Intra-Oral Applications of Platelet Concentrates: A Comprehensive Overview of Systematic Reviews
Received: 17-Mar-2018 / Accepted Date: 14-May-2018 / Published Date: 21-May-2018 DOI: 10.4172/2376-032X.1000233
Abstract
Aim: The aim of this systematic review is to provide a comprehensive overview of systematic reviews and metaanalyses on the intra-oral applications of platelet concentrates. The intra-oral applications of platelet concentrate were evaluated in the following areas: sinus augmentation, ridge preservation, implant therapy, periodontal treatment, endodontic treatment, and osteonecrosis treatment.
Materials and Methods: Electronic databases were searched for systematic reviews and meta-analyses on intra-oral applications of platelet concentrates. Reviews were AMSTAR rated for methodology quality.
Results: Twenty-three articles were selected, the articles reported on the use of platelet concentrates in more than one area. Nine articles reviewed platelet concentrates used in sinus augmentation, 4 articles in ridge preservation, 4 articles in implant therapy, 13 articles in periodontal treatment, 2 articles in endodontic treatment, and 3 articles in osteonecrosis treatment. The reviews were AMSTAR rated, 20 were high quality and 3 were moderate quality.
Conclusion: Within the limits of the included systematic reviews, this overview suggested that platelet concentrates can be used successfully in intra-oral procedures like sinus augmentation, ridge preservation, periodontal treatments, and treatment of osteoradionecrosis. This overview also suggested that platelet concentrates can accelerate wound healing and its effects may be more pronounced in the early post-operative stage. However, with the paucity of strong clinical evidence regarding the benefits on the use of autologous platelet concentrates, each clinician must proceed with caution should they decide to implement regenerative medicine into their clinical practice.
Keywords: Platelet concentrates; Platelet-rich fibrin; Platelet-rich plasma; Histomorphometric; Bone grafting
List of Abbreviations
AMSTAR: A Measurement Tool To Assess Systematic Reviews; APC: Autologous Platelet Concentrate; PCS: Platelet Concentrates; PRP: Platelet Rich Plasma; PRGF: Platelet Rich In Growth Factors; PRF: Platelet Rich Fibrin; TGF-Β1: Transforming Growth Factor Beta-1; PDGF-AB: Platelet Derived Growth Factor AB; VEGF: Vascular Endothelial Growth Factor; IL: Interleukin; OFD: Open Flap Debridement; PPD: Probing Pocket Depth; CAL: Clinical Attachment Level; CAF: Coronally Advanced Flap; CTG: Connective Tissue Graft; MRONJ: Medication-Related Osteonecrosis Of The Jaws; ISQ: Implant Stability Quotient
Introduction
Healing of the intraoral soft tissues occurs with a cascade of events mediated by different signaling molecules and growth factors. Growth factors in autologous platelets play a vital role in the acceleration of wound healing. Autologous platelet concentrates (APCs) available for surgical procedures include the following: platelet rich plasma (PRP), platelet rich in growth factors (PRGF), and platelet rich fibrin (PRF). The common feature of these heme products is their increased baseline platelet concentrations that has been demonstrated to increase wound healing at the surgical site. The effectiveness in enhanced wound healing is due to their ability to continually release various growth factors throughout the course of wound healing at various times [1,2].
Growth factors are capable of binding to specific cellular substrates on the cell surface that can regulate cellular events such as cell differentiation, proliferation, migration and synthesis of the extracellular matrix [3]. Such growth factors include the following: transforming growth factor beta-1 (TGF-β1), platelet derived growth factor AB (PDGF-AB), vascular endothelial growth factor (VEGF), interleukin (IL)-1beta, IL-4 and IL-6 [4]. All the growth factors are involved in differentiation and proliferation of osteoblasts, chondrocytes, endothelial cells and fibroblasts [5,6].
The two APCs produced, PRP and PRGF, requires an anticoagulant and bovine thrombin that acts as an activator, while PRF can be produced without either additive [7]. All variations of APCs require a sample of venous blood that is collected in a tube and immediately centrifuged. In terms of biologic activity, thrombin induces a rapid release of growth factors from the platelet alpha granules during the first 24 hours that slowly decreases by day 14 [8]. PRP has been shown to release the largest amount of TGF-β1 and PDGF-AB on the first day of surgical activity that slowly decreases by day 14 [9]. The effects of cytokine activity are very brief on wound healing, but have been demonstrated to increase bone regeneration by facilitating neogenesis, cell chemotaxis, mitosis, stem cell proliferation and osteo conduction [3,10].
In contrast, as PRF requires no thrombin activator during the centrifugation process to obtain a fibrin matrix, cytokine activity is prolonged as it is incorporated within the matrix in addition to platelets, leukocytes and stem cells [5,9,11]. PRF has been shown to stimulate wound healing at the site of tissue injury by the recruitment of cells, such as osteoblasts, endothelial cells, chondrocytes and fibroblasts. These specialized cells are involved in wound healing and angiogenesis [12,13].
As the fibrin matrix is slow to dissolve, it has been demonstrated that by the seventh day of wound healing, PRF releases the largest amount of PDGF-AB. By day 14, enormous quantities of TGF-β1 are observed [5]. PRF has been demonstrated to also contain substantial amounts of VEGF [4]. Therefore, PRF enhances epithelial healing, tissue vascularization and soft tissue regeneration. Such wound healing capacity may be due to fibrin matrix that contains the many growth factors and cytokines.
The aim of this systematic review is to provide a comprehensive overview of systematic reviews and meta-analyses on the intra-oral applications of platelet concentrates.
Materials and Methods
Focused question
What are the intra-oral applications of platelet concentrates?
Study design
A systematic search was conducted using PUBMED, EMBASE, Web of Science, Cochrane library, and Google Scholar for systematic reviews and meta-analyses of intra-oral applications of platelet concentrates published from inception until Dec 2016. The keywords used for the search were described in Table 1 . Gray literature was searched on Google Scholar using the advance search option to find articles with the word “platelet” and at least one of the words “systematic review” or “meta-analysis”. Hand searching was also done on the reference list of the selected systematic review.
| Database | Keywords |
|---|---|
| Pubmed | (“platelet concentrates”[tw] OR “platelet concentrate”[tw] OR “platelet rich fibrin”[tw] OR “platelet rich plasma”[tiab] OR “platelet*”[tw] OR “platelet-rich plasma”[MESH] OR “Blood Platelets”[MESH] OR “blood platelet”[tiab] OR “blood platelets”[tiab] OR thrombocyte*[tw] OR PRP[tw] OR PRF[tw] OR “second generation platelet concentrate”[tw] OR “autologous platelet gel”[tw] OR regeneration*[tw] OR “guided tissue regeneration, periodontal”[MESH] OR “guided tissue regeneration”[tiab]) AND ("systematic review"[tiab] OR "meta-analysis"[tiab] OR Review[pt]) AND ("oral*"[tw] OR intraoral*[tw] OR "periodont*"[tw]) AND ("soft tissue"[tw] OR “hard tissue”[tw] OR tissue*[tiab]) AND ("humans"[MESH]) |
| Web of Science | TOPIC: (platelet) AND TOPIC: (systematic) AND TOPIC: (human) NOT TOPIC: (animal) |
| Cochrane Library | Platelet |
| Google Scholar | With all of the words: platelet With the at least one of the words: systematic, meta |
Table 1: Database and keywords.
Inclusion and exclusion criteria
• Study must identify itself as a systematic review or meta-analysis.
• Study must review intra-oral applications of platelet concentrates in humans.
• Studies which included animal and human studies, but reported the human data separately were included.
• Must review at least 5 studies on platelet concentrates.
• Due to language limitations, only studies in English or have English translation.
• When other interventions are reviewed together with the use of platelet concentrates, only the data relevant to the use of platelet concentrates will be included.
• Based on AMSTAR checklist [14], only reviews scoring > 3 points out of 11 were included.
• In vitro studies, case reports, review articles that are not systematic reviews were excluded.
• Studies which included the use of other non-platelet derived biologics or recombinant platelet derived biologics in addition to the platelet concentrates were excluded.
Screening and data extraction
The “Title and Abstract” was screened by 2 reviewers (NST and MT) independently, articles that clearly did not meet the inclusion criteria were excluded. The full-text was then analyzed independently by 2 reviewers (NST and RK). A previously pilot tested data extraction sheet was used independently by 2 reviewers (RK, MT) for data extraction data. All disagreements were resolved via discussion with another reviewer (SMB).
