Case Report Open Access
Intracranial Tumorous Lesion as the First Manifestation of Langerhans Cell Histiocytosis: A Diagnostic and Therapeutic Challenge - Case Report
Jolanta Florczak Wyspianska1*, Michał Owecki1, Małgorzata Rzymkowska2, Halina Batura Gabryel2 and Wojciech Kozubski11Department of Neurology, Poznan University of Medical Sciences, Poznan, Poland
2Department of Pulmonology, Allergology, and Pulmonological Oncology, Poznan University of Medical Sciences, Poznan, Poland
- Corresponding Author:
- Jolanta Florczak Wyspianska
Poznan University of Medical Sciences
Department of Neurology, Przybyszewskiego
Poznan, Poland
Tel: 0048601878101
E-mail: jolaflorczak@op.pl
Received date: December 05, 2016; Accepted date: January 09, 2017; Published date: January 16, 2017
Citation: Wyspianska JF, Owecki M, Rzymkowska M, Gabryel HB, Kozubski W (2017) Intracranial Tumorous Lesion as the First Manifestation of Langerhans Cell Histiocytosis: A Diagnostic and Therapeutic Challenge - Case Report. J Alzheimers Dis Parkinsonism 7:298. doi:10.4172/2161-0460.1000298
Copyright: © 2017 Wyspianska JF. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Alzheimers Disease & Parkinsonism
Abstract
Langerhans cell histiocytosis (LHC) is a rare proliferative disease of unknown etiology characterized by bone marrow-derived histiocyte-like cell proliferation. The disease is confirmed by electron microscopy or immunohistochemical reactivity of histiocytes to the CD1a receptor, langerin (CD207) and/or S100 protein. The clinical spectrum ranges from an acute disseminated disease to solitary or few chronic lesions of bone or other organs. Central nervous system invasion in LCH patients has rarely been reported, especially in the adult population. We report a case of an intracranial tumorous lesion as the first manifestation of LCH in a 23 year old Caucasian man. The patient presented a 2 year history of cognitive decline and a 7 year history of diabetes insipidus, diabetes mellitus, hypothyroidism and obesity. The histopathological findings of a lesion located in the hypothalamic region confirmed the diagnosis of LCH.
Keywords
Langerhans cell histiocytosis; Central nervous system; Diabetes insipidus
Introduction
Langerhans cell histiocytosis (LCH) is a rare granulomatous disease of the monocyte -macrophage system which is caused by uncontrolled clonal proliferation of dendritic cells with Langerhans cell characteristics [1-3]. Cases of LCH are most often met in children under 15 years of age, but LCH can occur at any age, with the clinical presentation ranging from a benign single bone lesion to widespread multi-organ aggressive involvement [3,4]. A common site of LCH in adults, and typically the most involved location, is the respiratory system in which Langerhans cells are distributed beneath the epithelial lining of the tracheobronchial tree [5]. Central nervous system (CNS) LCH is a rare medical condition, especially as the first or only presentation of the disease, occurring in 1-11% of patients [4]. CSN-LCH can be subdivided in two subtypes, each with a distinct pathophysiology [4]. The first subtype is a neurodegenerative-like form with neuronal cell loss and progressive cerebellar ataxia, frequently combined with pyramidal syndrome and cognitive dysfunction [1]. It is hypothesized that neurological damage is due to CD8+ lymphocyte infiltration and cytokine production in the CNS [5]. The second subtype presents itself as a tumor lesion primarily involving the hypothalamic-pituitary region (HPR) and resulting in diabetes insipidus as well as anterior pituitary hormone deficiency. CSN-LCH can also affect the choroid plexus, meninges, pineal gland, brain stem, white- or grey-matter in the brain and rarely in the spinal cord [5-7]. Clinical presentations of CSN-LCH depend on process localization. Four groups can be clinically distinguished: 1) those with a disorder of HPR; 2) patients with site-dependent symptoms from space-occupying lesions; 3) those who exhibit a neurological dysfunction mostly connected with cerebellar-pontine pathway involvement; and 4) patients who present an overlap of the previously mentioned symptoms [6]. For a definite diagnosis a biopsy is necessary to reveal granulomas consisting of Langerhans cells, macrophages and T-lymphocytes, and a variable number of multinucleated giant cells and eosinophils [6]. The presence of intracellular Birbeck granules visualized by electron microscopy and immunohistochemical staining for the CD1a receptor, Langerin (CD207) [8], and/or S-100 protein constitute the gold standard for LCH diagnosing [2,9,10]. In addition, the cells are positive for CD11, vimentin, CD68 and HLA-DR [2,10].
