Research Article Open Access
Intrathecal Morphine Therapy for Chronic Non-malignant Pain Using a Constant Flow Infusion System
| Jairo Angelos, Martin Paiz, Christiane Pellegrino Rosa, Roberta Risso, Bernardo Monaco, Kleber Paiva Duarte, William Omar Contreras Lopez, Manoel Jacobsen Teixeira and Erich Talamoni Fonoff* | |
| Pain Center and Division of Functional Neurosurgery, Department of Neurology, School of Medicine, University of Sao Paulo, Sao Paulo, Brazil | |
| Corresponding Author : | Erich Talamoni Fonoff Division of Functional Neurosurgery Hospital das Clínicas, University of São Paulo Rua Dr Ovídio Pires de Campos 785, São Paulo, SP, Brazil, 01060-970 Tel: +55 11 2661 6402 Fax: +55 11 2661 6402 E-mail: fonoffet@usp.br |
| Received November 25, 2014; Accepted December 30, 2014; Published January 02, 2015 | |
| Citation: Angelos J, Paiz M, Rosa CP, Risso R, Monaco B, et al. (2015) Intrathecal Morphine Therapy for Chronic Non-malignant Pain Using a Constant Flow Infusion System. J Pain Relief 4:168. doi: 10.4172/2167-0846.1000168 | |
| Copyright: © 2015 Angelos J, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Journal of Pain & Relief
Abstract
Background: Implantable pumps for intrathecal opioid therapy in the last decades emerged as one of the most effective treatment for chronic non-malignant pain (CNMP) in selected patients. This study analyses reliability of intrathecal infusion morphine therapy for chronic and refractory pain not related to malignancy using an implantable constant flow pump.
Methods: we studied eleven patients (9 women and 2 men; age range 26-67 years), with medically unresponsive CNMP. Pain was classified as neuropathic or non-neuropathic based on clinical features. Patients were evaluated before and after pump implantation, measuring pain intensity and relief after intrathecal opioid treatment based on Visual Analogic Scale (VAS) scores.
Results: Pain significantly reduced in almost all patients (91%); mean pain scores improved from 8.9 ± 1.49 before implant to 66% improvement one month after infusion therapy (3.1 ± 1.60) and 41% after 18 months (5.2 ± 2.45).
Conclusion: The present data show that intrathecal morphine infusion at a constant flow proved to be a safe and suitable alternative for the treatment of CNMP.
Methods: we studied eleven patients (9 women and 2 men; age range 26-67 years), with medically unresponsive CNMP. Pain was classified as neuropathic or non-neuropathic based on clinical features. Patients were evaluated before and after pump implantation, measuring pain intensity and relief after intrathecal opioid treatment based on Visual Analogic Scale (VAS) scores.
Results: Pain significantly reduced in almost all patients (91%); mean pain scores improved from 8.9 ± 1.49 before implant to 66% improvement one month after infusion therapy (3.1 ± 1.60) and 41% after 18 months (5.2 ± 2.45).
Conclusion: The present data show that intrathecal morphine infusion at a constant flow proved to be a safe and suitable alternative for the treatment of CNMP.
As a therapeutic option for CNMP, intrathecal pain therapy provided by implantable infusion systems allows drug administration directly in cerebral spinal fluid (CSF) within the spinal canal in a safe and precise manner with long autonomy. They have been used in the management of selected cases of pain and severe spasticity. [3]
Recognized indications for intrathecal opioid delivery are failed back surgery syndrome (FBSS) [4], neuropathic pain, complex regional pain syndrome (CRPS), pain after spinal cord injury [5], pain resistant to spinal cord stimulation [6], spinal arachnoiditis, peripheral neuropathy, among others.
Authors have reported that chronic intrathecal morphine infusion is a safe and effective option [7-9] since it does not require a significant systemic drug concentration to provide a good pain relief [10]. Very low concentrations of drugs delivered intrathecally are found in systemic circulation, what lowers the occurrence of adverse effects [11].