Assessment of quality of systematic reviews and metaanalyses
The methodologic quality of the selected reviews was rated independently by 2 reviewers (NST and RK) using the AMSTAR checklist [14]. Studies were rated to be of high quality if scoring 8-11 out of a total of 11 points, of moderate quality if scoring 4-7 out of 11 points, or of low quality if scoring 0-3 out of 11 [15]. The AMSTAR ratings were verified by a third reviewer (MT) and any discrepancy was resolved via discussion with another reviewer (JBS). Studies scoring less than 3 out of 11 were excluded. Table 2 showed the selected reviews and their AMSTAR ratings.
| Study | Focused question/ Aims | Uses of platelet concentrates | AMSTAR Rating |
|---|---|---|---|
| Arora et al. [16] | To determine: 1) If PRP with bone and/or bone substitutes leads to more rapid and effective bone regeneration clinically, radiographically, and histologically with sinus augmentation procedures 2) Is there any clinical data parallel to animal experiments providing clinical evidence in sinus augmentation procedures? |
Sinus augmentation | High |
| Bae et al. [17] | To investigate if there were any positive effects of PRP on a sinus bone graft when used in conjunction with bone graft materials on bone regeneration | Sinus augmentation Implant therapy | High |
| Bastami and Khojasteh [18] | To evaluate the effects of L-PRF on bone regeneration in various human oral and maxillofacial surgeries | Sinus augmentation Ridge preservation Periodontal treatment Endodontic treatment Osteonecrosis treatment |
Moderate |
| Castro et al. [19] | “Does L-PRF promote periodontal wound healing in systemically healthy patients (ASA I) during periodontal surgery compared to traditional techniques?” | Periodontal treatment | High |
| Castro et al. [20] | “Does L-PRF promote regeneration in systemically healthy patients (ASA I) during guided bone regeneration techniques and implant surgery compared to traditional techniques?” | Sinus augmentation Ridge preservation Implant therapy |
High |
| Del Fabbro et al. [21] | To determine if the use of autologous platelet concentrates may improve the healing of extraction sockets | Ridge preservation | High |
| Del Fabbro et al. [22] | To determine whether the use of autologous platelet concentrates may affect the outcome of regenerative procedures for the treatment of periodontal defects and gingival recession | Periodontal treatment | High |
| Del Fabbro et al. [23] | 1) To determine if the use of autogenous platelet-derived growth factors may affect the survival rate of implants placed in the grafted maxillary sinus 2) To determine if a correlation between graft quality (based on histomorphometric data) and clinical outcome (based on implant survival) could be established |
Sinus augmentation | Moderate |
| Del Fabbro et al. [24] | “In patients undergoing tooth extraction, does the local application of autologous platelet concentrate improve clinical, radiographic and histological outcomes related to socket healing as compared to control?” | Ridge preservation | High |
| Del Fabbro et al. [25] | 1) To evaluate if the use of autologous platelet concentrates in patients under bisphosphonate treatment may be advantageous for: (a) improving the success of the surgical treatment of BRONJ; (b) reducing the incidence of BRONJ after oral surgery procedures 2) To evaluate if platelet concentrates may prove beneficial for: (a) improving healing of bone and soft tissue at the surgical site; (b) reducing the incidence of any other postsurgical complication and side-effect; (c) improving patients’ quality of life (by reducing pain, swelling and other common symptoms) in the postsurgical period |
Osteonecrosis treatment | High |
| Hou et al. [26] | To evaluate the efficacy of PRP in the surgical treatment of periodontal intrabony defects by comparing clinical outcomes between patients who received PRP as an adjunct to periodontal intrabony defect therapy and those who did not | Periodontal treatment | High |
| Kotsovilis et al. [27] | “What is the efficacy, with respect to clinical, radiographical and patient-centred outcomes, of combinations of PRP with other therapeutic bioactive agents/procedures, compared with the efficacy of the same agents/procedures without the adjunctive use of PRP in the therapy of periodontal intraosseous defects in patients with chronic periodontitis and without systemic diseases that could potentially influence the outcome of periodontal therapy?” | Periodontal treatment | High |
| Lemos et al. [28] | To assess bone formation in patients The null hypotheses: (a) The use of PRP in association with grafting has no effect on bone formation; (b) The use of PRP in association with grafting has no effect on implant survival rates |
Sinus augmentation Implant therapy |
High |
| Lopez-Jornet et al. [29] | “Is the application of platelet concentrates to the surgical bed effective in patients at risk for developing MRONJ or for patients already presenting MRONJ?” | Osteonecrosis treatment | High |
| Luo et al. [30] | To evaluate if adjunctive use of platelet concentrates could affect the outcomes of regenerative procedures for the treatment of gingival recession | Periodontal treatment | High |
| Martinez-Zapata et al. [31] | To evaluate the efficacy and safety of PRP in tissue regeneration | Periodontal treatment | High |
| Meschi et al. [32] | “What is the impact of APCs on the healing of soft and hard tissue and what are the possible adverse events in endodontic therapy using APCs when compared to traditional endodontic therapy?” | Endodontic treatment | High |
| Panda et al. [33] | 1) “What is the adjunctive effect of APCs over open flap debridement (OFD) in the treatment of periodontal intraosseous defects?” 2) “What is the adjunctive effect of APCs in the treatment of periodontal intraosseous defects when using graft materials?” 3) “What is the adjunctive effect of APCs in the treatment of periodontal intraosseous defects when using a combined graft/GTR protocol?” |
Periodontal treatment | High |
| Plachokova et al. [34] | To determine and to analyze structurally the reported effects of PRP on bone regeneration in humans | Sinus augmentation Periodontal treatment |
High |
| Pocaterra et al. [35] | To assess the effectiveness of PRP as an adjunctive material in the sinus floor elevation technique | Sinus augmentation | High |
| Rosello-camps et al. [36] | “Does PRP has a higher or similar efficacy in regenerating periodontal intrabony defects compared with other conventional periodontal regeneration treatments (e.g., bone grafts, barrier membranes)?” | Periodontal treatment | High |
| Schliephake [37] | To evaluate the clinical use and the efficacy of growth factors in different reconstructive procedures in the oral maxillofacial area | Periodontal treatment Sinus augmentation |
Moderate |
| Shah et al. [38] | To determine the clinical and radiographic outcomes of using platelet-rich fibrin (PRF) for the treatment of periodontal intra bony defects (IBDs) compared to open flap debridement (OFD) | Periodontal treatment | High |
Table 2: Characteristics of selected reviews on intraoral uses of platelet concentrates.
Results
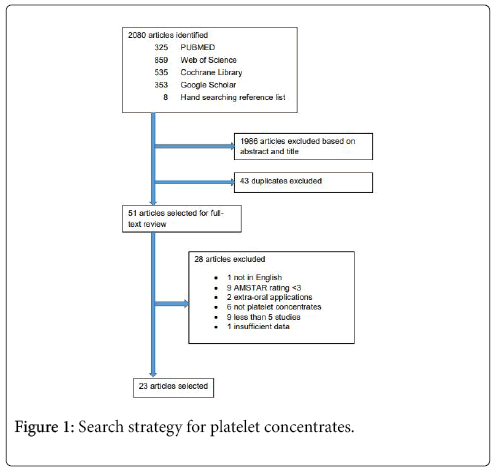
The initial search yielded 325 reviews in PUBMED, 859 in Web of Science, 535 in Cochrane Library, and 353 in Google Scholar. After abstract and title screening, 26 reviews were selected from PUBMED, 27 from Web of Science, 12 from the Cochrane Library, 34 from Google Scholar, and 8 from hand searching the reference list of the selected reviews. After duplicate elimination, a total of 51 reviews remained for full-text analysis. After full-text analysis and the elimination of 28 reviews, 23 were selected for data extraction (Figure 1).
Of the 23 articles selected (Table 2), some articles reported on the use of platelet concentrates in more than one area. A total of 9 articles reviewed platelet concentrates used in sinus augmentation [16-24], 4 articles in ridge preservation [22-26], 4 articles in implant therapy [17,20,28,35], 13 articles in periodontal treatment [17,21-23,27-35], 2 articles in endodontic treatment [23,36], and 3 articles in osteonecrosis treatment [23,37,38]. AMSTAR ratings of the selected reviews comprised of 20 high quality reviews and 3 moderate quality reviews.
Of the 9 reviews selected on the use of platelet concentrates in sinus augmentation (Table 3), 5 reported reduced healing time of soft and hard tissue [16,18,19,22,23], 1 reported improved handling of particulate graft [16], 3 reported no significant benefit [16,17,21], and 2 reported no significant difference in new bone formation between test and control groups [23,24].