Case Report
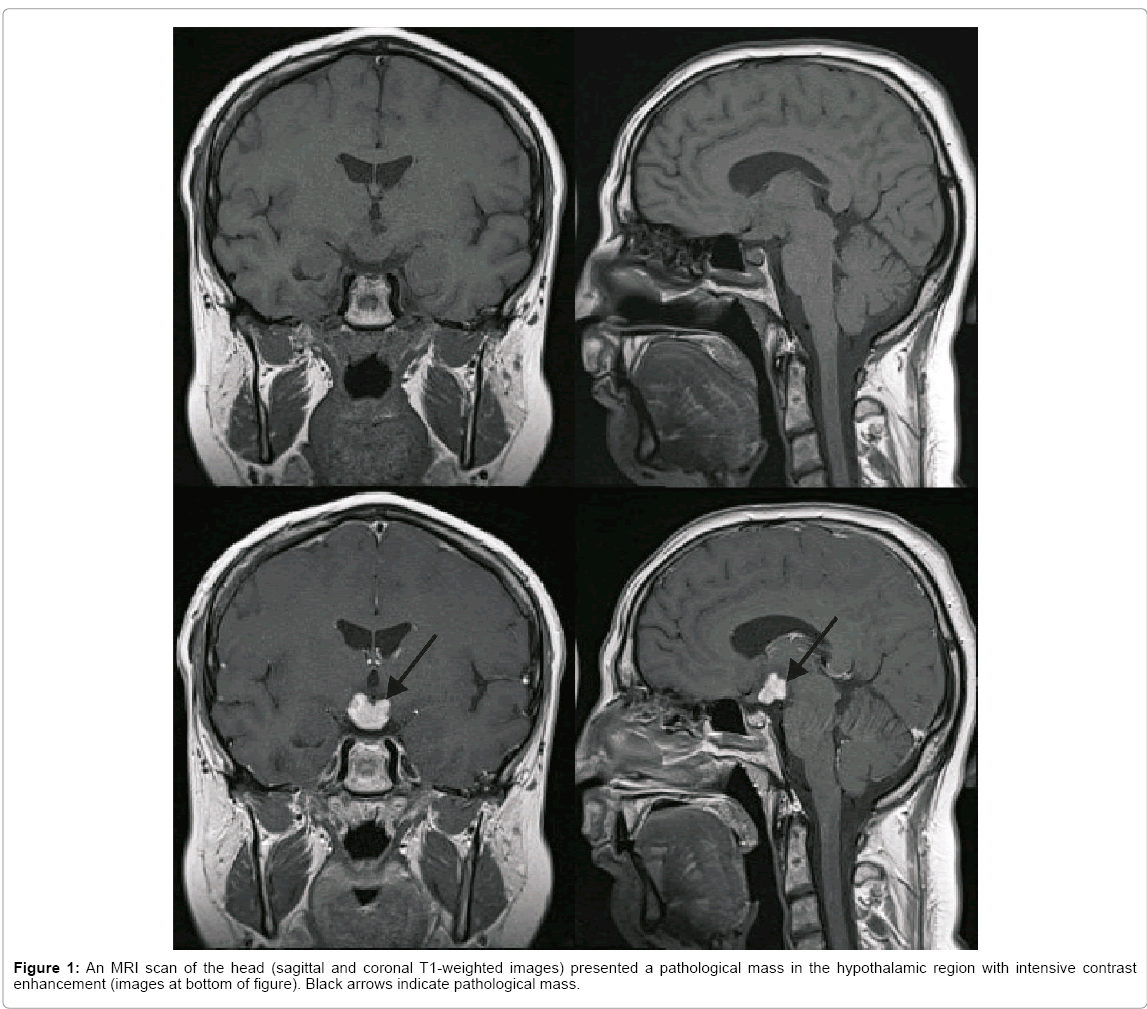
A 23 year old Caucasian man, a non-smoker, was admitted to the neurological ward in March of 2014 due to cognitive dysfunction, mainly memory and spatial orientation disturbances, which had gradually worsened over a period of two years. There was also a history of diabetes insipidus, diabetes mellitus, hypothyroidism and obesity for seven years. The patient was under an endocrinologist’s care due to the above disturbances. Chest X-rays that had been done several times over the period of seven years showed no abnormalities. MRI examinations of the head performed in July 2013 and January 2014 revealed a stable picture with the presence of a pathological, irregular mass (sized 13 mm/16 mm/18 mm) in the hypothalamic region with infiltration of the hypophysis stalk and compression of the optic chiasm, with intensive contrast enhancement (Figure 1). The radiological diagnosis was astrocytoma or craniopharyngioma, less possibly germinoma. In January 2014 a stereotactic biopsy of the tumor was done with histopathological diagnosis of a non-specific inflammatory process.
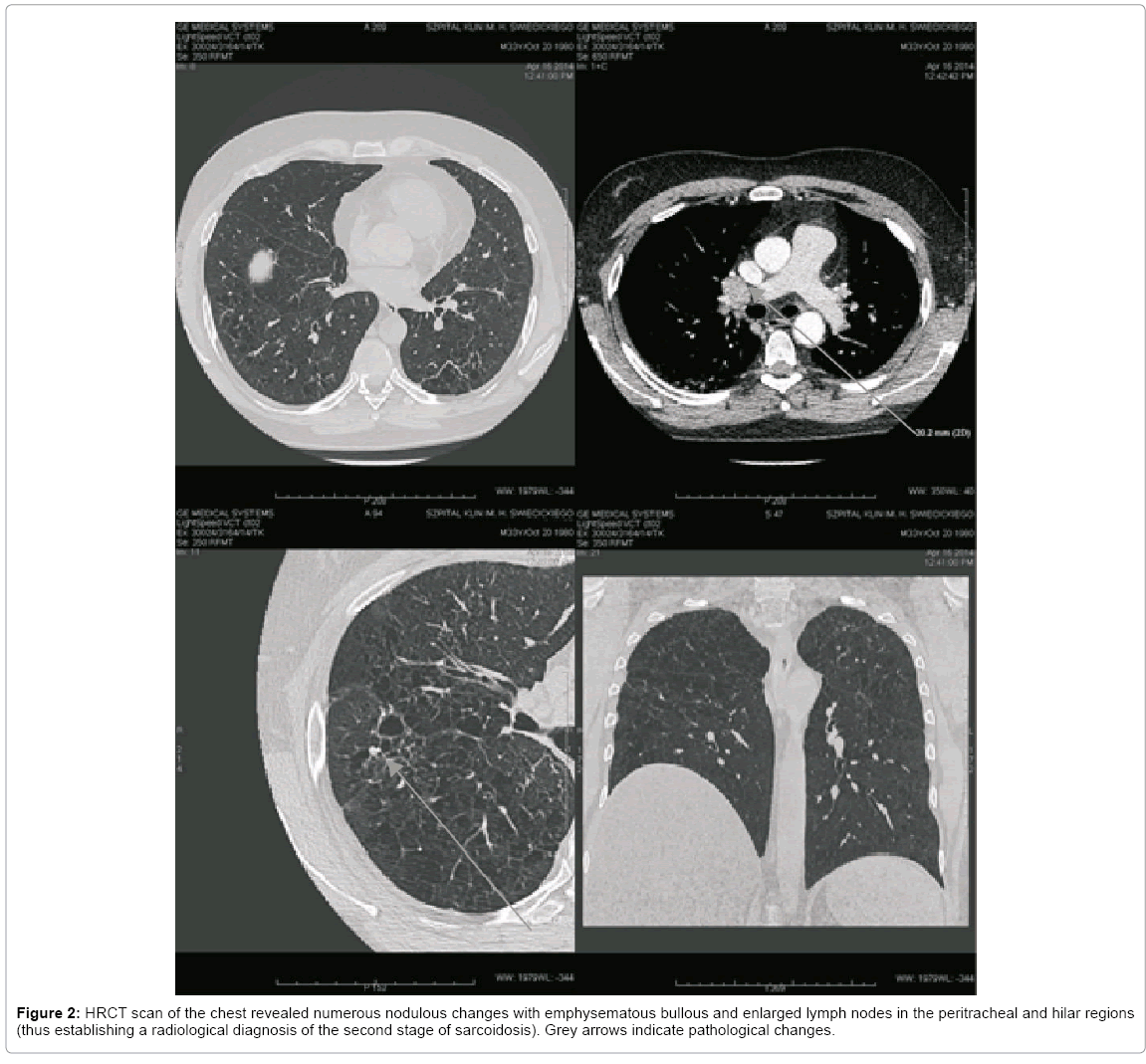
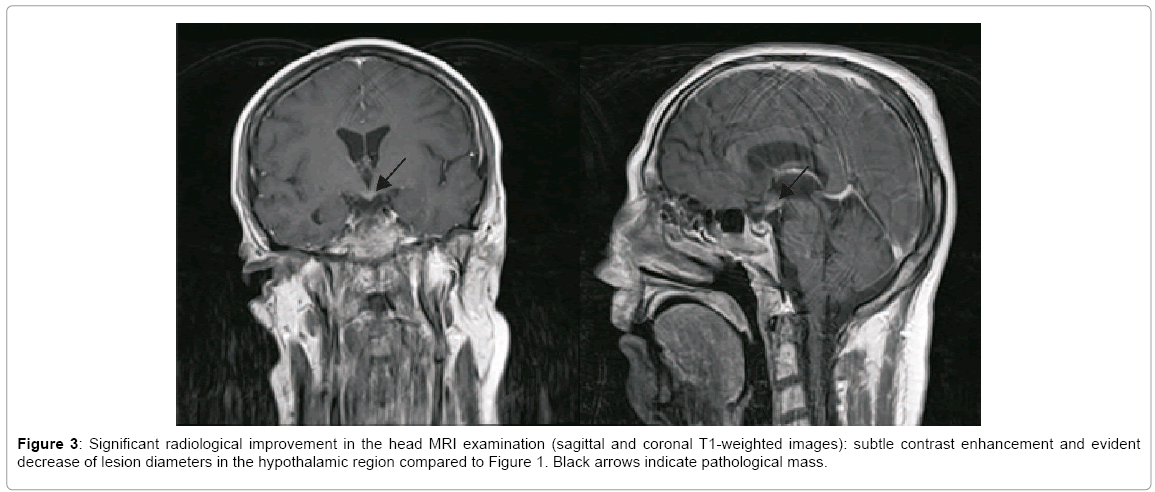
