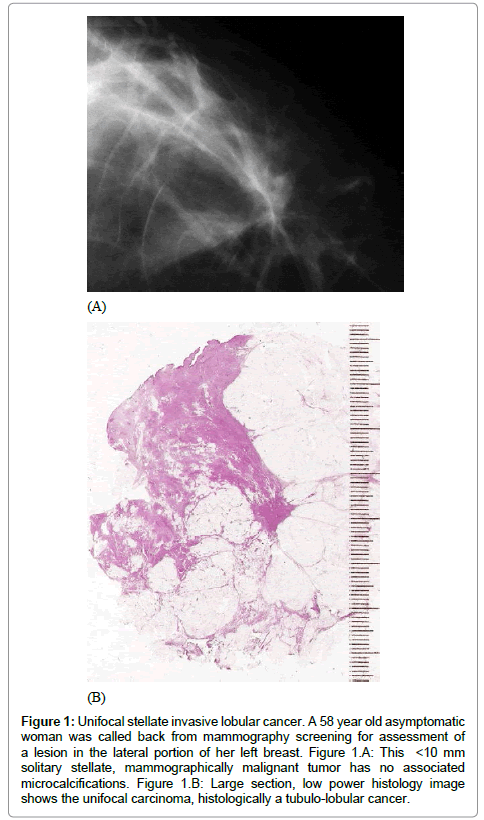
• A solitary or multifocal stellate tumor, corresponding approximately to the mixed pattern of ILC (Figure 1)
Figure 1: Unifocal stellate invasive lobular cancer. A 58 year old asymptomatic woman was called back from mammography screening for assessment of a lesion in the lateral portion of her left breast. Figure 1.A: This <10 mm solitary stellate, mammographically malignant tumor has no associated microcalcifications. Figure 1.B: Large section, low power histology image shows the unifocal carcinoma, histologically a tubulo-lobular cancer.
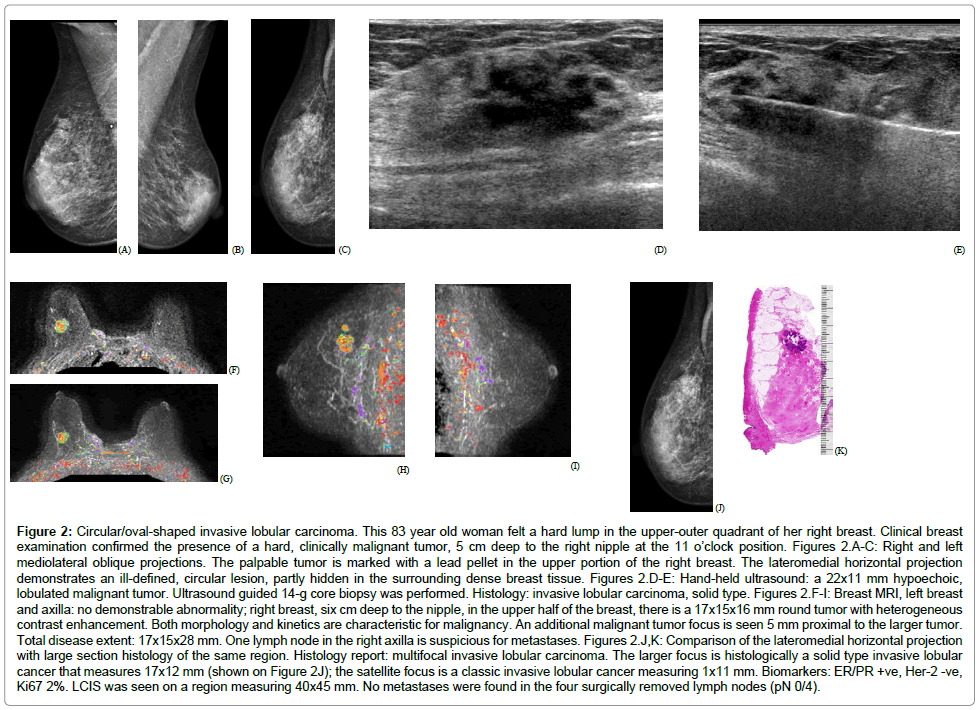
• A circular/oval-shaped tumor mass, corresponding approximately to the solid variant pattern of ILC (Figure 2)
Figure 2: Circular/oval-shaped invasive lobular carcinoma. This 83 year old woman felt a hard lump in the upper-outer quadrant of her right breast. Clinical breast examination confirmed the presence of a hard, clinically malignant tumor, 5 cm deep to the right nipple at the 11 o’clock position. Figures 2.A-C: Right and left mediolateral oblique projections. The palpable tumor is marked with a lead pellet in the upper portion of the right breast. The lateromedial horizontal projection demonstrates an ill-defined, circular lesion, partly hidden in the surrounding dense breast tissue. Figures 2.D-E: Hand-held ultrasound: a 22x11 mm hypoechoic, lobulated malignant tumor. Ultrasound guided 14-g core biopsy was performed. Histology: invasive lobular carcinoma, solid type. Figures 2.F-I: Breast MRI, left breast and axilla: no demonstrable abnormality; right breast, six cm deep to the nipple, in the upper half of the breast, there is a 17x15x16 mm round tumor with heterogeneous contrast enhancement. Both morphology and kinetics are characteristic for malignancy. An additional malignant tumor focus is seen 5 mm proximal to the larger tumor. Total disease extent: 17x15x28 mm. One lymph node in the right axilla is suspicious for metastases. Figures 2.J,K: Comparison of the lateromedial horizontal projection with large section histology of the same region. Histology report: multifocal invasive lobular