Make the best use of Scientific Research and information from our 700+ peer reviewed, Open Access Journals that operates with the help of 50,000+ Editorial Board Members and esteemed reviewers and 1000+ Scientific associations in Medical, Clinical, Pharmaceutical, Engineering, Technology and Management Fields.
Meet Inspiring Speakers and Experts at our 3000+ Global Conferenceseries Events with over 600+ Conferences, 1200+ Symposiums and 1200+ Workshops on Medical, Pharma, Engineering, Science, Technology and Business
Review Article Open Access
Intratumoral Heterogeneity of 64Cu-ATSM Uptake is a Prognostic Indicator in Patients with Cervical Cancer
| Albert J Chang1, Farrokh Dehdashti2,3, Barry A Siegel2,3, Michael J Welch3,4, Julie K Schwarz1,3,5 and Perry W Grigsby1,2,3,5* | |
| 1Department of Radiation Oncology, Mallinckrodt Institute of Radiology, USA | |
| 2Division of Nuclear Medicine, Mallinckrodt Institute of Radiology, USA | |
| 3Alvin J. Siteman Cancer Center, USA | |
| 4Division of Radiological Sciences, Mallinckrodt Institute of Radiology, USA | |
| 5Department of Obstetrics and Gynecology, Washington University School of Medicine, St. Louis, Missouri USA | |
| Corresponding Author : | Perry W Grigsby Washington University School of Medicine Department of Radiation Oncology–Campus Box 8224 Mallinckrodt Institute of Radiology, St. Louis, MO 63110, USA Tel: (314) 362-8502 Fax: (314) 747-9557 E-mail: pgrigsby@wustl.edu |
| Received March 20, 2013; Accepted May 15, 2013; Published May 21, 2013 | |
| Citation: Chang AJ, Dehdashti AJ, Siegel BA, Welch MJ, Schwarz JK, et al. (2013) Intratumoral Heterogeneity of 64Cu-ATSM Uptake is a Prognostic Indicator in Patients with Cervical Cancer. OMICS J Radiology 2:130. doi: 10.4172/2167-7964.1000130 | |
| Copyright: © 2013 Chang AJ, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Journal of Radiology
Abstract
Introduction: Intratumoral heterogeneity determined by FDG-PET is a poor prognostic factor in cervical cancer. Cu-
ATSM has been used to evaluate hypoxia in cervical cancer. In this study, FDG and 64Cu-ATSM uptake patterns were
compared and the prognostic significance of 64Cu-ATSM heterogeneity was determined.
Methods: 15 patients with cervical cancer who underwent pretreatment 64Cu-ATSM- and FDG-PET/CT were
included. The 64Cu-ATSM- and FDG-PET/CT images were co-registered and tumor volumes were autocontoured for
each image set in 10% increments of the SUVmax ranging from 40% to 80%. The hypoxic fraction defined by 64Cu-ATSM
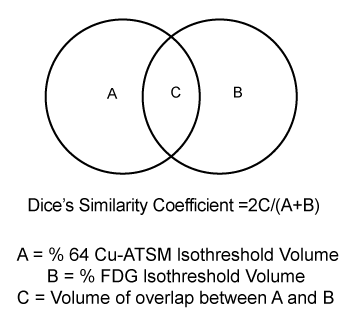
uptake was determined. Concordance between 64Cu-ATSM and FDG uptake was determined by Dice’s coefficient.
Heterogeneity of 64Cu-ATSM and FDG uptake was calculated as the variance of the 40-80% isothreshold volumes. The
association between heterogeneity of 64Cu-ATSM uptake with tumor-specific factors and outcomes was determined.
Results: The hypoxic fraction ranged from 0.773 ± 0.013 to 0.087 ± 0.010 as defined by the 40% to 80% Cu-ATSM
isothreshold volumes, respectively. Dice’s similarity coefficients for the FDG and 64Cu-ATSM 40 to 80% isothreshold
volumes ranged from 0.476 ± 0.012 to 0.112 ± 0.017. Greater 64Cu-ATSM heterogeneity was associated with increased
risk of lymph node metastasis at diagnosis (p<0.01), persistent disease after therapy, (p<0.01), and decreased median
progression-free survival (11 months vs. not reached, p=0.03).