On admission the patient was in good general condition with an efficient cardio-respiratory system and normal body temperature. On admission the neurological examination, except for the cognitive dysfunction, revealed no abnormalities. During hospitalization many diagnostic procedures were conducted. The ophthalmological and EEG examinations showed no abnormalities. Cerebrospinal fluid (CSF) analysis revealed: an elevated protein level 858 mg/l (normal, 200–400), 6 white blood cells per mm3 (normal, 0-5), decreased glucose level 62 mg/dl (corresponding serum level 160 mg/dl), and presence of oligoclonal bands, which were the same as in serum. Subsequent, extensive investigations of CSF for infection were all finally negative. High-resolution chest CT (HRCT) revealed an increased picture of lung stroma, multiple nodulous changes sized 3-6 mm with emphysematous bullous and enlarged lymph nodes in the peritracheal and hilar regions with radiological diagnosis of the second stage of sarcoidosis (Figure 2). Ultrasonography of the neck also showed enlarged lymph nodes. Thereafter the patient was hospitalized at the Pulmonology Department for further diagnostics with suspicion of sarcoidosis. An echocardiography and a bronchoscopy with bronchus biopsy and bronchoalveolar lavage (BAL) for CD4, CD8 and CD1a were conducted. The results of these examinations were not typical for sarcoidosis, thus the decision was taken to conduct a further and more extensive