carcinoma. The larger focus is histologically a solid type invasive lobular cancer that measures 17x12 mm (shown on Figure 2J); the satellite focus is a classic invasive lobular cancer measuring 1x11 mm. Biomarkers: ER/PR +ve, Her-2 -ve, Ki67 2%. LCIS was seen on a region measuring 40x45 mm. No metastases were found in the four surgically removed lymph nodes (pN 0/4).
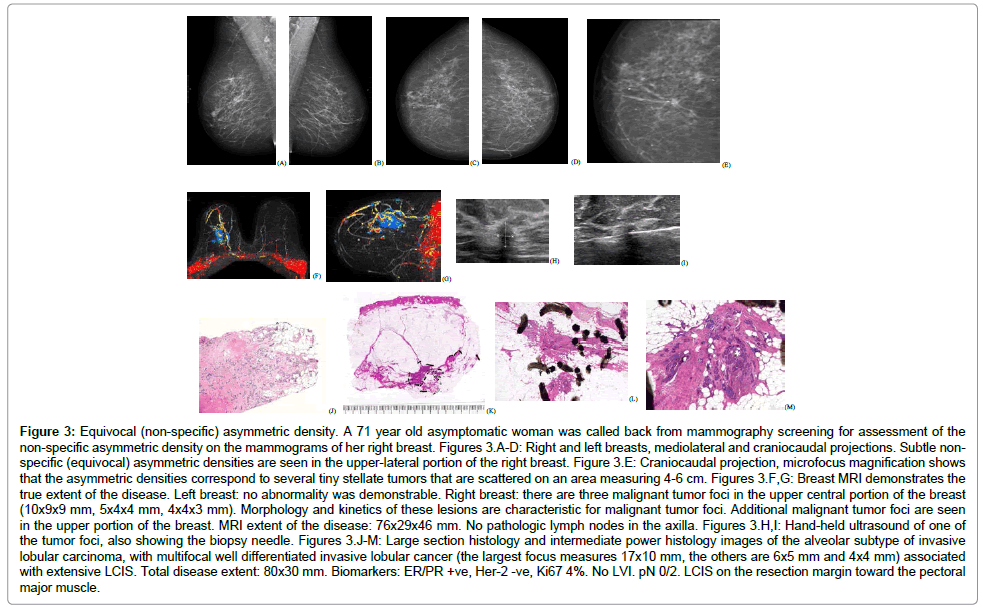
• Architectural distortion on the mammogram, corresponding to the classical subgroup of ILC (Figure 3)
Figure 3: Equivocal (non-specific) asymmetric density. A 71 year old asymptomatic woman was called back from mammography screening for assessment of the non-specific asymmetric density on the mammograms of her right breast. Figures 3.A-D: Right and left breasts, mediolateral and craniocaudal projections. Subtle nonspecific (equivocal) asymmetric densities are seen in the upper-lateral portion of the right breast. Figure 3.E: Craniocaudal projection, microfocus magnification shows that the asymmetric densities correspond to several tiny stellate tumors that are scattered on an area measuring 4-6 cm. Figures 3.F,G: Breast MRI demonstrates the true extent of the disease. Left breast: no abnormality was demonstrable. Right breast: there are three malignant tumor foci in the upper central portion of the breast (10x9x9 mm, 5x4x4 mm, 4x4x3 mm). Morphology and kinetics of these lesions are characteristic for malignant tumor foci. Additional malignant tumor foci are seen in the upper portion of the breast. MRI extent of the disease: 76x29x46 mm. No pathologic lymph nodes in the axilla. Figures 3.H,I: Hand-held ultrasound of one of the tumor foci, also showing the biopsy needle. Figures 3.J-M: Large section histology and intermediate power histology images of the alveolar subtype of invasive lobular carcinoma, with multifocal well differentiated invasive lobular cancer (the largest focus measures 17x10 mm, the others are 6x5 mm and 4x4 mm) associated with extensive LCIS. Total disease extent: 80x30 mm. Biomarkers: ER/PR +ve, Her-2 -ve, Ki67 4%. No LVI. pN 0/2. LCIS on the resection margin toward the pectoral major muscle.
