Inter-Threshold Agreement for Infarct Lesion Volume According to the Apparent Diffusion Coefficient
Received: 02-Oct-2020 / Accepted Date: 16-Oct-2020 / Published Date: 23-Oct-2020
Abstract
Background and purpose: ADC threshold-based computer mapping provide pertinent ischemic stroke volume. Indeed, pre-treatment lesional volume appears to be an independent predictor for functional outcome in AIS with proximal intracranial large vessel occlusion.
The aim of this study is to determine volumetric agreement between Olea Sphere® and our homemade software named Strike® using three different ADC thresholds (600, 615, 620 mm2/s) to evaluate the influence on functional outcomes of patients with AIS treated by Mechanical Thrombectomy Combined with Intravenous Thrombolysis (IVTMT).
Methods: 101 patients from THRACE (Mechanical thrombectomy after intravenous alteplase versus alteplase alone after stroke) were included. AIS were segmented from Olea Sphere® with an ADC threshold of 600 × 10-6 mm2/s, and with Strike® with 615 × 10-6 mm2/s and 620 × 10-6 mm2/s.
The primary study outcome was to compare the AIS’s mean volumes obtained with these different ADC thresholds. Secondary outcome was to determinate if this AIS volumes can be predicting functional prognosis which favourable outcomes: mRS=0-2 at 3 months.
Result: AIS’s volumes differences for each ADC thresholds were: Sphere® (600 mm2/s) and Strike® (615 mm2/s)=6.1200 (p-value: 0.6405), Sphere (600 mm2/s) and Strike® (620 mm2/s)=-11.0476 (p=0.0776) and Strike® (615 mm2/s) and Strike® (620 mm2/s)=-4.9276 (p=0.9562). The observed association between ADC 600 mm2/s (Sphere®) and functional outcome remained significant (p=0.0448). ADC 615 mm2/s and ADC 620 mm2/s remained not statistically significant (Respectively p=0.0987 and p=0.0692).
Conclusion: Several currently ADC thresholds for the measurement of AIS volume estimation are highly comparable and can be helpful before therapeutic decisions. However, some discrepancies can be found when correlating to the functional prognosis according to the ADC threshold for patients treated by Mechanical Thrombectomy Combined with Intravenous Thrombolysis (IVTMT).
Keywords: Apparent diffusion coefficient; Stroke; Thrombectomy; Volume; Software
Abbreviations
DWI: Diffusion Weighted Imaging; ADC: Apparent Diffusion Coefficient; AIS: Acute Ischemic Stroke; IVTMT: Intravenous Thrombolysis and Mechanical Thrombectomy; LVO: Large Vessel Occlusion; NIHSS: National Institutes of Health Stroke Scale; TICI scale: Thrombolysis in Cerebral Infarction Scale; mRS: Modified Rankin Scale
Introduction
Infarct lesion is defined on Diffusion Weighted Imaging (DWI) acquisition, especially through the generated Apparent Diffusion Coefficient (ADC) maps which is a pertinent method to rapidly evaluate an ischemic stroke.
DWI signal intensity varies with magnetic field strength, scanning parameters, and is susceptible to artefacts which are not the case of ADC map [1]. The ADC as for it is a measure of the diffusivity of water molecules in tissue. The cytotoxic oedema secondary to tissue’s ischemia tissue leads to a reduction in the ADC. It allows a better accuracy than visual, manual outlining or semi-automated determination of hyperintense region on DWI [2].
Multiple semi-automatic software such as Olea Sphere® (Olea Medical SAS, La Ciotat, France) and RAPID® (iSchemaView) were used for assessing lesional volume in randomized controlled trials [3,4]. However, computed segmentation of the infarct lesion is induced by the threshold applied on ADC map, thus volume could vary inducing over or underestimation of the DWI ischemic volume whereas pre- treatment lesional volume appears to be an independent predictor for functional outcome in acute ischemic stroke with proximal intracranial Large Vessel Occlusion (LVO) [5,6].