pathomorphological estimation of the brain bioptate. The immunohistochemical tests of the bioptate revealed the presence of CD68-positive macrophages and S100-positive glial cells. Finally, based on the clinical presentation, CT of the chest, MRI of the head and the biopsy of the intracranial lesion, a diagnosis of LCH was made and treatment with cladribine and steroids was introduced, although treatment with steroids had to be discontinued because of uncontrolled hyperglycemia. Six cycles of chemotherapy with cladribine and one stereotactic radiotherapy were administered with partial but significant improvement that was detectable in the MRI of the head (Figure 3). Poor improvement in the X-ray and HRCT of the chest examinations and slight improvement in the clinical status were observed. Better controlled diabetes mellitus and diabetes insipidus were the main benefits obtained from the applied treatment. There were no changes in the patient’s mental status. The patient remains currently under pulmonological, endocrinological and neurological care.
Discussion
LCH is a type of histiocytic syndrome characterized by infiltration of tissues with specific dendritic cells, i.e., the Langerhans cells. These are a sub-population of dendritic cells which are found in the skin, lymph nodes and beneath the epithelium of the tracheobronchial tree, where they serve as a primary line of defense by surveying antigens and becoming activated after an encounter with danger signals [2,9,11].
The etiology of LCH is unknown. LCH cells are clonal, and a cancerassociated mutation (BRAFV600E) was found in more than half of the investigated specimens, thus indicating that LCH may be more of a neoplastic disease than a reactive disorder [2,8,12]. Rizzo et al. claimed that LCH can be considered a systemic disease that originates in the bone marrow and affects the monocyte lineage [12], so that underlying myeloid abnormalities may drive the formation of LCH lesions upon specific local triggering [13]. Kepes pointed out that histiocytosis is a proliferative disease of the mesenchymal tissue and thus can arise in the brain, where mesenchymal structures such as the meninges or choroid plexus are present [14]. He also claimed that such lesions may sometimes penetrate the superficial or perivascular glia limitans and infiltrate the CSN parenchyma [14]. Reichmann et al. pointed out that the dendritic cells not only seem to be mainly recruited from the peripheral immune system but may in part differentiate from the local microglia as well [15]. A diagnosis of LCH should be made following a biopsy by means of a microscopic and immunophenotypic examination of the affected tissue [6,8,11].