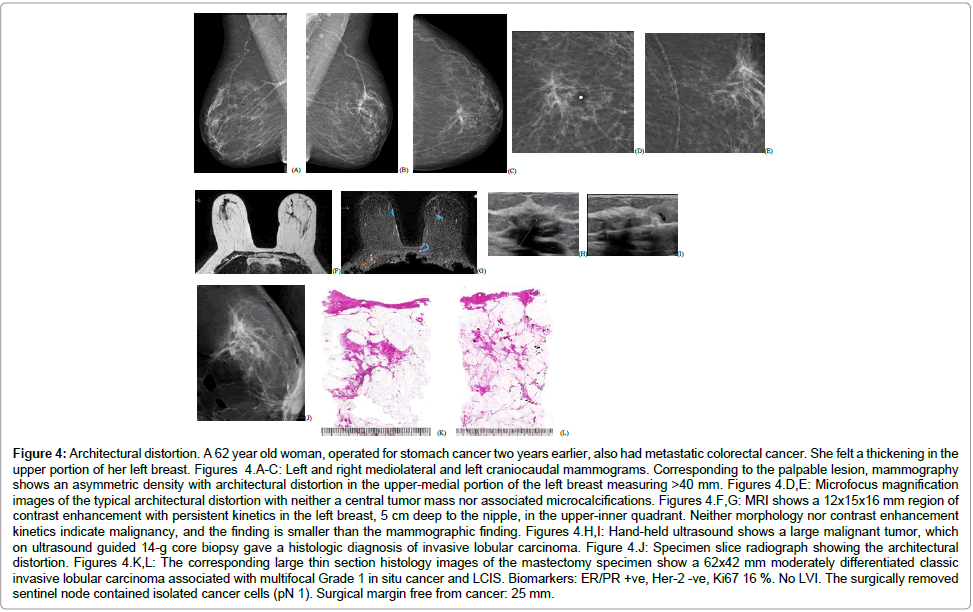
Equivocal asymmetric densities on the mammogram, corresponding to the alveolar subtype of ILC (Figure 4)
Figure 4: Architectural distortion. A 62 year old woman, operated for stomach cancer two years earlier, also had metastatic colorectal cancer. She felt a thickening in the upper portion of her left breast. Figures 4.A-C: Left and right mediolateral and left craniocaudal mammograms. Corresponding to the palpable lesion, mammography shows an asymmetric density with architectural distortion in the upper-medial portion of the left breast measuring >40 mm. Figures 4.D,E: Microfocus magnification images of the typical architectural distortion with neither a central tumor mass nor associated microcalcifications. Figures 4.F,G: MRI shows a 12x15x16 mm region of contrast enhancement with persistent kinetics in the left breast, 5 cm deep to the nipple, in the upper-inner quadrant. Neither morphology nor contrast enhancement kinetics indicate malignancy, and the finding is smaller than the mammographic finding. Figures 4.H,I: Hand-held ultrasound shows a large malignant tumor, which on ultrasound guided 14-g core biopsy gave a histologic diagnosis of invasive lobular carcinoma. Figure 4.J: Specimen slice radiograph showing the architectural distortion. Figures 4.K,L: The corresponding large thin section histology images of the mastectomy specimen show a 62x42 mm moderately differentiated classic invasive lobular carcinoma associated with multifocal Grade 1 in situ cancer and LCIS. Biomarkers: ER/PR +ve, Her-2 -ve, Ki67 16 %. No LVI. The surgically removed sentinel node contained isolated cancer cells (pN 1). Surgical margin free from cancer: 25 mm.