Applied threshold varies among software, but an optimal threshold for identification of ischemic core was suggested at 620 × 10-6 mm /s in literature [7].
Because IVTMT is regarded as gold standard therapy in patients with AIS caused by LVO, determining the best measurement method to quantification of the AIS volume is becoming an important factor to consider for patient selection [8-10].
The aim of this study is to compare the lesional acute infarct volume assessed on ADC computed map using different ADC threshold of 600, 615 and 620 × 10-6 mm2/s with two different software and to evaluate the influence on functional outcomes of patients treated by mechanical thrombectomy combined with intravenous thrombolysis.
Methodology
Outcomes
The primary study outcome was to compare the AIS’s means volumes obtained with three different thresholds.
Secondary outcome was to determinate if this AIS volumes can be predicting functional prognosis, favourable outcome is defined as a modified Rankin Scale (mRS) 0-2 at 3 months.
All data comprising Baseline characteristics (age, sex, vascular risk factors and comorbidities), baseline examination data (mRS at 3 months, baseline NIHSS) and symptom onset to recanalization with TICI status were extracted from THRACE database (Table 1).
| Variables | Total (n=101) |
|---|---|
| Median Age | 64 (49-73) |
| Hemorragic transfo (%) | |
| HI1 | 14 (14) |
| HI2 | 16 (16) |
| N/A | 1 (1) |
| NON | 53 (53) |
| PH1 | 8 (8) |
| PH2 | 8 (8) |
| Sex, M/F n (%) | 56 (55)/45 (45) |
| Diabete, n (%) | 96 (96) |
| HTA, n (%) | 57 (57) |
| Normal renal fct, n (%) | 97 (96) |
| ATCD AVC | 4 (4) |
| dyslipidemia n (%) | 39 (44) |
| Tabac, n (%) | 33 (38) |
| Coronary disease, n (%) | 11 (13) |
| AOMI, n (%) | 9 (9) |
| Median NIHSS | 18 |
| mRS 3 months n(%) | |
| 0 | 11 (13) |
| 1 | 22 (26) |
| 2 | 20 (23) |
| 3 | 11 (13) |
| 4 | 17 (20) |
| 5 | 5 (6) |
| TICI status | |
| 0 | 12 (14) |
| 1 | 3 (3) |
| 2A | 8 (9) |
| 2B | 46 (53) |
| 3 | 18 (21) |
Table 1: Baseline characteristics extracted from THRACE.
Image acquisition, segmentation and normalization
Pre-treatment DWI data were acquired on 1.5T or 3T MRI scanners (General Electric Healthcare, WI, USA; Philips medical systems, Best, Netherlands; Siemens, Erlangen, Germany) according to the local routine protocol in each participating center. Pre-treatment infarct lesions were semi-automatically segmented by both Olea Sphere® (Olea Medical SAS, La Ciotat, France) after applying a threshold of apparent diffusion coefficient (ADC) of 600 × 10-6 mm2/s on ADC maps, and with our homemade software named Strike® implementing RAPID® algorithm for DWI lesion segmentation (for more details see Appendix) after applying a threshold of ADC 615 and 620 × 10-6 mm2/s on ADC maps.
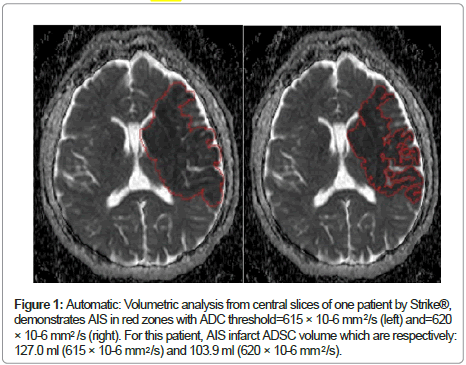
Figure 1 compares the two different thresholds used by our software Strike® (615 and 620 × 10-6 mm2/s) with ADC maps.