Conclusion: Significant fractions of cervical tumors are hypoxic. Regions of highest 64Cu-ATSM and FDG uptake
were discordant. Elevated 64Cu-ATSM heterogeneity may predict for increased risk of lymph node metastases, decreased
responsiveness to treatment, and decreased progression-free survival.
| Keywords |
| FDG-PET; Cervix cancer; IMRT; Brachytherapy |
| Introduction |
| Positron Emission Tomography (PET) with F-18 fluorodeoxyglucose (FDG) is indicated for the initial staging of advanced cervical cancer [1] and provides metabolic information about tumors that is not available with anatomic imaging techniques such as CT and MRI. FDG-PET has led to improved disease detection in the pelvic and para-aortic lymph nodes, as well as distant metastases [2]. Several features of FDG-PET images have been shown to correlate with prognosis, including primary tumor FDG uptake, as measured by Standardized Uptake Value (SUV), metabolic tumor volume, and tumor heterogeneity [3]. |
| Hypoxia has been correlated with poor patient survival, therapeutic resistance, and aggressive tumor phenotype. A promising agent for PET imaging of hypoxia is diacetyl-bis (N4- methylthiosemicarbazone) labeled with one of several copper radionuclides (Cu-ATSM). The association of tumor hypoxia and increased Cu-ATSM accumulation has been confirmed in animal studies using oxygen electrode measurements [4]. Clinical studies in patients with lung, rectal, and cervical cancer demonstrated that tumor hypoxia as determined by 60Cu-ATSM accumulation was a negative predictor for treatment response, progression-free survival, and cause-specific survival [5-7]. |
| In a previous study, metabolic heterogeneity on pretreatment FDG-PET/CT was suggested to be predictive of increased risk of lymph node involvement at diagnosis, poor response to therapy, and increased risk of pelvic recurrence in patients with cervical cancer [3]. The derivative of the volume-threshold function (dV/dT) from 40-80% was used as the measure of heterogeneity in that study [3]. A subsequent report suggested that this method was only a surrogate of tumor volume and inaccurate for measuring heterogeneity [8]. |
| Despite this suggestion, tumor heterogeneity has been implicated in disease progression, metastasis, and treatment resistance in many malignancies [9,10]. Hypoxia has been suggested to play a role in tumor heterogeneity leading to dedifferentiation of tumor cells and aggressive growth patterns in neuroblastoma and breast cancer [11]. The role of hypoxia in tumor heterogeneity and cervical cancer outcome is not well-defined. In the current study, we hypothesize that tumor heterogeneity as determined by 64Cu-ATSM uptake predicts for poor clinical outcome. The purpose of this study is to evaluate the relationship between tumor uptake of 64Cu-ATSM and FDG and explore the relationship between 64Cu-ATSM heterogeneity, tumor characteristics, and outcome. A method of measuring heterogeneity that accounts for tumor volume is proposed and evaluated in this study. |
| Patients |
| This is a secondary analysis of data from two separate prospective studies evaluating the role of 64Cu-ATSM-PET/CT in patients diagnosed with cervical cancer. The original studies were approved by the Institutional Review Board (IRB) at Washington University and conducted under IND 62,675 (IND study) on file with the Food and Drug Administration. The subjects participating in these studies gave written informed consent, which included consent to use the studyrelated data for future secondary analyses. The study population consisted of 15 patients with newly diagnosed, locally-advanced squamous cell carcinoma of the cervix [12]. This analysis