We investigated how these mammographic tumor features related to histological tumor size and to patient survival, adjusted for tumor size. We also compared survival according to the mammographic tumor features with survival according to the histological classification from 1982 (Figure 5), to assess possible improvements in survival of the ILC subtypes in the epoch of early detection and standardized therapy, from 1996 through 2010.
It should be noted that although tumor size had been measured for architecture distortion on the mammogram according to the concept of TNM stage, some analyses for this group were performed treating it as a separate category regardless of the measured tumor size, since the invasive extent was too great to be assessed by the measurement of maximum diameter. In a similar manner, this concept was also applied to invasive lobular carcinoma larger than 3 cm, which is expected to have as poor survival as does architecture distortion.
Breast cancer specific survival curves were calculated using the lifetable method. Crude and adjusted hazard ratios and their 95% CIs by mammographic appearance were estimated using the Cox proportional hazards regression model. We also applied the accelerated failure time model to compute adjusted hazard ratios and adjusted survival curves, making allowance for size, node, grade, focality, and treatment for the classification of mammographic appearance by tumor size smaller than 3 cm and larger than 3 cm.
Results
Classification of the four subtypes of invasive lobular carcinoma of the breast according to their mammographic appearance gave the following distribution: 66% (283) stellate tumors, 10% (44) circular tumors, 12% (52) showed architectural distortion without a tumor mass, and 12% (49) presented as equivocal asymmetric densities on the mammogram. The age distribution was similar for all the ILC subtypes (Table 1).
| Mammographic classification | Number of subjects | Mean (SD) age | Age range |
|---|---|---|---|
| Stellate tumor mass | 283 | 65 (12) | 36-92 |
| Circular tumor mass | 44 | 67 (14) | 42-92 |
| Architectural distortion | 52 | 67 (14) | 30-91 |
| Equivocal asymmetric density | 49 | 65 (12) | 42-92 |
| Total | 428 | 65 (12) | 30-92 |
Table 1: Mammographic classification and age distribution of 428 invasive lobular carcinoma cases, diagnosed 1996-2010, Falun, Sweden.
The relationship of the four ILC subtypes, classified according to mammographic tumor features, to the histologic tumor size showed that cases presenting as architectural distortion on the mammogram were overwhelmingly more likely (p<0.001) to be ≥30 mm than were the remaining three subgroups: 76% for architectural distortion compared with 21% for stellate, 25% for circular and 34% for equivocal asymmetric densities.
Correspondingly, only 6% of the architectural distortion cases were of size 1-14 mm compared to 29-34% for all other types (Table 2). The distribution of the four mammographic tumor features according to detection mode showed that the cases presenting with architectural distortion on the mammogram were found predominantly among the clinically-detected cancers (interval cancer, non-attendees, outside screening) (Table 3). Cancers detected outside of screening include women younger and older than the age limits of organized, populationbased mammography screening.
| Mammographic classification | Tumor size in mm | |||
|---|---|---|---|---|
| 1-14 | 15-29 | 30+ | Total | |
| Stellate tumor mass | 91 (32%) | 132 (47%) | 60 (21%) | 283 |
| Circular tumor mass | 15 (34%) | 18 (41%) | 11 (25%) | 44 |
| Architectural distortion | 3 (6%) | 9 (17%) | 40 (77%) | 52 |
| Equivocal asymmetric density | 14 (29%) | 17 (35%) | 18 (36%) | 49 |
| Total | 123 | 176 | 129 | 428 |
Table 2: Mammographic classification by tumor size in 428 invasive lobular carcinoma cases, diagnosed 1996-2010, Falun, Sweden.
| Mammographic classification | Screen-detected | Interval Cancer | Non-attendees | Outside Screening | Total |
|---|---|---|---|---|---|
| Stellate tumor mass | 143 | 46 | 7 | 84 | 280 |
| 51% | 16% | 3% | 30% | ||
| Circular tumor mass | 19 | 7 | 1 | 17 | 44 |
| 43% | 16% | 2% | 39% | ||
| Architectural distortion | 11 | 9 | 3 | 29 | 52 |
| 21% | 17% | 6% | 56% | ||
| Equivocal asymmetric density | 18 | 9 | 2 | 19 | 48 |
| 38% | 19% | 4% | 40% |
Table 3: Distribution of 424 out of 428 invasive lobular carcinoma cases according to detection mode.