As a drawback, the need of a subcutaneous tissue implant and intrathecal space catheterization can originate catheter related problems (dislocations, disconnections, rupture and obstruction), pump dysfunctions and inflammatory processes, which are rare in general [8].
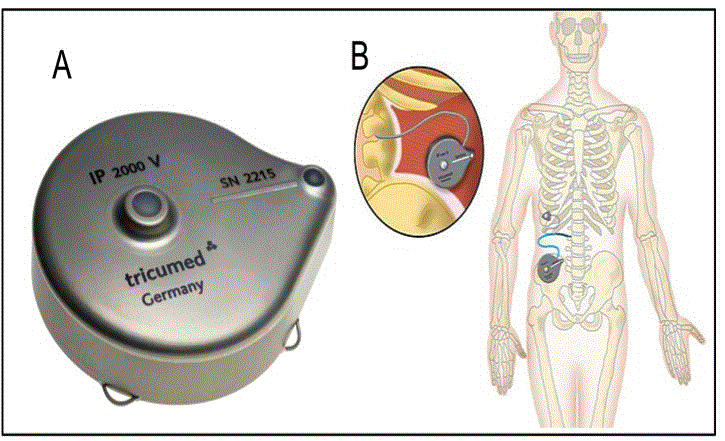
Tricumed infusion pump IP2000V (Tricumed Medizintechnik GmbH Röntgenstrasse 7a; 24143 Kiel, Germany, see Figure 1A) is an implantable device that is intended to store and deliver pharmacological agents at a constant rate. The IP2000V pump delivers fluid solutions from the reservoir to the device outlet through a combination of a pressurized fluid reservoir through a capillary chip and associated fluid channeling. It is supposed to be used for the delivery of morphine sulphate for continuous intrathecal pain therapy and intrathecal baclofen for spasticity.
Here we aimed to study clinical results, complications and side effects of intrathecal morphine therapy at a constant infusion flow in CNMP patients.
Etiology of pain and other aspects of patients are shown in Table 1.
Characteristics of pain were ascertained according neuropathic and non-neuropathic clinical features, like paresthesias, burning and continuous pain, allodynia and trigger points. In neurological examination, patients who exhibited the diagnosis of neuropathic pain had sensory abnormalities in the correspondent body segments. Outcome was verified clinically and with VAS scores: baseline (preoperative), one and eighteen months after surgery. Percentage of improve was analyzed setting VAS in a minimum of 50%.
Under general anesthesia, all 11 patients received a Tricumed infusion pump, with a reservoir volume of 60 ml, with a flow rate of 1 ml per 24 hours. Aseptic techniques were taken for lumbar and lateral and inferior abdominal region. After 1500 mg IV cefuroxime, patients were positioned in lateral decubitus and a lumbar puncture was made with a Tuohy needle between L2-L3 or L3-L4 vertebral space, avoiding medullary conus. An intrathecal catheter (Spinal Catheter Set 4000, Kiel, Germany) was inserted as the needle was withdrawn. Generally, the target of catheter was T6 to T9, depending on the highest level of pain, verified with a fluoroscopic view. After this, a small incision was done around the catheter output, in order to fix it on lumbar fascia. A transversal incision was performed in lateral-inferior abdominal region until muscle fascia for lodging IP2000V pump, which was connected with the tunneled spinal catheter (Figure 1B). Finally, pump was adequately fixed subcutaneously in its four suture loops.
Majority of patients (91%) exhibited a good clinical improve after pump implant. The mean pain scores (8.9 ± 1.49 before the implant) showed significant improvement of 66% after one month (3.1 ± 1.60) and 41% after 18 months (5.2 ± 2.45) (Figure 2).
Initial intrathecal morphine dose (10 mg/ml) ranged from 0.5 to 3.6 mg/day (average 1.75 mg/day), being titrated according to individual patient demand until 20% per medical consultation. In four cases morphine was associated with bupivacaine (2.86 mg/day average dose), which dose also was also adjusted if eventual pain worsening occurred. This drug combination was necessary not only for a better control of pain but due to morphine side effects, like itching (one patient) and urinary retention (one patient). In 18-month follow-up visit, the average intrathecal morphine dose was 5.5 mg/day (1.5-26.5 mg/day).