included data from 6 of the 10 patients enrolled in a study previously reported by Lewis et al. [13] (the patients for whom images were available at the time of this secondary analysis). The remaining 9 patients were enrolled in an ongoing multicenter trial (ACRIN 6682) [13]. In both protocols, subjects underwent pretreatment whole-body FDG-PET/ CT followed by 64Cu-ATSM PET/CT of the pelvis on a separate day. All patients were subsequently treated with concurrent chemotherapy and radiation. FDG-PET/CT images were used to define the extent of the metabolically active disease for radiation treatment planning. The radiation was based on standard Washington University treatment practices for cervical cancer, combining external intensity-modulated radiation therapy (IMRT) and high-dose-rate (HDR) brachytherapy [14]. Chemotherapy consisted of cisplatin (40 mg/m2 weekly for six cycles) administered concurrently with radiation therapy. The patient characteristics are listed in Table 1. |
| PET imaging |
| The details of FDG PET/CT have been previously described [3]. Imaging was performed approximately 60 minutes after administration of an average of 507 MBq (13.7 mCi) (range, 433-555 MBq) of FDG. FDG-PET/CT images were obtained from the base of skull through the proximal thighs, with imaging times of 2-4 minutes per bed position, depending on patient weight. To facilitate clearance of FDG activity from the bladder, a Foley catheter was placed and furosemide (20 mg intravenously) was administered 20 minutes after FDG injection. |
| For the 6 patients on the initial IND study, 64Cu-ATSM was produced as previously described [7]. These patients received a mean of 906 MBq (24.5 mCi) (range, 818-925 MBq) of 64Cu-ATSM and underwent conventional PET imaging on an ECAT HR+ scanner (Siemens-CTI). For the 9 patients on the ACRIN 6682 study, 64Cu- ATSM was produced using a kit formulation and PET/CT was performed as defined in the ACRIN 6682 protocol using a Siemens Biograph 40 scanner [13]. These 9 patients received a mean of 829 MBq (22.4 mCi) (range, 677-914 MBq). Images of the pelvis were acquired for 30 min beginning 30 min post-injection for both studies. |
| Image analysis |
| The 64Cu-ATSM images were co-registered to the FDG-PET/CT images using MIMVista 5 software (MIMVista Corp., Cleveland, OH) based on soft tissue and bony pelvic anatomy. Metabolic tumor volumes were autocontoured in 10% increments ranging from 40- 80% threshold values of the tumor SUVmax on both the 64Cu-ATSM and FDG image sets. Previous work demonstrated that the minimal threshold that represents the actual tumor volume for cervical cancer was 40% [15,16]; therefore, all values <40% were eliminated from the analysis (values <40% represent normal tissue background activity and not tumor). In addition, all values >80% were eliminated because the volumes were small and the partial volume effect was pronounced [17]. Typically, these volumes were <5 cm3. Contours were reviewed by a physician to ensure that any activity contributed by the bladder and bowel were not included in the region of interest. |
| To examine the relationship between intratumoral distribution of 64Cu-ATSM and FDG, Dice’s similarity coefficient was calculated as illustrated in Figure 1. The fraction of the 64Cu-ATSM 40%, 50%, 60%, 70%, and 80% isothreshold volumes within the 40% FDG isothreshold volume was also calculated for each patient (Figure 1). |
| Heterogeneity evaluation |