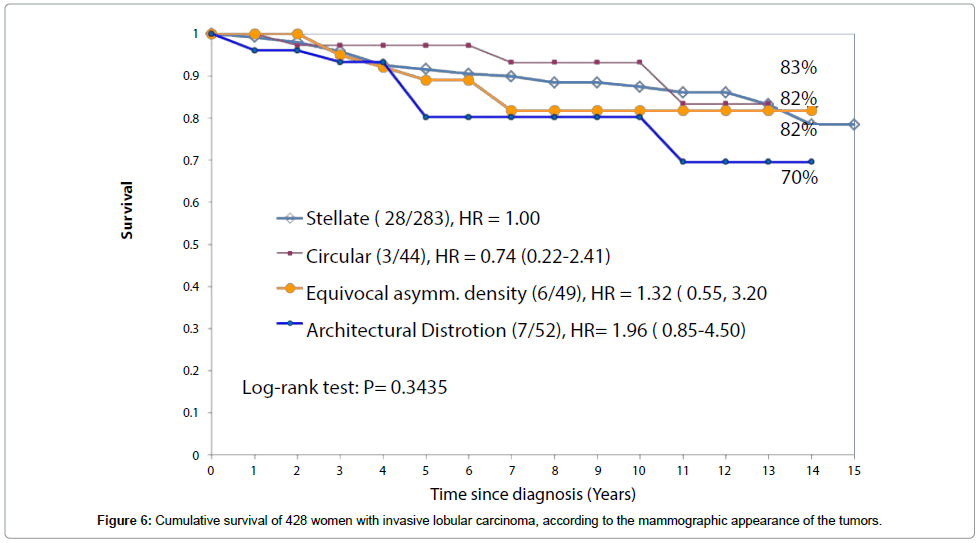
The long-term survival of patients with three of the four subtypes of ILC classified according to their mammographic appearance was considerably better during the mammography screening era (Figure 6) than it was before screening was introduced (Figure 5). At 14 years, the survival was 83% for the stellate ILC, 82% for the circular/oval-shaped ILC and 82% for the equivocal asymmetric densities group. These were all diagnosed during the screening era, irrespective of mode of detection, with all sizes included. However, the survival of patients with the fourth, diffuse subtype of ILC, presenting on the mammogram with architectural distortion, changed little, from 60% to 70%.
Discussion
Dixon et al. [10] classified a consecutive series of 103 ILC cases diagnosed during the 1960s into four histologic subgroups: classical, solid variant, alveolar variant, mixed group. They reported in 1982 that all four subtypes were mostly at advanced stages at diagnosis and had a very poor short-term and long-term outcome (Figure 5). Following the introduction of population based organized mammography screening in Falun, Sweden, our clinical impression was that the majority of ILC subtypes were much less advanced at the time of diagnosis, but one subgroup was still extensive and usually at an advanced stage at the time of diagnosis [13,14]. We correlated the mammographic tumor features of these ILC tumors with their large section histologic appearance and designed a study to assess the combined impact of mammography screening and the use of adjuvant therapeutic regimens on the survival of patients with these four ILC subtypes. We documented that the ILC cases with architectural distortion on the mammogram diagnosed in Falun Central Hospital in 1996-2010 were mostly clinically detected, extensive and diffusely infiltrating at the time of diagnosis, had frequent recurrences and a poor outcome that has changed little since the prescreening era. The other three ILC subtypes were significantly less advanced in the screening epoch. We also observed no age related differences in the incidence of the different ILC subtypes, similar to the findings of Stalsberg et al. [13].
There are good theoretical and empirical grounds for considering the cases with architectural distortion on the mammogram to represent a special subgroup of invasive lobular carcinoma [5,8,12,14]. Whereas the long-term survival of the remaining three ILC subtypes improved dramatically as a result of screening, there was no corresponding improvement for the architectural distortion cases despite screening and the introduction of new therapeutic regimens [14]. In breast cancer subtypes of apparently ductal origin, we have observed a similarly large tumor extent at the time of detection and a similarly poor survival [6,8,15].