One patient had pump dispositive withdrawn related to persistent CSF leak even after repeated surgical correcting procedures. No infection was observed in this patient, who had afterwards a diagnosis of pseudotumor, a condition that imposes difficulties into intra-spinal drug continuous administration due to high CSF pressure. This result also was computed in final analysis and considered a therapy failure.
There were no neurological complications after pump implant. One patient presented superficial skin infection, which was successfully treated with oral antibiotics. One patient exhibited transient sensory abnormalities over the pump pocket in the abdominal region with subsequent spontaneous resolution.
It was not possible to achieve intrathecal opioid therapy exclusively in any patient at the last follow-up, though nine had a significant relief after pump implant. Because of this, some adjuvant oral medications were kept as complementary treatment (Table 1).
Constant flow infusion systems fulfill requirements of continued drug delivery in CSF and provide a relatively high concentration of the drug close to opioid receptors in spinal cord [6]. This method is more likely to promote analgesia with doses significantly lower than oral and intravenous administration.
Only 3 products have been officially approved for long-term intrathecal administration: morphine, baclofen, and ziconotide. The efficacy of intrathecal ziconotide for the management of patients with severe chronic refractory non-cancer pain was illustrated in 3 placebo-controlled trials, showing a benefit over a short follow-up period for patients with pain due to cancer or AIDS. The efficacy of intrathecal opioid administration for the management of chronic noncancerous pain is mainly derived from prospective and retrospective non-controlled trials [12].
Our study analyses the benefits of intraspinal morphine in patients with CNMP of different etiologies. Although retrospective and with a small sample, the group of patients typifies the most frequent etiologies for intraspinal opioid therapy, 45% of these with FBSS.
Patients with psychiatric associated disease were excluded, as is extensively recommended in literature. In congruence with this recommendation and with recognized indications, we believe that the rigorous selection of patients is the key for the success of intra-spinal opioid therapy.
A recent consensus on intrathecal drug delivery (IDD) [13] highlights that analgesic response to IDD has been seen in patients with neuropathic, visceral, deafferentation, and mixed pain. Panelists noted that patients with some conditions – such as headache, fibromyalgia, atypical facial pain, noncancer head–neck pain, and borderline personality disorder – have not had good outcomes. Although patient selection varies widely among practitioners, the goal is to identify the patients whose pain states are likely to be relieved by intrathecal medication.
Literature diverges on a strict method in trialing patients for intrathecal opioid use, some authors [14] using both intrathecal and epidural morphine. But all agree that a general improvement of at least 50% in pain condition after opioid test favors a definitive device implant.
Most of studies concerning intrathecal opioid treatment consider the use of an electronic device, what indeed appears to control pain in a better way, easily meeting pain demands by changing infusion program [15]. However, in our experience using a gas driven constant flow infusion pump, we also could obtain optimal doses during the day and manage adverse effects without difficult.
In the two largest studies published by Winkelmüller and Winkelmüller [16] with 120 patients and that by Paice et al. [17] with 429 patients, two-thirds of patients showed a good to an excellent response after morphine pump implant. In our series, we observed that 91% of patients presented a general improve in pain scores after one month of the implant, showing a decrease on VAS around 66%, though did not achieved a permanent decrease on VAS in the last follow-up (41%).
Our rate of improvement is consistent with other reports in their last patient observation: 57.5% (16 patients, 49-month) [18], 36% (30 patients, 24-month) [14], 62% (132 patients, 12-month) [19]. Like we did, these studies also relate a decrease in the grade of improvement in a final following, but any specific clinical or individual condition was associated to this outcome.