LCH is a rare entity that can be difficult to recognize because of a variety of presenting manifestations, especially when the only presentation are neurological or endocrinological symptoms. Most cases of LCH are found in children with involvement of the bones, skin, lymph nodes, liver and spleen [8,16]. A common site of LCH is the respiratory system [5]. Pulmonary involvement in LCH (PLCH) is often in adults and may be the sole organ involved (>80% of patients) or may be a part of a multisystem disease [17]. About twothirds of patients are symptomatic upon presentation with dyspnea and unproductive cough as the most common symptoms at diagnosis [9]. CD1a and S-100 stains are very helpful in the identification of Langerhans cells, although the mere presence of these cells does not establish the diagnosis of PLCH [9]. The chest radiograph is almost always abnormal, with reticulonodular infiltrates in early disease, whereas cystic lesions dominate in advanced disease [9]. Primary pulmonary LCH is a reactive disorder connected with smoking, and smoking cessation is the main and first line of therapy in adult smokers with PLCH [9,17]. If smoking cessation fails, systemic steroid or/and 2-CDA therapy are another option [8]. In 10-15% of adults diagnosed with PLCH, symptoms due to extra-pulmonary disease may be present [17]. In our patient, pulmonary involvement in the course of the disease was later than CNS involvement, which resulted in making a proper diagnosis more difficult. CNS-LCH lesions have been classified into tumorous/granulomatous lesions, non-tumorous/non-granulomatous lesions and brain atrophy [3]. A solitary intraparenchymal tumorous CSN lesion without signs of systematic disease predominantly occurs in the HPR with diabetes insipidus (DI) as a key manifestation [1,3]. Less frequently, granulomatous lesions may also localize in the meninges, choroid plexus, pineal gland, or cerebral parenchyma [1]. In our patient the solitary tumorous mass in the HPR was found, giving typical for this localization clinical picture. Isolated central DI (CDI), as was the case in our patient, may be the first manifestation of CNS-LCH, requiring further differentiation from other neoplastic or inflammatory brain lesions [18]. In pediatric and adolescent patients, up to 50% of CDI cases are idiopathic. The most commonly known causes of CDI are: germ cell tumors, autoimmune infundibuloneurohypophysitis, leukemia, granulomatous diseases, e.g. sarcoidosis and LCH [6,19]. In CNS-LCH, the presence and number of CD1a+ cells may be variable, dominating in some lesions, scarce or even absent in others or partly reflecting the stage of granuloma formation [1].
On MRI examination, tumorous CNS-LCH lesions manifest themselves as contrast-enhancing masses, which are iso- or hypointense on T1-weighted imaging and hyperintense on T2-weighted imaging [3]. MRI examinations of the head in the case of our patient performed twice, in July 2013 and January 2014 revealed the presence of a pathological, irregular mass in the hypothalamic region with infiltration of the hypophysis stalk and compression of the optic chiasm, with intensive contrast enhancement. Excluding HPR disease, the second most frequent presentation of CSN-LCH is a combination of neurodegenerative changes localized in the cerebellum, basal ganglia and/or pons with characteristic but non-pathognomonic MRI patterns [3,6]. Similar neurodegenerative findings are observed in patients with corticobasal degeneration, toxic demyelination, chronic liver failure or long-term parenteral nutrition [6]. Other MRI changes in CSNLCH include frequently observed dilated Virchow-Robin spaces and much less a common leukoencephalopathy pattern [1,6]. Long-term follow-up with attention to bone, skin and lung disorders may lead to a diagnosis of LCH [18].
The mainstay of therapy for solitary intraparenchymal CSN-LCH, when possible, is complete surgical excision [3]. If such treatment is not available, chemotherapy and/or radiotherapy are recommended [3,8,13]. Cladribine (2-chlorodeoxyadenosine- 2CDA) and vinblastine are active drugs in patients with CNS-LCH, and have shown good response in tumorous lesion of the CSN due to LCH [3,4,20]. As for the recommendations, in our patients combined therapy with steroids, cladribine and stereotactic radiotherapy was used with regression of the pathological mass in the HPR region and slight improvement of the clinical status. For multisystem LCH (MS-LCH) in adults when risk organs such as the hematopoietic system, spleen, liver and/or CNS are involved, also monotherapy with cladribine or vinblastine is dedicated as a first-line therapy [4,8]. In case of further progression, especially in CNS involvement, cytarabine may be added to 2-CDA [8]. In the rare case of the most aggressive course of disease, a hematopoietic stem cell transplant might be successful [2,4,8]. Radiotherapy is an effective treatment option for adult patients with LCH in selected situations such as an isolated unresectable lesion, e.g. a CNS, bone, recurrent or progressive lesion in the case of minor response to standard systemic therapy [8,19]. Also, Gamma Knife surgery can be used with good response to brainstem LCH treatment [1,3]. In the case we present a combined therapy was used: six cycles of chemotherapy with cladribine and one stereotactic radiotherapy. Partial but significant improvement was detectable in the MRI of the head (Figure 3). Better controlled diabetes mellitus and diabetes insipidus were the main benefits obtained from the applied treatment. In neurodegenerative CSN-LCH, retinoid acid and intravenous immunoglobulin may stabilize such patients.