There are several major clinical implications which can be deduced from these observations. There is a need to revise the classification of the ILC subtypes to reflect their apparent sites of origin, their imaging and subgross histologic observations, their response to treatment and their long-term outcome. Furthermore, improvements in early detection and therapeutic regimens have vastly improved the prognosis of those ILC subtypes (stellate and circular/oval-shaped) that have mammographic presentations characteristic for cancers originating from the acini of the terminal ductal lobular units (TDLUs) [8].
On the other hand, the ILC subtype appearing as architectural distortion on the mammogram has a diffuse tumor distribution, a large size at diagnosis and a poor outcome at long-term follow-up, similar to those breast cancer subtypes that originate in the major ducts and propagate diffusely. Furthermore, all these subtypes appear to be largely unaffected by the adjunctive treatment methods in current use and continue to have a poor survival. We therefore suggest that the ILC subtype with architectural distortion on the mammogram and ILC tumors larger than 3 cm with equivocal density on mammogram should be designated as a separate entity, because their prognosis cannot be reliably evaluated according to the current TNM classification system.
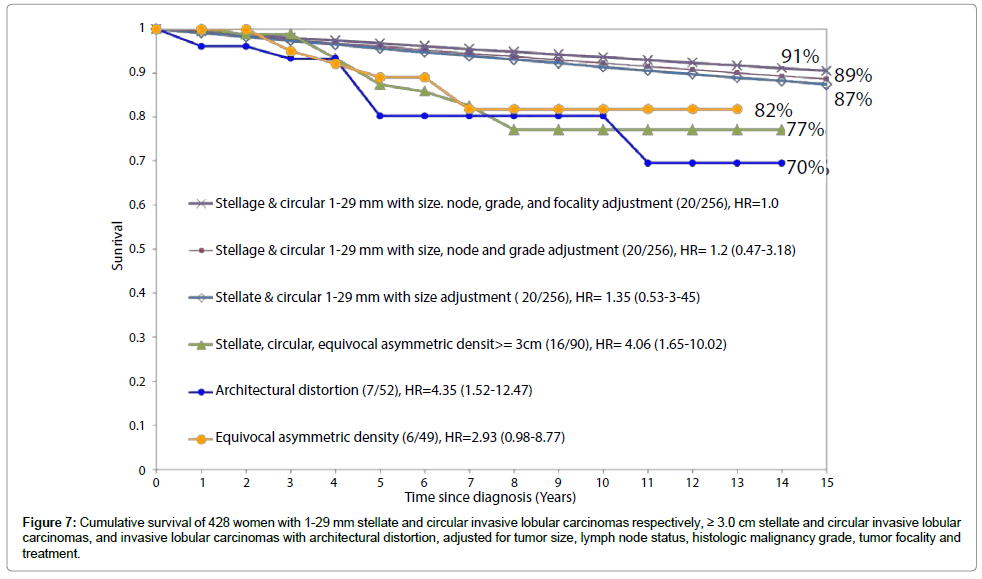
The data presented on Figure 7 suggest that architectural distortion and ILC tumors larger than 3 cm should be considered a single entity. A similar phenomenon was also noted in a recently published study demonstrating a 16-fold higher rate of local recurrence in breast cancer cases of ductal origin presenting with casting type calcifications on the mammogram [16]. In the new era of breast cancer control, breast cancers of ductal origin with or without associated castingtype calcifications on the mammogram and associated with 1-14 mm invasive acinar adenocarcinoma of the breast (AAB) account for the majority of breast cancer deaths, although they comprise a small minority of the breast cancer cases [6,15,17,18]. There is a clear need to further develop diagnostic and management strategies to decrease mortality from the breast cancers of ductal origin and the diffusely infiltrating special subtypes of ILC described above.
Figure 7: Cumulative survival of 428 women with 1-29 mm stellate and circular invasive lobular carcinomas respectively, ≥ 3.0 cm stellate and circular invasive lobular carcinomas, and invasive lobular carcinomas with architectural distortion, adjusted for tumor size, lymph node status, histologic malignancy grade, tumor focality and treatment.
Acknowledgements
The authors thank Tibor Tot, M.D., Ph.D., Chairman of the Department of Pathology and Clinical Cytology, Falun Central Hospital, Sweden, for his valuable comments and histopathologic photomicrographs. This work was supported by the American Cancer Society through a grant from the Longaberger Company’s Horizon of Hope Campaign®. We thank the editor and publishers of the journal Histopathology for permission to reproduce from our reference 10.