| Study | Inclusion and exclusion criteria/ Quality assessment | Type/ Preparation of platelet concentrate | Results/ Conclusion |
|---|---|---|---|
| Arora et al. [16] | Inclusion: - published/unpublished studies and randomized controlled trials or clinical controlled trials - patients with edentulous posterior maxilla who require sinus augmentation - PRP with bone or bone substitutes Exclusion: - studies that did not involve randomization, a control group, included PRP alone as an intervention or were not clear in the outcome variables were excluded Quality: - no assessments were made |
PRP | - PRP can lead to early regeneration and reduction in healing time of soft and hard tissues - no significant statistical or clinical benefit was reported - no human study strongly supports the benefit of using PRP in sinus augmentation - no obvious positive effects of PRP on healing of bone graft material in maxillary sinus augmentation - PRP improved the handling of the particulate bone grafts |
| Bae et al. [18] | Inclusion: - only controlled clinical trials that had intervention and control groups in humans that reported the effects of PRP on sinus bone grafts for dental implants - >8 sinus bone grafts - mean follow-up was >12 months of implant placement for implant survival and >3 months of sinus bone graft for bone formation and bone-to-implant contact - the data of implant survival, bone formation, and bone-to-implant contact in the article were clearly indicated and sufficient for a meta-analysis. - only articles written in English Exclusion: - case reports, case series, editorials, in vitro studies, animal experiments, and review papers Quality: - Cochrane Collaboration’s tool for assessing risk of bias[39] |
PRP | - bone formation was significantly greater in the intervention group treated with PRP compared to the control group based on the random-effects model - PRP reduced the healing time of sinus lifting and increased the early stage bone formation |
| Bastami and Khojasteh [23] | Inclusion: - English papers - at least 4 weeks follow-up - studies reporting clinical, radiographic and histological findings Quality: - no assessments were made |
L-PRF | - L-PRF was shown to be effectively used to accelerate and enhance new bone formation - no statistically significant new bone formation between test and control groups |
| Castro et al. [22] | Inclusion: - systemically healthy humans (ASA I) with loss of periodontal tissues - use of L-PRF as sole biomaterial or in combination to other biomaterials in periodontal surgery - open flap debridement with or without grafting, periodontal plastic surgery via coronally advanced flap, with or without connective tissue graft Quality: - Cochrane Collaboration’s tool for assessing risk of bias [39] - mostly moderate risk of bias |
L-PRF (L-PRF protocol 2700 r.p.m./12 min. or 3000 r.p.m./10 min.) |
- when L-PRF was added to a bone substitute during sinus floor elevation bone healing occurred faster, with 1.4 times more new -formed bone in the L-PRF group - when L-PRF was used in a trans-alveolar approach, histologically a faster bone healing was observed |
| del Fabbro et al. [19] | Inclusion: - randomized controlled trials and case control trials assessing the efficacy of platelet concentrates on sinus augmentation procedures - clearly reported implant survival or histomorphometric results - the mean follow-up for implant survival was more than 6 months after placement Exclusion: - case series, case reports, technical studies, animal studies, and reviews Quality: - none mentioned |
PRP PRF Plasma rich in growth factors (PRGF) | - no consistent data if the possible advantage of platelet concentrates in the early phases of graft healing is also reflected in a better treatment outcome in the medium long term - no clear advantage of platelet concentrates |
| Lemos et al. [20] | Inclusion: - randomized controlled trial or prospective study; articles published in the English language xclusion: - in vitro studies, animal studies, reviews, retrospectives studies, and studies evaluating the association of PRP but without a comparison between graft only and graft with PRP Quality: - PRISMA criteria, five-point Jadad scale |
PRP | - no significant difference in implant stability, marginal bone, alveolar bone height, implant survival, or bone formation - the meta-analysis indicates no influence of PRP with bone graft on bone formation and implant survival in maxillary sinus augmentation |
| Plachokovaet al. [21] | Inclusion: - studies reporting treatment outcomes of PRP and controls on bone healing - studies with at least five patients - follow-up of at least 3 months - only papers written in English Quality: - the quality assessment items were derived from two guidelines for systematic reviews by Clarke and Oxman, and Montenegro et al. [40] - majority do not meet current recommendations on study quality related to sample size calculation, randomization methods, allocation concealment, examiner blinding, validity of statistical methods as well as validity of outcomes and estimation |
PRP | - no significant difference in the treatment outcome was observed between sinus elevation procedures with and without PRP |
| Pocaterra et al. [24] | Inclusion: - only randomized controlled clinical trials comparing PRP as an adjunctive material to a control group without PRP - adult human subjects (age >18 years) with no systemic disease Quality: - CONSORT based quality assessment |
PRP | - of the studies identified, only one reported a significant difference in bone augmentation in favorof the adjunctive use of PRP, while four studies did not find any significant difference - none of the studies included reported a significant difference in the implant survival rate |
| Schliephake [17] | Inclusion: - studies that reported the clinical use of autogenous and human recombinant growth factors in the oral maxillofacial and craniofacial area Exclusion: - studies on the use of animal-derived growth factor extracts Quality: - not reported |
PRP | - no benefit for the final outcome could be shown for the use of PCs neither in sinus lift procedures nor in lateral / vertical crest augmentations - clinical efficacy of growth factors in reconstructive procedures in the oral and maxillofacial area is limited |
Table 3: Platelet concentrates used in sinus augmentation.
Of the 4 reviews selected on the use of platelet concentrates in ridge preservation (Table 4), 2 reported less post-extraction pain [22,25], 2 reported improved alveolar ridge preservation [22,23], 1 reported better soft tissue healing and less buccal bone resorption [22], 2 reported insignificant acceleration of osseous healing [23,25], and 1 reported limited histological evidence of better bone quality [26].
| Study | Inclusion and exclusion criteria/ Quality assessment | Type/ Preparation of platelet concentrate | Results/ Conclusion |
|---|---|---|---|
| Bastami and Khojasteh [23] | Inclusion: - English papers - at least 4 weeks follow-up - studies reporting clinical, radiographic and histological findings Quality: - no assessments were made |
L-PRF | -significant effect on intrinsic bone quality - bone volume/ total volume (BV/TV) ratio of L-PRF group at 8 weeks was not significantly greater than L-PRF with mucosal flap or control - when L-PRF was mixed with cortico-cancellousporcine bone and collagen membrane, bone level significantly improved after five months in the distal sites |
| Castro et al. [22] | Inclusion: - systemically healthy humans (ASA I) with loss of periodontal tissues - use of L-PRF as sole biomaterial or in combination to other biomaterials in periodontal surgery - open flap debridement with or without grafting, periodontal plastic surgery via coronally advanced flap, with or without connective tissue graft Quality: - Cochrane Collaboration’s tool for assessing risk of bias [39] - mostly moderate risk of bias |
L-PRF (L-PRF protocol 2700 r.p.m./12 min. or 3000 r.p.m./10 min.) |
- L-PRF improved the preservation of the alveolar ridge and resulted in less buccal bone resorption compared to the natural healing - better soft tissue healing and less post-extraction pain was frequently reported - scintigraphic analyses after 4 and 8 weeks did not show enhanced bone healing in L-PRF sites - adverse events were reported in four out of the 8 articles - less pain for L-PRF sites compared to control sites was reported in all studies reviewed |
| del Fabbro et al. [25] | Inclusion: - randomized clinical trials and controlled clinical trials assessing the efficacy of platelet concentrates for healing in patients undergoing dental extractions - no limitation on the number of patients treated or the follow-up duration - studies were included only if a test group using platelet concentrates was compared with a control group in which platelet concentrates were not used - platelet concentrates could be used alone or in combination with other materials but they had to be the only difference between test and control Exclusion: - case series, case reports, technical reports, animal studies and reviews Quality: - randomization method: low, medium and high risk of bias |
PRGF PRP | - use of platelet concentrates may reduce postoperative pain and inflammation - no systematic acceleration of osseous healing at the post -extraction site |
| del Fabbro et al. [26] | Inclusion: - randomized controlled trials and split-mouth design assessing the efficacy of platelet concentrates for healing and regeneration of hard tissues in patients undergoing tooth extraction - studies were included only if a test group using platelet concentrates was compared with a control group in which platelet concentrates were not used - platelet concentrates could be used alone or in combination with other materials, but had to be the only difference between test and control - any type of platelet concentrates - at least 10 patients or at least 5 patients per group - studies with follow-up duration of at least 3 months Quality: - Cochrane risk of bias assessment |
PRP PRGF | - a positive effect of platelet concentrates in accelerating bone healing is only suggested but could not be clearly demonstrated by radiographic assessment - limited histological and histomorphometric evidence of better bone quality or greater bone formation in the first 3 months after tooth extraction - no clear evidence of the beneficial effect of platelet concentrates on soft tissue healing after tooth extraction - there is limited evidence that the use of platelet concentrates is associated with the reduction of patients’ pain perception in the first postoperative week |
Table 4: Platelet concentrates used in ridge preservation.
Of the 13 reviews selected on the use of platelet concentrates in periodontal treatment (Table 5), 2 reported more improved periodontal parameters at follow-up than at baseline with significantly greater probing depth reduction and clinical attachment gain [23,34], and 4 reported some beneficial effects on clinical and radiographic outcomes for regeneration of periodontal intrabony defects [17,29,31,33]. However, when platelet concentrates were combined with guided tissue regeneration (GTR) techniques the beneficial effects are negligible [29,31]. Thus, the specific selection of specific agents or procedures combined with platelet concentrates could influence outcome [32]. In the treatment of gingival recession, platelet concentrates produced no significant benefit [28]. However, in chronic periodontitis, platelet concentrates improved gingival recession but not the clinical attachment [30].