Moreover, an early onset of cytarabine is recommended as first line therapy [8].
The course of LCH is unpredictable, with a spectrum of spontaneous regression [19], chronic progression, chronic recurrences, or a rapidly fatal deterioration [3,7,8,10]. When LCH infiltrates into a risk organ, such as the liver, spleen or bone marrow, the prognosis is poor; liver dysfunction is the poorest prognostic factor [10]. A few follow-ups with respect to CSN-LCH indicates that patients with extrahypothalamic LCH may have better prognosis than those with hypothalamic LCH [21,22]. Intracranial lesions have been observed during the course of disease in patients with a history of proved LCH and also as the first and presenting LCH manifestation, in which case they can cause a considerable diagnostic challenge [3,6,23].
In conclusion, it is important to consider CSN-LCH in differential diagnosis of a solitary tumorous intracranial lesion, especially when it is located in the hypothalamic-pituitary region. Long-term follow-up with close attention to lung, skin, and bone disorders may lead to the diagnosis of LCH. Pronounced inflammation was noted in all types of CNS-LCH, as well as in granulomatous or diffuse lesions so the need of biopsy and immunohistochemical tests are crucial in making proper diagnosis and treatment.
References
- Grois N, Prayer D, Prosch H, Lassmann H; CNS LCH Co-operative Group (2005) Neuropathology of CNS disease in Langerhans cell histiocytosis. Brain 128: 829-838.
- Lewandowski K (2013) Zalecenia postepowania diagnostyczno-terapeutycznego w nowotworach zlosliwych 2013. VM Media, VM Group 2: 975-980.
- Savardekar A, Tripathi M, Bansal D, Vaipahei K, Gupta SK (2013) Isolated tumorous Langerhans cell histiocytosis of the brainstem: A diagnostics and therapeutic challenge. J Neurosurg Pediatrics 12: 258-261.
- Tin WS, Martin-Duverneuil N, Idbaih A, Garel C, Ribeiro M et al. (2011) Efficacy of vinblastine in central nervous system Langerhans cell histiocytosis: A nationwide retrospective study. Orphanet J Rare Dis 6: 83.
- Krause ML, Patch RK, Caples SM (2012) A 36 year old man with seizures and multiple cystic pulmonary nodules. Chest 142: 256-259.
- Gabbay LB, Leite Cda C, Andriola RS, Pinho Pda C, Lucato LT (2014) Histiocytosis: A review focusing on neuroimaging findings. Arq Neuropsiquiatr 72: 548-558.
- Yamagata T, Takami T, Yamamoto N, Tanaka S, Wakasa K et al. (2013) Primary intramedullary Langerhans cell histiocytosis of the thoracic spinal cord - Case report. Neurol Med Chir (Tokyo) 53: 245-248.
- Girschikofsky M, Arico M, Castillo D, Chu A, Doberauer C, et al. (2013) Management of adult patients with Langerhans cell histiocytosis: recommendations from an expert panel on behalf of Euro-Histio-Net. Orphanet J Rare Dis 8: 72.
- Suri HS, Yi ES, Nowakowski GS, Vassallo R (2012) Pulmonary Langerhans cell histiocytosis. Orphanet J Rare Dis 7: 16.