References
- Lakhani SR, Ellis IO, Schnitt SJ, Tan PH, van de Vijver MJ (2012) WHO Classification of Tumours of the Breast. (4thedn), International Agency for Research on Cancer, Lyon.
- Weigelt B, Geyer FC, Reis-Filho JS (2010) Histological types of breast cancer: how special are they? MolOncol 4: 192-208.
- Simpson PT, Reis-Filho JS, Lakhani SR (2010) Breast pathology: beyond morphology. Seminars DiagnPathol 27: 91-96.
- Tabar L, Dean PB, Lindhe N, Ingvarsson M (2012) The ongoing revolution in breast imaging calls for a similar revolution in breast pathology. Int J Breast Cancer
- Tabár L, Dean PB, Chen T, Yen A, Chiu S, et al. (2013) The impact of mammography screening on the diagnosis and management of early phase breast cancer. Springer, New York.
- Tabár L, Chen HHT, Yen MFA, Tot T, Tung TH, et al. (2004) Mammographic tumor features can reliably predict the long-term outcome of women with 1-14 mm invasive breast cancer: suggestions for revision of current therapeutic practice and the TNM classification system. Cancer 101: 1745-1759.
- Pekar G, Hofmeyer S, Tabar L, Tarján M, Chen TH, et al. (2013) Multifocal breast cancer documented in large-format histology sections: long-term follow-up results by molecular phenotypes. Cancer 119: 1132-1139.
- Tabár L, Dean PB, Yen AMF, Tarján M, Chiu SYH, et al. (2014) A proposal to unify the classification of breast and prostate cancers based on the anatomic site of cancer origin and on long-term patient outcome. Breast Cancer (Auckl) 8: 15-38.
- Iorfida M, Maiorano E, Orvieto E, Maisonneuve P, Bottiglieri L, et al. (2012) Invasive lobular breast cancer: subtypes and outcome. Breast Cancer Res Treatment 133: 713-723.
- Dixon JM, Anderson TJ, Page DL, Lee D, Duffy SW (1982) Infiltrating lobular carcinoma of the breast. Histopathol 6: 149-161.
- Martinez V, Azzopardi JG (1979) Invasive lobular carcinoma of the breast: incidence and variants. Histopathol 3: 467-488.
- Mendelson EB, Harris KM, Doshi N, Tobon H (1989) Infiltrating lobular carcinoma: mammographic patterns with pathologic correlation. AJR Am J Roentgenol 153: 265-271.
- Stalsberg H, Thomas DB (1993) Age distribution of histologic types of breast carcinoma. Int J Cancer 54: 1-7.
- Tot T (2003) The diffuse type of invasive lobular carcinoma of the breast: morphology and prognosis. Virchows Arch 443: 718-724.
- Tabár L, Tot T, Dean PB (2007) Breast cancer: Early Detection with Mammography. Casting Type Calcifications: Sign of a Subtype with Deceptive Features.Stuttgart, New York.
- Holmberg L, Wong YN, Tabár L, Ringberg A, Karlsson P, et al. (2013) Mammography casting-type calcification and risk of local recurrence in DCIS: analyses from a randomised study. Br J Cancer108: 812-819.
- Palka I, Ormándi K, Gaál S, Boda K, Kahán Z (2007) Casting-type calcifications on the mammogram suggest a higher probability of early relapse and death among high-risk breast cancer patients. ActaOncol 46: 1178-1183.
- Zunzunegui RG, Chung MA, Oruwari J, Golding D, Marchant DJ, et al. (2003) Casting-type calcifications with invasion and high-grade ductal carcinoma in situ: a more aggressive disease? Arch Surg 138: 537-540.
Citation: Tabár L, Dean PB, Chen SL, Chen HH, Yen AM, et al. (2014) Invasive Lobular Carcinoma of the Breast: The Use of Radiological Appearance to Classify Tumor Subtypes for Better Prediction of Long-Term Outcome. J Clin Exp Pathol 4:179. DOI: 10.4172/2161-0681.1000179
Copyright: © 2014 Tabár L, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 16509
- [From(publication date): 9-2014 - Apr 04, 2025]
- Breakdown by view type
- HTML page views: 11892
- PDF downloads: 4617