| Study | Inclusion and exclusion criteria/ Quality assessment | Type/ Preparation of platelet concentrate | Results/ Conclusion |
|---|---|---|---|
| Bastami and Khojasteh [23] | Inclusion: - English papers - at least 4 weeks follow-up - studies reporting clinical, radiographic and histological findings Quality: - no assessments were made |
L-PRF | - periodontal parameters significantly improved in follow -ups than base -line evaluations - probing depth reduction and clinical attachment gain were significantly greater in test than in control groups |
| Castro et al. [27] | Inclusion: - randomised controlled clinical trials or controlled clinical trials studies regarding periodontal surgery - L-PRF prepared following selected L-PRF protocol - studies conducted in humans - no limitation in publication date nor in follow-up duration and only English Exclusion: - other applications of L-PRF in Medicine (Traumatology, Ophthalmology, Dermatology, etc.) or in Dentistry (Endodontics) - animal studies, in vitro studies - other types of platelet concentrates (fibrin glues, PRP, PRGF, A-PRF, I-PRF) - studies regarding bone augmentation procedures, ridge preservation or implant surgery - case reports, case series, retrospective studies Quality: - Cochrane Collaboration’s tool for assessing risk of bias. |
L-PRF (L-PRF protocol 2700 rpm/12 min or 3000 rpm/10 min) | - significant probing depth reduction (PD), clinical attachment level (CAL) gain and bone fill were found when comparing L-PRF to open flap debridement (OFD) in intra-bony defects (IBDs) - for furcation defects, significant PD reduction, CAL gain and bone fill were reported when comparing L-PRF to open flap debridement (OFD) - when L-PRF was compared to a connective tissue graft, similar outcomes were recorded for PD reduction, CAL gain, keratinized tissue width and recession reduction - concluded that L-PRF enhanced periodontal wound healing |
| Castro et al. [22] | Inclusion: - randomised controlled clinical trials or controlled clinical trials - studies regarding periodontal surgery: intra-bony defects, furcation defects and periodontal plastic surgery - L-PRF prepared following a selected protocol - studies conducted in humans - no limitation in publication date nor in follow-up duration and only English Exclusion: - other applications of L-PRF in Medicine (Traumatology, Ophthalmology, Dermatology, etc.) or in Dentistry (Endodontics) - animal studies, in vitro studies other types of platelet concentrates (fibrin glues, PRP, PRGF, A-PRF, I-PRF…) - studies regarding bone augmentation procedures, ridge preservation or implant surgery - case reports, case series, retrospective studies Quality: - Cochrane Collaboration’s tool for assessing risk of bias |
L-PRF (L-PRF protocol: 2700 rpm/12 min or 3000 rpm/10 min) | - L-PRF might have a positive effect on bone regeneration and osseointegration, evidence not strong - better implant stability over time and less marginal bone loss were reported after the use of L-PRF in implant therapy - reported more probing depth reduction at 3 and 6 months, and clinical attachment level gain |
| del Fabbro et al. [28] | Inclusion: - randomized controlled trials assessing the efficacy of platelet concentrates for healing and regeneration of hard and soft tissues in patients undergoing surgical procedures for the treatment of periodontal defects and gingival recession - no limitation was placed regarding the number of patients treated - studies were only included if a test group using platelet concentrates was compared to a control group in which platelet concentrates were not used Exclusion: - case series, case reports, retrospective studies, technical studies, animal studies, and reviews - maxillary sinus augmentation procedure - articles reporting on any other oral surgical intervention like tooth extraction, inlay and onlay grafts for the treatment of jawbone defects, treatment of odontogenic cysts, and periapical surgery Quality: - guidelines reported in the Cochrane Handbook Forest plots - Funnel plots were also used to assess the publication bias |
PRP | - for the treatment of intra-bony defects, may be advantageously used as an adjunct to graft procedures, but not in combination with guided tissue regeneration - no significant benefit of platelet concentrates was found for gingival recession |
| Hou et al. [29] | Inclusion: - randomized controlled clinical trial in which an intervention group receiving PRP was compared with a control group not receiving PRP - the patients with no systemic illness or abnormal platelet counts that could affect the clinical outcome of periodontal therapy - a follow-up period of at least 6 months Exclusion: - inadequate comparison of the results of PRP for the treatment of periodontal intra-bony defects - PRP administered to both the intervention and control groups - the use of a biologic material that would hamper meaningful comparisons - reviews, case reports, and animal studies Quality: - quality of the selected RCTs was assessed using the Risk of Bias tool according to the Cochrane Handbook for Systematic Reviews of Interventions [39] |
PRP | - adjunctive use of PRP together with conventional grafting procedures may be a beneficial treatment approach - when combined with the use of a regenerative technique, such as GTR, the beneficial effect of PRP on the treatment of intra-bony defects is negligible |
| Kotsovilis et al. [32] | Inclusion: - any language - randomized control trial (RCT), either of a parallel group or of a split-mouth design - chronic periodontitis of any extent and severity - patients with no systemic diseases that could affect periodontal therapy - the presence of at least one experimental group, in which PRP was clinically applied as an adjunct to other therapeutic bioactive agents/procedures for the therapy of periodontal intraosseous defects - appropriate non-PRP control group - follow-up period of at least 6 months Exclusion: - mixed RCT design - use of historical control group - history of periodontal therapy within the preceding 12 months or less - periodontal intraosseous defect(s) extending into furcation area(s) or located around teeth presenting furcation involvement(s) - patients receiving any medication reported to interfere with wound healing, cause gingival overgrowth or known to affect the number or function of platelets over a period of 3 months or less prior to the baseline of the RCT - patients with abnormal platelet counts - patients receiving antibiotics at the baseline of the RCT and/or during the previous 3 months or less - history of radiotherapy in the head and neck region of the patients - teeth presenting endodontic problems at the baseline of the RCT - presence of an aggressive periodontitis Quality: - different criterias for overall estimation of plausible risk of bias |
PRP | - the clinical use of PRP is an entirely safe procedure, causing no adverse events or postoperative complications - diverse outcomes have been reported for the efficacy of PRP combined with various therapeutic bioactive agents/procedures, reflecting the limited and heterogeneous data available |
| Luo et al. [35] | Inclusion: - only randomized controlled trials with a follow-up ≥3 months were included - patients diagnosed as localized or multiple recession-type defects and only gingival defects sites classified as Miller Class I or II - platelet concentrates used in certain regenerative procedures of gingival recessions Exclusion: - studies in which the patients had previous surgical treatment to correct the gingival recession - studies involving teeth with intrabony defects - studies that failed to provide the mean value and standard deviation Quality: - assessed with guidelines in the Cochrane Handbook [39] |
PRP PRF Platelet derived growth factors (PDGF) | - platelet concentrates might exert a positive adjunctive effect in the treatment of gingival recession and also wound healing - results highlight the potential benefits of platelet concentrates as a supportive intervention in the treatment of gingival defects |
| Martinez-Zapata et al. [30] | Inclusion: - randomized controlled trials (RCTs) that assess the efficacy and/or safety of PRP for healing and regeneration of hard and soft tissues in all surgical procedures - split-mouth-design RCTs - crossover studies were also included when results regarding the first treatment period were reported Quality: - Jadad scale used to assess quality |
PRP Plasma rich in growth factors (PRGF) | - PRP improves the gingival recession but not the clinical attachment level in chronic periodontitis. |
| Panda et al. [31] | Inclusion: - clinical trials, either of a parallel group or of a split-mouth design - presence of at least one experimental group in which APCs were clinically applied as an adjunct to surgical procedures alone or in combination with bone grafting materials or guided tissue regeneration (GTR) procedures - presence of an appropriate non-APC control group - patients with intra-bony defects, no systemic diseases that could potentially influence the outcome of periodontal therapy - articles having follow-up period of at least 9 months Exclusion: - periodontal intra-bony defects extending into furcation areas of teeth - intra-bony defects extending apically with endodontic involvements Quality: - methodological quality assessment |
Autologous platelet concentrates (APCs) | - platelet concentrates may be advantageously used as a cost -effective adjunct to surgical regenerative therapy, even in combination with bone grafts, although they did not show any advantage when used together with GTR - platelet-rich fibrin proved to be effective as a sole regenerative material for treatment of intra-bony defects, in combination with open flap debridement (OFD) |
| Plachokova et al. [21] | Inclusion: - studies reporting treatment outcomes of PRP and controls on bone healing - studies with at least five patients - follow-up of at least 3 months - only papers written in English Quality: - the quality asssement items were derived from two guidelines for systematic reviews by Clarke and Oxman, and Montenegro et al. [40] - majority do not meet current recommendations on study quality related to sample size calculation, randomization methods, allocation concealment, examiner blinding, validity of statistical methods as well as validity of outcomes and estimation |
PRP | - more studies reported PRP treatments of periodontal defects were significantly more effective compared with their controls |
| Rosello-camps et al. [33] | Inclusion: - prospective clinical trials with 10 or more human patients, reporting the radiographic and clinical outcomes of PRP for regeneration of periodontal defects Exclusion: - case reports or case series with less than 10 patients, systematic reviews, preclinical studies, and human retrospective trials or randomized trials with missing information Quality: - assessed using a modification of the randomized clinical trial checklist of the Cochrane Center and the CONSORT (Consolidated Standards of Reporting Trials) statement |
PRP | - high heterogeneity among studies made it difficult to draw clear conclusions - PRP might offer some beneficial effects on clinical and radiographic outcomes for regeneration of periodontal intra-bony defects |
| Schliephake [17] | Inclusion: - studies that reported the clinical use of autogenous and human recombinant growth factors in the oral maxillofacial and craniofacial area Exclusion: - studies on the use of animal-derived growth factor extracts Quality: - not reported |
PRP | - the use of autogenous growth factors in platelet concentrates (PCs) has shown to be beneficial in the treatment of intra-bony pockets at a reasonable level of evidence by improving probing depth and clinical attachment levels as well as linear bone fill within the limits of the observation periods - the application in conjunction with non-autogenous graft materials has been superior with the use of PCs - no benefits have been shown for the use of PCs in recession treatment - when used in furcation treatment, probing depth, clinical attachment level and linear bone fill have been reported to improve significantly, but without clinical benefit |
| Shah et al. [34] | Inclusion: - studies investigating the effect of PRF in the treatment of periodontal intraosseous defects - studies having a test group using PRF alone and a control group with open flap debridement (OFD) alone - no limitations were placed regarding the number of patients treated but follow-up of minimum 6 months was required - only chronic periodontitis patients Exclusion: - study designs such as case series, case reports, retrospective studies, technical studies, animal studies and reviews Quality: - internal quality of trials was assessed based on the Cochrane collaborations’ tool for assessment of bias, but no study was excluded for its risk of bias. - all criteria were judged as adequate, unclear, or not adequate |
PRF | - statistically significant difference for intra-bony defects (IBD) but not for CAL was reported - the meta-analysis result for gain in CAL was found to be 0.95 mm - the difference between PRF with PRP was non-significant for CAL as well as IBD - the meta-analysis showed clinically significant improvements in the periodontal parameters like CAL, IBD and reduction in PD when IBDs were treated with PRF alone compared to OFD |
Table 5: Platelet concentrates used in periodontal treatment.