Some aspects should be considered when explaining the partial and non-permanent decrease of VAS scores with intrathecal morphine for CNMP, not only in this series. In general, these patients have experienced a pain condition difficult to manage, generally they`ve had poor access to rehabilitation therapies and finally may present psychological issues as a consequence of dealing with pain for a long time [18]. In this aspect, intrathecal morphine pump implant or other therapeutic procedure opposes against only one aspect of the disease, giving rise to unsatisfying results because other important factors are not being treated.
Despite the absence of a control group and the concomitant use of oral analgesics in the course of intrathecal morphine therapy, the evident differences on VAS after pump implant show the benefits this therapy could bring for our patients. Although this study did not explore important outcomes like return to work and quality of life indices, when questioned about the importance of intrathecal morphine in the roll of pain therapy, patients who benefited from it reported a positive change in daily life activities after implant.
At first, the percentage of decrease on VAS in our study may not mean a strong improve evoking criticisms in proposing patients a chronic intra-spinal opioid therapy. However, ratifying the comments of Slavin [18], these patients had already failed all other available analgesic interventions, such that intrathecal opioids for them may be the last resort, even though half of patients exhibit a good response.
We added bupivacaine to morphine in 45% of patients, mainly in order to diminish morphine adverse effects without loss of analgesia. Anderson and Burchiel [14] did the same in 20% of cases.
The practice of associating other drugs to opioids in an intra-spinal deliver is not new. Krames and Larming [20] studied the combination of bupivacaine and morphine in 20 of 26 patients with CNMP verifying good analgesia and reduction of adverse effects due to morphine. Posteriorly, Rainov, et al. [21] also viewed the same benefit when morphine was combined with bupivacaine, midazolam and clonidine in different settings, while studying 10 patients with pain of spinal origin.
Two patients (18%, both female ones) had inadequate analgesia with intrathecal morphine, even after managing oral medications. We believe that in these cases, in which pump was also been withdrawn, indications for surgery should be revisited (generalized pain and coccidinya). Rates of explanted patients varied among authors, 6.66% [14], 12.5% [18], but the main reasons generally were the same (inadequate analgesia and intolerably opioid side effects).
This outcome shows that a specific population of implanted patients will not experience the benefits of intrathecal opioids, despite well managed. Randomized and controlled studies are needed to show who such individuals are.
The low infection rate in our case series, the absence of drug overdose and the effective control of pain reaffirm the safety and reliability of infusion systems for intra-spinal drug administration.
Here we showed the possibility of using an infusion system different from electronic devices and reproduce equivalent good results, with security and with a low rate of complications. This reinforces the importance of gas driven pumps as another option to intra-spinal therapy in CNMP patient’s population, known for having a long operating life, maximum drug capacity in a minimum pump size with no battery to ever be replaced.
Apart from finding the best candidate for IDD, we emphasize the attention to the well-accepted exclusion criteria for this therapy that can predict a bad outcome if ignored.
References
- Winn HR (2011) Youmans Neurological Surgery. Elsevier Saunders, Philadelphia.
- Manchikanti L, Ailinani H, Koyyalagunta D, Datta S, Singh Vet (2011) A systematic review of randomized trials of long-term opioid management for chronic non-cancer pain. Pain Physician 14: 91-121.
- Deer TR, Smith HS, Cousins M, Doleys DM, Levy RM (2010) Consensus guidelines for the selection and implantation of patients with noncancer pain for intrathecal drug delivery. Pain Physician 13: E175–213.
- Teixeira MJ, Yeng LT, Garcia OG, Fonoff ET, Paiva WS (2011) Failed back surgery pain syndrome: therapeutic approach descriptive study in 56 patients. Rev. Assoc. Médica Bras 57: 282-287.
- Teixeira MJ, Paiva WS, Assis MS, Fonoff ET, Bor-Seng-Shu E (2013) Neuropathic pain in patients with spinal cord injury: report of 213 patients. Arq. Neuropsiquiatr 71: 600-603.
- Lara NA Jr, Teixeira MJ, Fonoff ET (2011) Long term intrathecal infusion of opiates for treatment of failed back surgery syndrome. ActaNeurochir. Suppl 108: 41-47.