| To determine FDG and 64Cu-ATSM tumor heterogeneity, the 40%, 50%, 60%, 70%, and 80% isothreshold volumes were determined on each scan. FDG and 64Cu-ATSM heterogeneity was defined as the variance of the 40-80% isothreshold volumes (as demonstrated by the numerator of the equation shown below). The variance for each tumor was normalized to total tumor volume as defined by the 40% isothreshold volume on FDG-PET to account for the role of volume in tumor heterogeneity. |
| Tumors with large variances in the 40-80% isothreshold volumes were noted to be more heterogeneous than tumors with low variances in isothreshold volumes. |
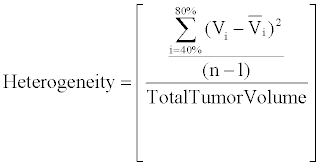 |
| n=≠ Isothreshhold Intervals |
| Vi=Volume at each isothreshold interval |
| Vi=average of isothreshhold volumes |
| Outcome evaluation |
| Initial staging, tumor size, and lymph node involvement was determined by clinical examination and by diagnostic FDG-PET/CT. After completion of therapy, patients underwent follow-up physical examinations approximately every 2 months for the first 6 months, every 3 months for the next 2 years, and then every 6 months. FDGPET/ CT was repeated approximately 3 months after completion of treatment and then yearly or when warranted by clinical examination or symptoms. Disease status, including persistent disease 3 months after completion of chemoradiation therapy, pelvic recurrences and distant metastatic disease, were recorded. |
| Statistical analysis |
| Regression analysis was used to correlate 64Cu-ATSM heterogeneity with tumor volume. Mann-Whitney non-parametric analysis was used to evaluate the correlation between SUVmax for FDG and 64Cu- ATSM and risk of lymph node metastasis at diagnosis, treatment response, and risk of pelvic recurrence. Logistic modeling was used to determine an appropriate cutoff value for 64Cu-ATSM heterogeneity as a predictor of persistent disease as determined by the 3-month posttreatment FDG-PET/CT, as previously described [18]. Chi-square contingency table testing was used to evaluate the correlation between ATSM heterogeneity and FIGO stage, lymph node involvement at diagnosis, and metabolic response on post treatment FDG-PET/CT. Analysis of Progression-Free Survival (PFS) was performed using the Kaplan-Meier method and a log-rank (Mantel-Cox) test. PFS was measured from the completion of radiation treatment. p<0.05 was set as the threshold for significance for all study outcomes. All statistical calculations were performed using Stat View for Windows (Version 5.0.1, SAS Institute, Cary, NC). |
| Results |
| Patient characteristics |
| The median age of the patient cohort was 57 years (range, 40- 79 years). The median follow-up time for all patients was 16 months (range 5-63 months). The numbers of women with International Federation of Gynecology and Obstetrics (FIGO) clinical stage IB2, IIA, IIB, IIIA, IIIB, and IVA disease were 2, 1, 4, 2, 4, and 2, respectively. In 7 of the 15 patients, the pelvic nodes were positive on pretreatment FDG PET/CT. Para-aortic lymph node involvement was noted in 4 of the patients with pelvic lymph node involvement. Four patients developed recurrent and/or progressive disease with one of these patients dying from disease. Six patients had persistent FDG uptake on their 3-month post-treatment FDG PET/CT scan. Detailed patient characteristics are listed in Table 1. |
| Tumor characteristics |
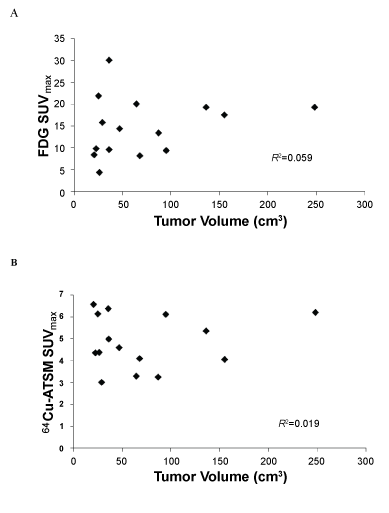
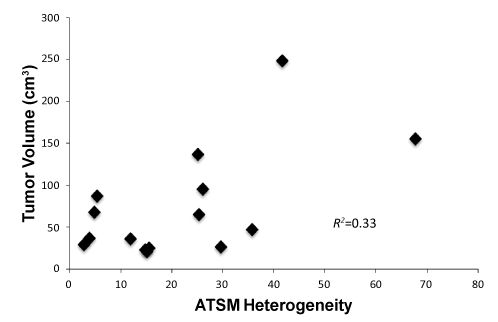
| The mean SUVmax for FDG for all tumors was 14.8 ± 6.7 (range 8.2-30.0) and that for 64Cu-ATSM was 4.8 ± 1.2 (range 3.0-6.5). There was no significant correlation between the SUVmax for FDG and that for 64Cu-ATSM (R2=0.011; p=0.90). The SUVmax values for 64Cu-ATSM and FDG uptake of each patient are listed in Table 1. There was no significant correlation between tumor volume and the SUVmax for 64Cu- ATSM (R2=0.019; p=0.63) or for FDG (R2=0.059; p=0.38) (Figure 2). SUVmax for FDG-predicted for persistent disease after chemoradiation treatment (p=0.049) but did not predict for the presence of lymph node metastases at diagnosis or disease recurrence (p>0.05). No association was observed between SUVmax for 64Cu-ATSM and lymph node involvement, treatment response as determined by the 3-month follow-up PET/CT and clinical examination, nor disease recurrence (p>0.05) (Figure 2). |