Of the 4 reviews selected on the use of platelet concentrates in implant therapy (Table 6), 1 reported significantly higher implant stability quotients (ISQ values) measured through resonance frequency analysis [22], 1 reported no significant effect on the bone-to-implant contact [18], and 3 reported no significant effect on implant survival [18,20,24].
| Study | Inclusion and exclusion criteria/ Quality assessment | Type/ Preparation of platelet concentrate | Results/ Conclusion |
|---|---|---|---|
| Bae et al. [18] | Inclusion: - only controlled clinical trials that had intervention and control groups in humans that reported the effects of PRP on sinus bone grafts for dental implants - >8 sinus bone grafts - mean follow-up was > 12 months of implant placement for implant survival and > 3 months of sinus bone graft for bone formation and bone-to-implant contact - the data of implant survival, bone formation, and bone-to-implant contact in the article were clearly indicated and sufficient for a meta-analysis. - only articles written in English Exclusion: - case reports, case series, editorials, in vitro studies, animal experiments, and review papers Quality: - Cochrane Collaboration’s tool for assessing risk of bias [39] |
PRP | - the meta-analysis of the three selected studies on implant-based implant survival reported that implant survival was not significantly different in the test group - bone-to-implant contact was not significantly different in the PRP group compared to the control group - no significant effect on the implant survival and bone-to-implant contact |
| Castro et al. [22] | Inclusion: - systemically healthy humans (ASA I) with loss of periodontal tissues - use of L-PRF as sole biomaterial or in combination to other biomaterials in periodontal surgery - open flap debridement with or without grafting, periodontal plastic surgery via coronally advanced flap, with or without connective tissue graft Quality: - Cochrane Collaboration’s tool for assessing risk of bias - mostly moderate risk of bias [39] |
L-PRF (L-PRF protocol 2700 rpm/12 min. or 3000 rpm/10 min.) |
- significantlyhigher implant stability quotients (ISQ values) measured through resonance frequency analysis |
| Lemos et al. [20] | Inclusion: - randomized controlled trial or prospective study; articles published in the English language Exclusion: - in vitro studies, animal studies, reviews, retrospectives studies, and studies evaluating the association of PRP but without a comparison between graft only and graft with PRP Quality: - PRISMA criteria, five-point Jadad scale |
PRP | - no significant effect on implant survival |
| Pocaterra et al. [24] | Inclusion: - only randomized controlled clinical trials comparing PRP as an adjunctive material to a control group without PRP - adult human subjects (age > 18 years) with no systemic disease Quality: - CONSORT based quality assessment |
PRP | - no significant effect on implant survival |
Table 6: Platelet concentrates used in implant therapy.
Of the 2 reviews selected on the use of platelet concentrates in endodontic treatment (Table 7), both reported complete bone regeneration of periapical lesions [23,36], and 1 reported faster healing of peri-apical lesions [36].
| Study | Inclusion and exclusion criteria/ Quality assessment | Type/ Preparation of platelet concentrate | Results/ Conclusion |
|---|---|---|---|
| Bastami and Khojasteh [23] | Inclusion: - English papers - studies with at least 4-week follow-up - studies reporting clinical, radiographic and histological findings Quality: - no assessments were made |
L-PRF | - L-PRF showed appropriate outcomes in the treatment of peri-apical and endo-periodontal defects - complete bone regeneration of periapical lesions after 6 months |
| Meschi et al. [36] | Inclusion: - Dutch and English - all types of clinical trials (interventional and observational), case series, and case reports - all types of endodontic treatments involving the application of autologous platelet concentrates (APCs) - only teeth are included Exclusion: - studies including patients with bleeding disorders, malignancy and history of chemotherapy, metabolic bone diseases, and radiation in the head and neck region - reviews/systematic reviews/meta-analysis - studies involving the dermatologic application of APCs - animal studies, in vitro studies Quality: - quality assessment with the Cochrane Collaboration’s risk-of-bias assessment tool [39] - four highly biased studies and one study with an unclear risk of bias - only two RCTs presented low risk of bias in all domains |
APCs | - sparse evidence that the addition of APCs to endodontic treatment modalities might accelerate postoperative bone healing, improve the patients’ quality of life in the early postoperative period, aid further root development, and support maintenance or regaining of pulp vitality |
Table 7: Platelet concentrates used in endodontic treatment.
Of the 3 reviews selected on the use of platelet concentrates in osteonecrosis treatment (Table 8), 2 reported complete bone closure and resolution of lesion [23,38], and 1 reported a beneficial effect for preventing the post-surgical occurrence or recurrence of bisphosphonate related osteonecrosis of the jaw [37].
| Study | Inclusion and exclusion criteria/ Quality assessment | Type/ Preparation of platelet concentrate | Results/ Conclusion |
|---|---|---|---|
| Bastami and Khojasteh [23] | Inclusion: - English papers - at least 4 weeks follow-up - studies reporting clinical, radiographic and histological findings Quality: - no assessments were made |
L-PRF | - total bone closure was observed in majority of studies |
| del Fabbro et al. [37] | Inclusion: - studies that report clinical results of oral surgery procedures in patients under bisphosphonate therapy, in which autologous platelet concentrate was used - articles reporting on the treatment of an existing condition of bisphosphonate related osteonecrosis of the jaw (BRONJ), and studies reporting on the incidence/onset of BRONJ in patients undergoing oral surgery procedures - limited to clinical studies involving humans - restrictions were not placed regarding language - prospective and retrospective studies - no limitation on sample size - studies had to provide details on the type of bisphosphonate taken, the indication for bisphosphonate therapy and the duration of the treatment at the time of surgery - studies also had to provide clear definitions of the clinical outcomes for considering success or failure of the procedure, and details on the type of platelet concentrate used Exclusion: - studies not dealing with original clinical cases (e.g. reviews, technical reports) - multiple publications of the same pool of patients Quality: - methodological quality of the selected studies was evaluated |
Autologous Platelet Concentrates (APCs) | - suggestive of beneficial effect for preventing the postsurgical occurrence or recurrence of bisphosphonate related osteonecrosis of the jaw (BRONJ) |
| Lopez-Jornet et al. [38] | Inclusion: - diagnosis of BRONJ in accordance with American Society of Bone and Mineral Research (ASBMR) definitions - original studies - clinical studies and case reports with at least five cases treated with APCs - studies conducted in humans - studies in English. - review articles, systematic reviews, prospective clinical trials, nonrandomized and randomized studies, as well as observational studies Exclusion: - in vitro studies - experimental animal studies - letters, editorials, doctoral theses, or abstracts Quality: - assessed with NewcastleOttawa scale (NOS) |
PRP Plasma rich in growth factors (PRGF) L-PRF | - no published scientific data to sufficiently support any specific treatment protocol, including the use of APCs together with surgical debridement, for the management of MRONJ (medication related osteonecrosis of the jaw) - most studies report complete resolution of lesion |
Table 8: Platelet concentrates used in osteonecrosis treatment.
The selected systematic reviews for each application (Table 9) were further categorized into the type of platelet concentrates (PCs) evaluated. Commonly evaluated forms of PCs included PRP, L-PRF, and unspecified compositions of PCs. The specific significant benefits of each form of PCs, as well as nonsignificant applications were outlined in Table 9. In sinus augmentation, more benefits of PRP [16,18], L-PRF [22,23], and PCs [19] were reported in the early postoperative. But in the longer term follow-up, there were no significant clinical benefits [16,17,20,21,23,24]. In ridge preservation, the use of LPRF [22] and PCs [25,26] contributed to immediate post-operative benefits. The use of L-PRF [22,23] can contribute to significant increase in bone quality and bone level, but it was not significant with respect to bone volume [23].
| Platelet concentrates used in sinus augmentation | |
|---|---|
| PRP | Early post-operative Decreased healing time [16,18] Improved handling of particulate bone [16] Significantly increased early stage bone formation [18] Follow-up No significant clinical benefits [16,17,20,21,24] |
| L-PRF | Early post-operative Increased speed and quality of bone formation [22,23] Improved bone healing [22,23] Follow-up No significant bone formation [23] |
| PCs | Benefits for early phases of graft healing [19] Inconclusive benefits for long-term follow-up [19] |
| Platelet concentrates used in ridge preservation | |
| L-PRF | Significant increase in intrinsic bone quality [23] Significant bone level improvement when used with biomaterials [22,23] Less post-operative pain reported [22] Improved soft tissue healing [22] No significant increase in bone volume [23] |
| PCs | Decreased post-operative pain and inflammation [25,26] Some evidence of accelerated bone healing [26] No significant clinical effect [25] |
| Platelet concentrates used in periodontal treatment | |
| PRP | Beneficial effects reported [17,21,28,29,33] No beneficial effects when used with guided tissue regeneration techniques [28,29] When used in gingival recession, conflicting results, some report no significant effect [17,28] and others report improvements [30] |
| L-PRF | When used in surgical management of periodontitis, there is significant decrease in probing depth, significant gain in clinical attachment, and significant increase in bone fill [23,27,34] When use in furcation involved teeth, there is also significant decrease in probing depth, significant gain in clinical attachment, and significant increase in bone fill [27] When used in gingival recession, there is no significant effect [27] |
| PCs | Positive adjunctive effect in the treatment of gingival recession [35] No beneficial effects when used with guided tissue regeneration techniques [31] |
| Platelet concentrates used in implant therapy | |
| PRP | No significant effect on implant survival [18,20,24] No significant effect on bone-to-implant contact [18] |
| L-PRF | Significantly higher implant stability quotient (ISQ values) [22] |
| Platelet concentrates used in endodontic treatment | |
| L-PRF | Bone fill in peri-apical defects [23] |
| PCs | Sparse evidence [36] |
| Platelet concentrates used in osteonecrosis treatment | |
| L-PRF PCs | Total bone closure observed in the majority of studies [23,38] |
Table 9: Intraoral applications of different forms of platelet concentrates derived from this overview of the selected systematic reviews.