- Yuasa M, Fujiwara S, Oh I, Yamaguchi T, Fukushima N et al. (2012) Rapidly progressing fatal adult multi-organ Langerhans cell histiocytosis complicated with fatty liver disease. J Clin Exp Hematopathol 52: 121-126.
- Lajolo C, Campisi G, Deli G, Littarru C, Guiglia R, et al. (2012) Langerhans's cell histiocytosis in old subjects: two rare case reports and review of the literature. Gerodontology 29: e1207-1214.
- Badalian-Very G, Vergilio JA, Fleming M, Rollins BJ (2013) Pathogenesis of Langerhans cell histiocytosis. Annu Rev Pathol 8: 1-20.
- Rizzo FM, Cives M, Simone V, Silvestris F (2014) New insights into the molecular pathogenesis of Langerhans cell histiocytosis. Oncologist 19: 151-163.
- Kepes JJ (1979) Handbook of neurology. Elsevier Publishing Company, New York 38: 93-117.
- Reichmann G, Schroeter M, Jander S, Fischer HG (2002) Dendritic cells and dendritic-like microglia in focal cortical ischemia of the mouse brain. J Neuroimmunol 129: 125-132.
- Pogrzeba J, Rudkowska-Kazanowska A, Wróbel T (2005) Langerhans cell histiocytosis as a diagnostic problem – a two cases study. Acta Hematol Pol 36: 441-446.
- Vassallo R, Ryu JH, Schroeder DR, Decker PA, Limper AH (2002) Clinical outcomes of pulmonary Langerhans'-cell histiocytosis in adults. N Engl J Med 346: 484-490.
- Marchand I, Barkaoui MA, Garel C, Polak M, Donadieu J, et al. (2011) Central diabetes insipidus as the inaugural manifestation of Langerhans cell histiocytosis: Natural history and medical evaluation of 26 children and adolescents. J Clin Endocrinol Metab 96: E1352-E1360.
- Nagasaki K. Tsumanuma I, Yoneoka Y, Ogawa Y, Kikuchi T, et al. (2009) Spontaneous regression of isolated neurohypophyseal Langerhans cell histiocytosis with diabetes insipidus. Endocr J 56: 721-725.
- Dhall G, Finlay JL, Dunkel IJ, Ettinger LJ, Kellie SJ, et al. (2008) Analysis of outcome for patients with mass lesions of the central nervous system due to Langerhans cell histiocytosis treated with 2- chlorodeoxyadenosine. Pediatr Blood Cancer 50: 72-79.
- Cagli S, Oktar N, Demirtas E (2004) Langerhans' cell histiocytosis of the temporal lobe and pons. Br J Neurosurg 18: 174-180.
- Rodriguez-Pereira C, Borrra´s-Moreno JM, Pseudo-Martinez JV, Vera-Roma´n JM (2005) Cerebral solitary Langerhans cell histiocytosis: Report of two cases and review of the literature. Br J Neurosurg 19: 192-197.
- Perren F, Fankhauser L, Thiévent B, Pache JC, Delavelle J, et al. (2011) Late adult onset of Langerhans cell histiocytosis mimicking glioblastoma multiforme. J Neurol Sci 301: 96-99.
Relevant Topics
- Advanced Parkinson Treatment
- Advances in Alzheimers Therapy
- Alzheimers Medicine
- Alzheimers Products & Market Analysis
- Alzheimers Symptoms
- Degenerative Disorders
- Diagnostic Alzheimer
- Parkinson
- Parkinsonism Diagnosis
- Parkinsonism Gene Therapy
- Parkinsonism Stages and Treatment
- Stem cell Treatment Parkinson
Recommended Journals
Article Tools
Article Usage
- Total views: 4957
- [From(publication date):
February-2017 - Apr 02, 2025] - Breakdown by view type
- HTML page views : 4044
- PDF downloads : 913