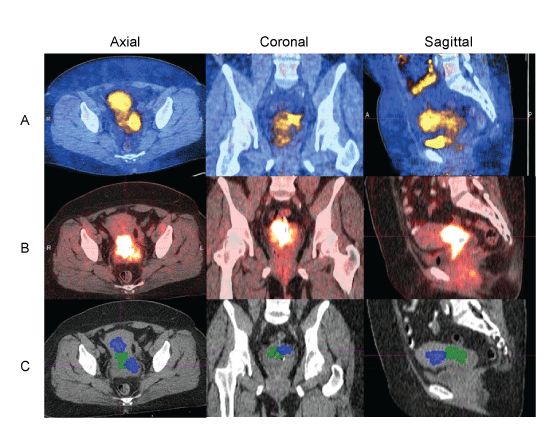
| The similarity between 64Cu-ATSM and FDG uptake volumes was calculated for each lesion using Dice’s coefficient (Table 2). A coefficient of 0.113 ± 0.017 (mean ± standard error mean [SEM]) was observed with metabolic tumor volumes generated using the 80% threshold value on the ATSM and FDG images with minimal overlap of regions with high 64Cu-ATSM and FDG uptake in the primary tumor. A representative example is depicted in (Figures 3a and 3b) with the 70% isothreshold volumes contoured in (Figure 3c). When the thresholding was decreased to 40% for both image sets, the Dice’s similarity coefficient was 0.477 ± 0.012 suggesting areas with moderate uptake have a higher degree of overlap. The hypoxic fraction within the primary lesion was also evaluated by analyzing the volume of uptake as defined by the 40%, 50%, 60%, 70%, and 80% isothreshold volumes within the 40% FDG isothreshold volume (Table 3). Areas of significantly greater hypoxia as defined by the 80% and 70% 64Cu-ATSM isothreshold volume accounted for 8.7 ± 1% and 20.2 ± 1.8% of the tumor volume, respectively. Regions of moderate hypoxia as defined by the 60% and 50% isothreshold volume accounted for 35.7 ± 2.4% and 53.6 ± 2.3% of the tumor volume. |
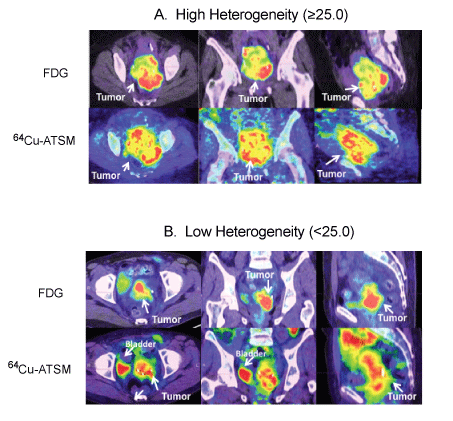
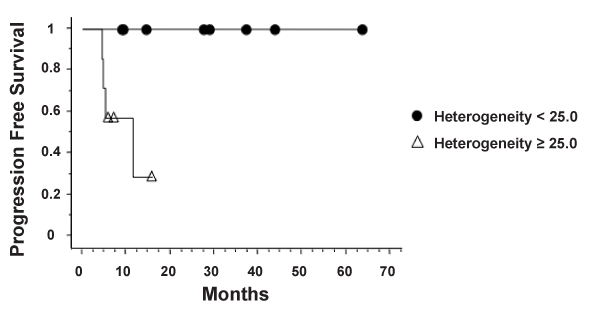
| Analysis of intratumoral FDG and 64Cu-ATSM uptake heterogeneity was performed. Figure 4 demonstrates examples of patients with high and low FDG and 64Cu-ATSM heterogeneity. The median heterogeneity of 64Cu-ATSM and FDG uptake was 15.6 (range, 2.8-41.6) and 11.1 (range, 2.6-42.2), respectively. Tumor size did not correlate with 64Cu-ATSM heterogeneity (R2=0.33, p=0.16) (Figure 5). No relationship was seen between SUVmax and heterogeneity for 64Cu- ATSM images. Logistic analysis was used to separate 64Cu-ATSM heterogeneity into high heterogeneity (= 25.0) and low heterogeneity (<25.0) groups. The association between 64Cu-ATSM heterogeneity and patient characteristics is summarized in Table 4. High 64Cu- ATSM heterogeneity was not correlated with tumor grade (p=0.80) or FIGO clinical stage (p=0.73). Greater 64Cu-ATSM heterogeneity was associated with the presence of lymph node involvement at diagnosis (p<0.01) and the presence of residual disease as determined by persistent FDG uptake on the posttreatment FDG-PET/CT scan (p<0.01) (Figure 5). In addition, elevated 64Cu-ATSM heterogeneity was associated with poorer PFS. The median progression free survival was 11 months for patients with 64Cu-ATSM heterogeneity = 25.0 and not reached for patients <25.0 (p=0.03, Figure 6). |
| Discussion |
| We have evaluated the relationship between FDG and 64Cu- ATSM uptake within primary cervical tumors and the prognostic significance of 64Cu-ATSM heterogeneity. Large fractions of cervical tumors are hypoxic, with regions of greatest hypoxia exhibiting low glucose metabolism as suggested by the inverse relationship between intratumoral 64Cu-ATSM and FDG uptake. To our knowledge, this is the first imaging study to evaluate the relationship between hypoxia and tumor heterogeneity. In a previous report, we found that elevated tumor heterogeneity as determined by FDG uptake was a poor prognostic factor [3]. In the current study, 64Cu-ATSM heterogeneity predicted for increased risk of lymph node involvement at diagnosis, decreased treatment response to chemoradiation, and poorer PFS independent of clinical stage and tumor uptake, as measure by SUVmax. These findings demonstrate that tumor heterogeneity, as defined by the variance in 64Cu-ATSM uptake, is a poor prognostic factor. |