In periodontal treatment, beneficial effects were reported for PRP [17,21,28,29,33], and L-PRF [22,23,27,34]. Conflicting effects were reported for gingival recession [17,27,28,35]. Interestingly, the beneficial effects of PRP and PCs were negated when used with guided tissue regeneration [27,31]. In implant therapy, the use of PRP did not have any significant effect on the implant survival nor the bone to implant contact [18,20,24]. Although the use of L-PRF reported significantly higher implant stability [22]. In endodontic treatment, there is sparse evidence on the use of PCs. In treatment of osteonecrosis, use of L-PRF and PCs can aid in total bone closure of osteonecrotic lesions [23,38].
Discussion
The goal of this comprehensive overview was to evaluate the available systematic reviews regarding the benefits of using APCs in all specialties of dental medicine on surgical wound healing and tissue regeneration. In many of the systematic studies reviewed, the effectiveness of APCs was compared to conventional treatment. It is hypothesized that APCs with their growth factors modulate wound healing by signals directed to specific target cell that bind to receptors [3,4,6,41,42]. However, the results of this comprehensive overview illustrate the heterogeneity of the different studies using APCs with regards to the study design, sample size, data reporting, surgical technique, graft material, endpoint variables, healing time; and most importantly, the method of preparation of the autologous concentrate and the type of centrifuge used in the study.
The use of APCs in sinus elevation procedures with bone graft augmentation is well recognized, but there is a lack of consistent clinical evidence regarding its healing potential. In this overview, the use of PRP in sinus elevation accelerated the healing and significantly increased early bone formation in the short-term and early postoperative [16,18]. The meta-analysis by Bae et al. [18]. consisting of 8 controlled clinical studies concluded that there were significant positive effects on early bone formation with the use of PRP when used with bone graft materials. This is consistent with PRP studies that showed beneficial effects in the first 3 to 6 months of graft maturation [3,4,6]. In 2000, Rosenberg and Torosian [43] further reported the benefits of PRP on bone quality and bone maturation in sinus grafting. However, studies with longer follow-up reported no significant clinical benefits of PRP in the healed maxillary sinus [16,17,20,21,24]. The systematic study by Arora et al. [16]. Determined that the use of PRP with autogenous bone and other graft substitute materials had no significant or clinical benefit on bone regeneration despite the increase in vertical bone height.
In comparison to PRP, the use of PRF in sinus graft procedures is relatively new. In an animal study using dogs, Xuan et al. [44]. showed that using a L-PRF membrane had a significant positive effect on bone formation in the grafted maxillary sinus. In a systematic review by Bastami and Khojasteh [23], PRF was shown to accelerate and increase new bone formation in humans. Similarly, in several sinus graft studies [45-47] conducted without the use of a graft material, the authors concluded that PRF alone improved bone healing and increased vertical bone height (7.5 mm to 10.4 mm) in the posterior maxilla when dental implants were simultaneously placed. The authors concluded that the use of PRF without bone graft materials may prove to be an alternative procedure that can correct atrophy in the vertical dimension for dental implants in the posterior maxilla [45-47].
On the contrary, a study consisting of 60 patients reported use of PRF with natural bovine graft material resulting in less new bone formation when compared to bovine bone alone [48]. Furthermore, the systematic review by Bastami and Khojasteh [23] could not find convincing evidence for the use of L-PRF on new bone formation. Other studies [11,49,50] also revealed that there were no significant differences when PRF was used alone, or with a bone graft material, to increase the vertical bone height to support dental implants in the posterior maxilla.
The conflicting results of PCs could be due to the cumulation of both shorter term and longer term follow-up results. Therefore, the use of PRP, L-PRF, and PCs for bone regeneration in the sinus required more randomized clinical trials focusing on the early post-operative and shorter term clinical effectiveness of PRP. This overview suggested that the application of PCs in the maxillary sinus can accelerate bone formation and tissue healing; and once the healing is complete, the final bone formed was the same as the control. This may be of significance because faster bone formation and healing may equate to early implant placement and shorter rehabilitation time.
The use of implant supported restorations to replace missing teeth is now becoming the primary treatment modality after tooth extraction. To prevent alveolar dimensional changes in the extraction site that occurs within 8 weeks after tooth extraction, the use of PRP and PRF has been investigated. Hauser et al. [51]. reported that the use of PRF prior to implant surgery reduced alveolar dimensional changes compared to natural wound healing. The use of PRF in extraction sites can improve soft tissue healing [22], improve bone quality [23], and reduce post-operative pain [22]. And when used in conjunction with biomaterials can significantly improve the bone level [22,23], but not the bone volume [23].
Although a systematic review of 8 studies by Del Fabbro et al. [25]. concluded that the use of APCs had a minimal positive effect on postextraction ridge preservation; the authors together with another study [26] also reported decreased post-operative pain and inflammation with PCs. In addition, the other study [26] also reported some evidence of accelerated bone healing.
The use of autologous platelet concentrates for the regeneration of periodontal bony defects has been well documented [31,34,52-54]. Similarly, the systematic reviews in this overview also reported beneficial effects for PRP used in surgical periodontal therapy [17,21,28,29,33]. The primary objective in periodontal treatment is to restore the health and function of the periodontium. Conventional open flap debridement (OFD) is unable to completely regenerate lost hard tissue destroyed from disease. Plachokova et al. [21] demonstrated the beneficial effects of PRP on bone regeneration when used for intra-bony defects. However, these beneficial effects were not significant when guided tissue regeneration techniques were used with PRP [28,29] or PCs [31]. Another systematic review [17], comparing PRP to open flap debridement was shown to have a moderate to high degree of clinical significance in four clinical variables of measurement consisting of pocket depth reduction, clinical attachment level, linear bone fill and gingival margin level.
Other clinical studies confirmed the benefits of PRF when used to manage intra-bony defects compared to conventional OFD [54,55]. The benefits of PRF was especially observed when used with bone grafting [31]. A systematic review and meta-analysis of 24 articles by Castro et al. [27]. compared the use of PRF and open flap debridement (OFD) in the management of intra-bony defects, it reported successful regeneration of osseous tissue. Improvements in probing pocket depth (PPD) reduction and clinical attachment level (CAL) gains were observed post-periodontal therapy. The authors concluded that the addition of L-PRF enhances periodontal wound healing. Overall, the use of L-PRF, in the surgical management of periodontitis and teeth with furcation involvement, can produced a significant decrease in probing depth, a significant gain in clinical attachment, and a significant increase in bone fill [22,23,27].
An explanation for the benefits of all forms of PCs can be attributed to the stimulatory effects of fibroblasts on gain in clinical attachment levels [3]. These findings suggest that the use of PCs may be beneficial in the use of surgical regenerative therapy in the treatment of periodontal defects. But, when evaluating clinical attachment level, the amount of bone formation compared to connective tissue formation cannot be determined. This is because a biopsy specimen of human histology would be required, and this is not possible in human studies where the goal is periodontal regeneration.
Surgical procedures were used to treat gingival recession to create a stable zone of attached gingival tissue. There is increased interest in the use of APCs to cover the exposed root, as growth factors may upregulate cellular activity to facilitate regeneration of periodontal tissue. Root coverage for gingival recession using PRP and the polymerized fibrin matrix of PRF has been reported [35]. Luo et al. [35] conducted a systematic review and their results concluded that the use of APCs may have a positive effect on gingival tissue regeneration, especially with recession depth and clinical attachment level.
Six studies compared the use of coronally advanced flap (CAF) to CAF and PRF. Four studies reported an increase in root coverage [30,56-58], while two other studies found no significant differences. In their review, Del Fabbro et al. [28] and Schliephake [17] concluded that autogenous platelet concentrates do not exert a positive effect on root coverage.
Comparing the use of connective tissue grafts (CTGs), it was reported that the use of PRF had similar success rates in Miller class I and II defects [59]. However, increased keratinized tissue was observed with the use of CTGs compared to using PRF as a fibrin matrix. Therefore, there is no consensus on the use of PRP or PRF in root coverage procedures for gingival recession. The overview reported conflicting outcomes for PRP [17,28,30], no significant effect for LPRF [27], and positive adjunctive effect for unspecified PCs [31]. Due to the diverse outcomes, APCs have limited benefit to improve root coverage compared with other treatment options.
At present, there are no accepted standard treatment protocol for medication-related osteonecrosis of the jaws (MRONJ) that results in a predictable favorable result, including the use of APCs [60]. MRONJ is associated with antiresorptive medications used to treat various bone disorders, such as osteoporosis and metastatic bone disease [61,62]. The lesion is characterized by exposed bone in the maxillofacial region that has been present for 8 weeks or more and the patient has had no exposure to radiation in the maxillofacial region. The pathophysiology remains unclear and there are no accepted standard treatment protocols [60]. However, the use of APC has been reported to be an effective treatment for MRONJ [63-66]. APCs contain the various growth factors that enhance wound healing and stimulates collagen production and angiogenesis and was shown to be effective in the treatment of MRONJ, many subsequent reports soon followed [63,65] demonstrating the effectiveness of APCs as an adjunctive treatment in the management of MRONJ [41,67]. Most recently, L-PRF has been shown to be an effective adjunctive treatment in the treatment of MRONJ [68]. In their study, 26 out of 34 patients had complete resolution of MRONJ.