- Cousins MJ, Mather LE (1984) Intrathecal and epidural administration of opioids. Anesthesiology 61: 276-310.
- Deer T, Winkelmüller W, Erdine S, Bedder M, Burchiel K (1999) Intrathecal therapy for cancer and nonmalignant pain: patient selection and patient management. Neuromodulation J. Int. Neuromodulation Soc. 2: 55-66
- Krames ES (1993) Intrathecal infusional therapies for intractable pain: patient management guidelines. J. Pain Symptom Manage 8: 36-46.
- Angel IF, Gould HJ Jr, Carey ME (1998) Intrathecal morphine pump as a treatment option in chronic pain of nonmalignant origin. Surg. Neurol 49: 92-98
- Koulousakis A, Kuchta J, Bayarassou A, Sturm V (2007) Intrathecal opioids for intractable pain syndromes. ActaNeurochir. Suppl. 97: 43-48.
- VerDonck A, Vranken JH, Puylaert M, Hayek S, Mekhail N (2014) Intrathecal drug administration in chronic pain syndromes. Pain Pr. Off. J. World Inst. Pain 14: 461-476.
- Prager J, Deer T, Levy R (2014) Best Practices for Intrathecal Drug Delivery for Pain: Intrathecal Drug Delivery. Neuromodulation Technol. Neural Interface 17: 354-372.
- Anderson VC, Burchiel KJ (1999) A prospective study of long-term intrathecal morphine in the management of chronic nonmalignant pain. Neurosurgery 44: 289-300.
- Ilias W, Todoroff B (2008) Optimizing pain control through the use of implantable pumps. Med. Devices Auckl. NZ 1: 41.
- Winkelmüller M, Winkelmüller W (1996) Long-term effects of continuous intrathecal opioid treatment in chronic pain of nonmalignant etiology. J Neurosurg 85: 458-467.
- Paice JA, Penn RD, Shott S (1996) Intraspinal morphine for chronic pain: a retrospective, multicenter study. J. Pain Symptom Manage 11: 71-80.
- Kumar K, Kelly M, Pirlot T (2001) Continuous intrathecal morphine treatment for chronic pain of nonmalignant etiology: long-term benefits and efficacy. Surg. Neurol 55: 79-86
- Deer T, Chapple I, Classen A, Javery K, Stoker V (2004) Intrathecal drug delivery for treatment of chronic low back pain: report from the National Outcomes Registry for Low Back Pain. Pain Med. Malden Mass 5: 6-13.
- Krames ES, Lanning RM (1993) Intrathecal infusional analgesia for nonmalignant pain: analgesic efficacy of intrathecal opioid with or without bupivacaine. J. Pain Symptom Manage 8: 539-548.
- Rainov NG, Heidecke V, Burkert W (2001) Long-term intrathecal infusion of drug combinations for chronic back and leg pain. J. Pain Symptom Manage 22: 862-871
Tables and Figures at a glance
| Table 1 |
Figures at a glance
 |
 |
| Figure 1 | Figure 2 |
Relevant Topics
- Acupuncture
- Acute Pain
- Analgesics
- Anesthesia
- Arthroscopy
- Chronic Back Pain
- Chronic Pain
- Hypnosis
- Low Back Pain
- Meditation
- Musculoskeletal pain
- Natural Pain Relievers
- Nociceptive Pain
- Opioid
- Orthopedics
- Pain and Mental Health
- Pain killer drugs
- Pain Mechanisms and Pathophysiology
- Pain Medication
- Pain Medicine
- Pain Relief and Traditional Medicine
- Pain Sensation
- Pain Tolerance
- Post-Operative Pain
- Reaction to Pain
Recommended Journals
Article Tools
Article Usage
- Total views: 15874
- [From(publication date):
January-2015 - Apr 27, 2025] - Breakdown by view type
- HTML page views : 11274
- PDF downloads : 4600