| Several methods have been proposed to evaluate intratumoral hypoxia. The use of Eppendorf oxygen-sensitive electrodes to measure oxygenation status is invasive and provides information limited to the region directly surrounding the probe [19]. Pimonidazole staining requires a biopsy specimen and also provides information limited to the area of biopsy rather than a global picture of intratumoral hypoxia [20]. PET with 18F-fluoromisonidazole (FMISO) has been well characterized as an imaging agent for assessment of hypoxia. However, the relatively low FMISO accumulation within a tumor coupled with delayed clearance from normoxic background tissues results in a low tumor-to-background ratio (<2:1) [21]. Imaging with 64Cu-ATSM results in rapid delineation of tumor hypoxia (in less than 1 h) with high tumor-to-background tissue ratios (tumor-to-blood ratios >>2) [22]. When compared to FMISO, 64Cu-ATSM has increased selectivity for tissues with low oxygen tension [23]. As demonstrated in this study, PET imaging with 64Cu-ATSM is a non-invasive method that provides images detailing the relative severity of hypoxia within different regions of the tumor. |
| 64Cu-ATSM and FDG volumes were autocontoured using multiple isothresholds of the SUVmax to compare the spatial distribution of the radiotracers. In a study by Lohith et al. [24,25], regions of interest were manually drawn to evaluate the differences in 64Cu-ATSM and FDG uptake within the tumor [24]. Because generation of the contours was automated in the current study, this method can be easily reproduced in other studies to compare the intratumoral distribution of various tracers and reduces potential observer bias. |
| Spatial mismatching of tumor 64Cu-ATSM and FDG uptake has been reported in rodent models of gliosarcoma, melanoma and liver, colon, and lung tumors [22,25-27] and in squamous cell lung cancer in humans [24]. Because of the disorganized growth of the tumor vasculature, regional differences in blood perfusion and oxygenation are observed within a tumor [28]. Because FDG uptake may be dependent on blood perfusion, regions of elevated FDG uptake may represent areas that are well vascularized, perfused, and oxygenated [29,30]. In contrast to FDG, 64Cu-ATSM may not be limited by vascular perfusion and can diffuse to greater distances to poorly vascularized and hypoxic regions. This phenomenon has been suggested by an inverse correlation between blood perfusion and 62Cu-ATSM uptake [31]. The differences on the dependence on blood perfusion in tracer uptake may explain the spatial mismatch between FDG and 64Cu- ATSM. |
| In a previous study, we reported that elevated 60Cu-ATSM uptake predicted for poor prognosis in patients in cervical cancer [32]. Areas of elevated Cu-ATSM and low FDG uptake have been associated with hypovascular regions rich in CD133+ cancer stem cells [26,27]. Cancer stem cells are involved in tumor growth, maintenance, and metastases and have been found to be more resistant to radiotherapy and chemotherapy [33]. Cancer stem cells regulate tumor angiogenesis by production of Vascular Endothelial Growth Factor (VEGF) [34,35]. These phenomena may explain the increased risk of lymph node involvement and worse outcomes in patients with greater 64Cu-ATSM heterogeneity in the current study. |