The management of MRONJ remains controversial. A systematic review by Lopez-Jornet and colleagues [40], which due to an absence of published randomized clinical trials, consisted of studies with small sample sizes. This systematic review concluded that although published scientific data do not sufficiently support the use of APCs in the treatment of MRONJ, it and another study [25] reported that the majority of their included studies reported total bone closure. Thus, the use of APCs may have potential in the treatment of MRONJ due to its reported benefits of modulating cellular events in both soft and hard tissues.
The clinical value and strengths of this overview are the APC applications for the periodontal therapies [19,23-25,29-37] and for the clinical management of MRONJ [25,39,40]. Beneficial clinical outcome for periodontal soft tissue procedures and regeneration using bone grafting procedures appear to be consistent. However, it is interesting to note that APC in combination with GTR provides negligible benefits. MRONJ is consistently one of the most challenging infections in dentistry impacting on implants, site preparation, extractions, periodontal and endodontic therapies for patients taking bisphosphonates. The 3 reviews [25,39,40] were uniform in demonstrating a benefit for resolution of MRONJ lesions.
The weaknesses of this overview are that it is limited by the selected systematic reviews and the heterogeneity of their included studies. There is a variation of the clinical outcomes for implant surgeries [20,22,24,26]. The use of implant stability quotient (ISQ) values for implant stability showed consistent positive results for APC but is a relatively crude assessment of clinical success [24]. Furthermore, the lack of evidence for APC for endodontic therapies [25,38] do not permit conclusive evidence for clinical direction for this field.
Conclusion
Within the limits of this comprehensive overview, platelet concentrates can have benefits in intraoral tissue healing. It can be used successfully in intra-oral procedures like sinus augmentation, ridge preservation, periodontal treatments, and treatment of osteoradionecrosis. This overview suggested that the application of PCs in the oral cavity can accelerate bone formation and tissue healing; and once the healing is complete, the final bone or tissue formed was the same as the control. This may be of clinical significance because faster bone formation and healing may equate to early treatment interventions and shorter rehabilitation time. Of significance are the beneficial clinical outcome for resolution of MRONJ lesions, and for the periodontal soft tissue procedures and regeneration using bone grafting procedures. However, it has been difficult to state with absolute certainty that the use of APCs can benefit hard or soft tissue healing. Perhaps, the existence of conflicting and non-significant results may be a misunderstanding of which time point in the healing process, PCs outcomes should be evaluated. Thus, with a paucity of consistent clinical evidence regarding the benefits on the use of APCs, each clinician must proceed with caution should they decide to implement regenerative medicine into their clinical practice. Further research studies using randomized clinical trials evaluating the early postoperative APCs outcomes are needed to fully evaluate the clinical benefits of APCs on wound healing in the oral and maxillofacial region.
Competing Interests
MT, NST, RK, SMB, CYSL, and JBS declare no competing interests regarding the content of this manuscript.
References
- Anitua E, Tejero R, Zalduendo MM, Orive G (2013) Plasma rich in growth factors promotes bone tissue regeneration by stimulating proliferation, migration, and autocrine secretion in primary human osteoblasts. J Periodontol 84: 1180-1190.
- Han J, Meng HX, Tang JM, Li SL, Tang Y, et al. (2007) The effect of different platelet-rich plasma concentrations on proliferation and differentiation of human periodontal ligament cells in vitro. Cell Prolif 40:241-252.
- Marx RE, Carlson ER, Eichstaedt RM, Schimmele SR, Strauss JE, et al. (1998) Platelet-rich plasma: growth factor enhancement for bone grafts. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 85:638-646.
- Dohan DM, Choukroun J, Diss A, Dohan SL, Dohan AJ, et al. (2006) Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part II: platelet-related biologic features. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 101:e45-50.
- He L, Lin Y, Hu X, Zhang Y, Wu H (2009) A comparative study of platelet-rich fibrin (PRF) and platelet-rich plasma (PRP) on the effect of proliferation and differentiation of rat osteoblasts in vitro. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 108:707-713.
- DohanEhrenfest DM, Diss A, Odin G, Doglioli P, Hippolyte MP, et al. (2009) In vitro effects of Choukroun's PRF (platelet-rich fibrin) on human gingival fibroblasts, dermal prekeratinocytes, preadipocytes, and maxillofacial osteoblasts in primary culture. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 108:341-352.
- Dohan DM, Choukroun J, Diss A, Dohan SL, Dohan AJ, et al. (2006) Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part I: technological concepts and evolution. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 101:e37-44.
- Tsay RC, Vo J, Burke A, Eisig SB, Lu HH, et al. (2005) Differential growth factor retention by platelet-rich plasma composites. J Oral Maxillofac Surg 63:521-528.
- Kobayashi E, Fluckiger L, Fujioka-Kobayashi M, Sawada K, Sculean A, et al. (2016) Comparative release of growth factors from PRP, PRF, and advanced-PRF. Clin Oral Investig 20:2353-2360.
- Marx RE (2004) Platelet-rich plasma: evidence to support its use. J Oral Maxillofac Surg 62:489-496.
- Choukroun J, Diss A, Simonpieri A, Girard MO, Schoeffler C, et al. (2006) Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part IV: clinical effects on tissue healing. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 101:e56-60.
- Roy S, Driggs J, Elgharably H, Biswas S, Findley M, Khanna S, et al (2011) Platelet-rich fibrin matrix improves wound angiogenesis via inducing endothelial cell proliferation. Wound Repair Regen 19:753-766.
- Chen FM, Wu LA, Zhang M, Zhang R, Sun HH (2011) Homing of endogenous stem/progenitor cells for in situ tissue regeneration: promises, strategies, and translational perspectives. Biomaterials 32:3189-3209.
- Shea BJ, Grimshaw JM, Wells GA, Boers M, Andersson N, et al. (2007) Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews. BMC Med Res Methodol 7:10.
- Sharif MO, Janjua-Sharif FN, Ali H, Ahmed F (2013) Systematic reviews explained: AMSTAR-how to tell the good from the bad and the ugly. Oral Health Dent Manag 12:9-16.
- Arora NS, Ramanayake T, Ren YF, Romanos GE (2010) Platelet-rich plasma in sinus augmentation procedures: a systematic literature review: Part II. Implant Dent 19:145-157.
- Bae JH, Kim YK, Myung SK (2011) Effects of platelet-rich plasma on sinus bone graft: meta-analysis. J Periodontol 82: 660-667.
- Bastami F, Khojasteh A (2016) Use of leukocyte-and platelet-rich fibrin for bone regeneration: a systematic review. Regeneration Reconstruction Restoration J 1: 47-68.
- Castro AB, Meschi N, Temmerman A, Pinto N, Lambrechts P, et al. (2017) Regenerative potential of leucocyte- and platelet-rich fibrin. Part A: intra-bony defects, furcation defects and periodontal plastic surgery. A systematic review and meta-analysis. J Clin Periodontol 44: 67-82.
- Castro AB, Meschi N, Temmerman A, Pinto N, Lambrechts P, et al. (2017) Regenerative potential of leucocyte- and platelet-rich fibrin. Part B: sinus floor elevation, alveolar ridge preservation and implant therapy: a systematic review. J Clin Periodontol 44: 225-234.
- Del Fabbro M, Bortolin M, Taschieri S (2011) Is autologous platelet concentrate beneficial for post-extraction socket healing? A systematic review. Int J Oral Maxillofac Surg 40: 891-900.
- Del Fabbro M, Bortolin M, Taschieri S, Weinstein R (2011) Is platelet concentrate advantageous for the surgical treatment of periodontal diseases? A systematic review and meta-analysis. J Periodontol 82: 1100-1111.
- Del Fabbro M, Bortolin M, Taschieri S, Weinstein RL (2013) Effect of autologous growth factors in maxillary sinus augmentation: a systematic review. Clin Implant Dent Relat Res 15: 205-216.
- Del Fabbro M, Corbella S, Taschieri S, Francetti L, Weinstein R (2014) Autologous platelet concentrate for post extraction socket healing: a systematic review. Eur J Oral Implantol 7: 333-344.
- Del Fabbro M, Gallesio G, Mozzati M (2015) Autologous platelet concentrates for bisphosphonate-related osteonecrosis of the jaw treatment and prevention: A systematic review of the literature. European Journal of Cancer 51: 62-74.
- Hou X, Yuan J, Aisaiti A, Liu Y, Zhao J (2016) The effect of platelet-rich plasma on clinical outcomes of the surgical treatment of periodontal intrabony defects: a systematic review and meta-analysis. BMC Oral Health 16: 71.
- Kotsovilis S, Markou N, Pepelassi E, Nikolidakis D (2010) The adjunctive use of platelet-rich plasma in the therapy of periodontal intraosseous defects: a systematic review. J Periodontal Res 45: 428-443.
- Lemos CA, Mello CC, dos Santos DM, Verri FR, Goiato MC, et al. (2016) Effects of platelet-rich plasma in association with bone grafts in maxillary sinus augmentation: a systematic review and meta-analysis. Int J Oral MaxillofacSurg45: 517-525.
- Lopez-Jornet P, Perez AS, Mendes RA, Tobias A (2016) Medication-related osteonecrosis of the jaw: Is autologous platelet concentrate application effective for prevention and treatment? A Systematic review. Journal of Cranio-Maxillo-Facial Surgery 44: 1067-1072.
- Luo HY, Li RM, Wang CL, Peng L, Ye L (2015) The adjunctive use of platelet concentrates in the therapy of gingival recessions: a systematic review and meta-analysis. J Oral Rehabil 42: 552-561.
- Martinez-Zapata J, Marti-Carvajal A, Sola I, Bolibar I, Exposito JA, et al. (2009) Efficacy and safety of the use of autologous plasma rich in platelets for tissue regeneration: a systematic review. Transfusion Practice 49: 44-56.
- Meschi N, Hilkens P, Lambrichts I, Van den Eynde K, Mavridou A, et al. (2016) Regenerative endodontic procedure of an infected immature permanent human tooth: an immunohistological study. Clin Oral Investig 20: 807-814.
- Panda S, Doraiswamy J, Malaiappan S, Varghese SS, Del F abbro M (2016) Additive effect of autologous platelet concentrates in treatment of intrabony defects: a systematic review and meta-analysis. J Investig Clin Dent 7: 13-26.
- Plachokova AS, Nikolidakis D, Mulder J, Jansen JA, Creugers NH (2008) Effect of platelet-rich plasma on bone regeneration in dentistry: a systematic review. Clin Oral Implants Res 19: 539-545.
- Pocaterra A, Caruso S, Bernardi S, Scagnoli L, Continenza MA, et al. (2016) Effectiveness of platelet-rich plasma as an adjunctive material to bone graft: a systematic review and meta-analysis of randomized controlled clinical trials. Int J Oral MaxillofacSurg 45: 1027-1034.
- Rosello-Camps A, Monje A, Lin GH, Khoshkam V, Chavez-Gatty M, et al. (2015) Platelet-rich plasma for periodontal regeration in the treatment of intrabony defects: a meta-analysis on prospective clinical trials. Oral Surg Oral Med Oral Pathol Oral Radiol 120: 562-574.
- Schliephake H (2015) Clinical efficacy of growth factors to enhance tissue repair in oral and maxillofacial reconstruction: a systematic review. Clin Implant Dent Relat Res 17: 247-273.
- Shah M, Deshpande N, Bharwani A, Nadig P, Doshi V, et al. (2014) Effectiveness of autologous platelet-rich fibrin in the treatment of intra-bony defects: a systematic review and meta-analysis. J Indian Soc Periodontol 18: 698-704.
- Higgins JPT, Green S (2011) Cochrane handbook for systematic reviews of interventions version 5.1.0. The Cochrane Collaboration.
- Montenegro R, Needleman I, Moles D, Tonetti M (2002) Quality of RCTs in periodontology--a systematic review. J Dent Res 81: 866-870.
- Gawai KT, Sobhana CR (2015) Clinical evaluation of use of platelet rich plasma in bone healing. J Maxillofac Oral Surg 14: 67-80.
- Kumar YR, Mohanty S, Verma M, Kaur RR, Bhatia P, et al. (2016) Platelet-rich fibrin: the benefits. Br J Oral Maxillofac Surg 54: 57-61.
- Rosenberg ES, Torosian J (2000) Sinus grafting using platelet-rich plasma -- initial case presentation. Pract Periodontics Aesthet Dent 12: 843-850.
- Xuan F, Lee CU, Son JS, Jeong SM, Choi BH (2014) A comparative study of the regenerative effect of sinus bone grafting with platelet-rich fibrin-mixed Bio-Oss(R) and commercial fibrin-mixed Bio-Oss(R): an experimental study. J CraniomaxillofacSurg 42: e47-50.
- Mazor Z, Horowitz RA, Del Corso M, Prasad HS, Rohrer MD, et al. (2009) Sinus floor augmentation with simultaneous implant placement using Choukroun's platelet-rich fibrin as the sole grafting material: a radiologic and histologic study at 6 months. J Periodontol 80: 2056-2064.
- Simonpieri A, Choukroun J, Del Corso M, Sammartino G, DohanEhrenfest DM (2011) Simultaneous sinus-lift and implantation using microthreaded implants and leukocyte- and platelet-rich fibrin as sole grafting material: a six-year experience. Implant Dent 20: 2-12.
- Tajima N, Ohba S, Sawase T, Asahina I (2013) Evaluation of sinus floor augmentation with simultaneous implant placement using platelet-rich fibrin as sole grafting material. Int J Oral Maxillofac Implants 28: 77-83.
- Tatullo M, Marrelli M, Cassetta M, Pacifici A, Stefanelli LV, et al. (2012) Platelet Rich Fibrin (P.R.F.) in reconstructive surgery of atrophied maxillary bones: clinical and histological evaluations. Int J Med Sci 9: 872-880.
- Zhang Y, Tangl S, Huber CD, Lin Y, Qiu L, et al. (2012) Effects of Choukroun's platelet-rich fibrin on bone regeneration in combination with deproteinized bovine bone mineral in maxillary sinus augmentation: a histological and histomorphometric study. Journal of Cranio-Maxillofacial Surgery 40: 321-328.
- Inchingolo F, Tatullo M, Marrelli M, Inchingolo AM, Scacco S, et al. (2010) Trial with platelet-rich fibrin and bio-oss used as grafting materials in the treatment of the severe maxillar bone atrophy: clinical and radiological evaluations. Eur Rev Med PharmacolSci 14: 1075-1084.
- Hauser F, Gaydarov N, Badoud I, Vazquez L, Bernard JP, et al. (2013) Clinical and histological evaluation of postextraction platelet-rich fibrin socket filling: a prospective randomized controlled study. Implant Dent 22: 295-303.
- Pradeep AR, Rao NS, Agarwal E, Bajaj P, Kumari M, et al. (2012) Comparative evaluation of autologous platelet-rich fibrin and platelet-rich plasma in the treatment of 3-wall intrabony defects in chronic periodontitis: a randomized controlled clinical trial. J Periodontol 83: 1499-1507.
- Ajwani H, Shetty S, Gopalakrishnan D, Kathariya R, Kulloli A, et al. (2015) Comparative evaluation of platelet-rich fibrin biomaterial and open flap debridement in the treatment of two and three wall intrabony defects. J Int Oral Health 7: 32-37.
- Agarwal A, Gupta ND, Jain A (2016) Platelet rich fibrin combined with decalcified freeze-dried bone allograft for the treatment of human intrabony periodontal defects: a randomized split mouth clinical trail. ActaOdontolScand 74: 36-43.
- Elgendy EA, Abo Shady TE (2015) Clinical and radiographic evaluation of nanocrystalline hydroxyapatite with or without platelet-rich fibrin membrane in the treatment of periodontal intrabony defects. J Indian SocPeriodontol 19: 61-65.
- Aroca S, Keglevich T, Nikolidakis D, Gera I, Nagy K, et al. (2010) Treatment of class III multiple gingival recessions: a randomized-clinical trial. J Clin Periodontol 37: 88-97.
- Padma R, Shilpa A, Kumar PA, Nagasri M, Kumar C, et al. (2013) A split mouth randomized controlled study to evaluate the adjunctive effect of platelet-rich fibrin to coronally advanced flap in Miller's class-I and II recession defects. J Indian SocPeriodontol 17: 631-636.
- Agarwal SK, Jhingran R, Bains VK, Srivastava R, Madan R, et al. (2016) Patient-centered evaluation of microsurgical management of gingival recession using coronally advanced flap with platelet-rich fibrin or amnion membrane: a comparative analysis. Eur J Dent 10: 121-133.
- Sculean A, Gruber R, Bosshardt DD (2014) Soft tissue wound healing around teeth and dental implants. J Clin Periodontol 41: S6-22.
- Ruggiero SL, Dodson TB, Fantasia J, Goodday R, Aghaloo T, et al. (2014) American association of oral and maxillofacial surgeons position paper on medication-related osteonecrosis of the jaw-2014 update. J Oral Maxillofac Surg 72: 1938-1956.
- Rogers MJ, Watts DJ, Russell RG (1997) Overview of bisphosphonates. Cancer 80: 1652-1660.
- Greenberg MS (2004) Intravenous bisphosphonates and osteonecrosis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 98: 259-260.
- Lee CY, David T, Nishime M (2007) Use of platelet-rich plasma in the management of oral biphosphonate-associated osteonecrosis of the jaw: a report of 2 cases. J Oral Implantol 33: 371-382.
- Curi MM, Cossolin GS, Koga DH, Zardetto C, Christianini S, et al. (2011) Bisphosphonate-related osteonecrosis of the jaws--an initial case series report of treatment combining partial bone resection and autologous platelet-rich plasma. J Oral MaxillofacSurg 69: 2465-2472.
- Adornato MC, Morcos I, Rozanski J (2007) The treatment of bisphosphonate-associated osteonecrosis of the jaws with bone resection and autologous platelet-derived growth factors. J Am Dent Assoc 138: 971-977.
- Mozzati M, Gallesio G, Arata V, Pol R, Scoletta M (2012) Platelet-rich therapies in the treatment of intravenous bisphosphonate-related osteonecrosis of the jaw: a report of 32 cases. Oral Oncol 48: 469-474.
- Park JH, Kim JW, Kim SJ (2017) Does the addition of bone morphogenetic protein 2 to platelet-rich fibrin improve healing after treatment for medication-related osteonecrosis of the jaw? J Oral Maxillofac Surg 75: 1176-1184.
- Kim JW, Kim SJ, Kim MR (2014) Leucocyte-rich and platelet-rich fibrin for the treatment of bisphosphonate-related osteonecrosis of the jaw: a prospective feasibility study. Br J Oral Maxillofac Surg 52: 854-859.
Citation: Ting M, Tadepalli NS, Kondaveeti R, Braid SM, Lee CYS, et al. (2018) Intra-Oral Applications of Platelet Concentrates: A Comprehensive Overview of Systematic Reviews. J Interdiscipl Med Dent Sci 6: 233. DOI: 10.4172/2376-032X.1000233
Copyright: © 2018 Ting M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 12819
- [From(publication date): 0-2018 - Apr 29, 2025]
- Breakdown by view type
- HTML page views: 11711
- PDF downloads: 1108