| A relationship between tumor volume and heterogeneity has been suggested. A previous measure of tumor heterogeneity with FDG-PET was reported using the derivative (dV/dT) of the volume-threshold function from 40% to 80% [3]. This method of measuring tumor heterogeneity was suggested to be inaccurate and only a surrogate for tumor volume [8]. A difficulty exists in separating tumor volume from tumor heterogeneity as, inherently, large volume tumors have a greater potential to become heterogeneous when compared to small tumors. Also, the possibility exists that tumors with greater heterogeneity may grow larger. Hypoxia has been suggested to play a role in tumor heterogeneity [9,20,36]. Under hypoxic conditions, tumors develop more aggressive phenotypes with low apoptotic potential, which can lead to larger tumors [37-39]. Similar to tumor heterogeneity, tumor volume has been associated with clinical stage, with larger tumors presenting at an advanced stage. |
| To further clarify the relationship between tumor heterogeneity and tumor volume and to evaluate the role of tumor heterogeneity in predicting patient outcome, a novel method of evaluating heterogeneity was proposed in the current study by calculating the variance of the 40-80% isothreshold volumes within the tumor. To account for tumor volume, this variance was normalized to tumor volume. With the proposed measure of heterogeneity, heterogeneous tumors had a speckled pattern of uptake in comparison to tumors with low heterogeneity. We found an association between greater 64Cu-ATSM heterogeneity and the presence of lymph node metastases at diagnosis. Furthermore greater 64Cu-ATSM heterogeneity was associated with shorter PFS independent of FIGO stage. Because of the limited number of patients and follow-up interval, further studies will be needed to validate this measure. |
| Conclusion |
| 64Cu-ATSM-PET and FDG-PET provide complementary information about the biology of the tumor. The differential uptake of 64Cu-ATSM and FDG within tumors of the uterine cervix demonstrates that areas of hypoxia have low glucose metabolism and areas of high metabolic activity are better oxygenated. PET imaging with 64Cu-ATSM provides a comprehensive picture of the diversity of hypoxia within a cervical tumor. A novel measure of heterogeneity was defined by calculating the variance of 64Cu-ATSM uptake normalized to tumor volume. With this measure, the degree of 64Cu-ATSM intratumoral heterogeneity predicted for lymph node involvement and decreased PFS. This measure of heterogeneity will be validated in future studies. |
| Acknowledgements |
| This work was supported by R01 CA136931-03, DOE grant DE-FG02- 87ER60512 and the ACRIN NCI grant U01 CA079778. |
References |
|
Tables and Figures at a glance
| Table 1 | Table 2 | Table 3 | Table 4 |
Figures at a glance
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 |
 |
 |
 |
| Figure 4 | Figure 5 | Figure 6 |
Post your comment
Relevant Topics
- Abdominal Radiology
- AI in Radiology
- Breast Imaging
- Cardiovascular Radiology
- Chest Radiology
- Clinical Radiology
- CT Imaging
- Diagnostic Radiology
- Emergency Radiology
- Fluoroscopy Radiology
- General Radiology
- Genitourinary Radiology
- Interventional Radiology Techniques
- Mammography
- Minimal Invasive surgery
- Musculoskeletal Radiology
- Neuroradiology
- Neuroradiology Advances
- Oral and Maxillofacial Radiology
- Radiography
- Radiology Imaging
- Surgical Radiology
- Tele Radiology
- Therapeutic Radiology
Recommended Journals
Article Tools
Article Usage
- Total views: 13731
- [From(publication date):
July-2013 - Apr 01, 2025] - Breakdown by view type
- HTML page views : 9357
- PDF downloads : 4374