All the image post-processing results and lesion masks were checked and were manually corrected if necessary, by a junior neuroradiologist (5 years’ experience) blind to clinical information.
Statistical analysis
Quantitative variables are expressed as mean ± standard deviation or median (interquartile range) and categorical variables are expressed as numbers (percentage). For volume comparisons, mean infarct volumes were calculated and tabulated in a summary statistic (Table 2).
| Thresholds | Mean (ml) | Standard deviation |
|---|---|---|
| ADC 600 | 37.4664 | 3.4712 |
| ADC 615 | 31.3464 | 3.4712 |
| ADC 620 | 26.4188 | 3.5061 |
Table 2: Means infarct volume obtained from different ADC thresholds.
ANOVA analysis was conducted on mean infarct variability for each ADC thresholds using Bonferoni method, which uses critical values from Student’s t-distribution after an adjustment to compensate for multiple comparisons (Table 3).
| Groups to compare (ADC) | Mean difference (ml) | 95% confidence interval | p-value |
|---|---|---|---|
| 620/600 | -11.048 | [-22.9260, 0.8308] | 0.0776 |
| 620/615 | -4.9276 | [16.8060, 6.9508] | 0.9562 |
| 600/615 | 6.12 | [-5.6988, 17.9389] | 0.6405 |
Table 3: Mean infarct differences between the different ADC thresholds.
Main baseline characteristics and outcomes were compared between different ADC thresholds in uni and multivariable models. In multivariable models, association of ADC volumes were adjusted for pre-specified confounding factors (age, haemorrhagic transformation, and recanalization status) by using mixed binary logistic regression models for binary outcomes, mixed ordinal logistic regression model for mRs.
Data were analysed using statistical software R® (version 3.6.2). A p-value of less than 0.05 was considered statistically significant.
Results
The gross evaluation of the gallbladder revealed a saccular 9.0 × 4.5 cm gallbladder with multiple gallstones and red to tan velvety mucosa with a firm mass measuring 5.0 × 3.0 × 1.5 cm on the lateral fundus, 5.0 cm away from the cystic duct margin [1]. The external surface serosa is smooth and glistening. Within the gallbladder, there were approximately 20 hard black faceted gallstones, 4 × 4 × 3 cm in aggregate. No gallstone was lodged within the cystic duct. Histologic examination demonstrated unusual gall bladder adenosquamous carcinoma (Figure 1). Submitted entirely, the carcinoma infiltrated the fundus wall locally but was confined within the serosa. The liver margin was negative for carcinoma cells, while tumor invaded peri muscular connective tissue on the peritoneal side without involvement of the serosa (visceral peritoneum). The remainder of the gallbladder mucosa showed changes of chronic cholecystitis and multiple gallbladder cystic diverticula (Rokitansky Aschoff sinuses).
Figure 1: Automatic: Volumetric analysis from central slices of one patient by Strike®, demonstrates AIS in red zones with ADC threshold=615 × 10-6 mm /s (left) and=620 × 10-6 mm /s (right). For this patient, AIS infarct ADSC volume which are respectively: 127.0 ml (615 × 10-6 mm /s) and 103.9 ml (620 × 10-6 mm /s).
Patients selection
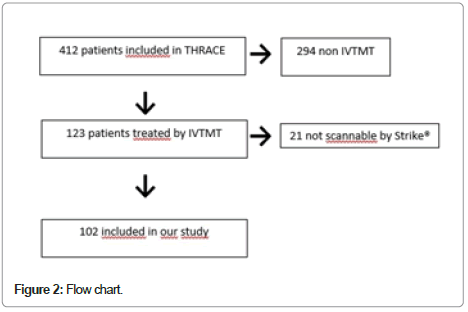
We included 101 patients from THRACE (Figure 2) (Mechanical thrombectomy after intravenous alteplase versus alteplase alone after stroke a prospective, multicenter, randomized controlled trial comparing efficacy and adverse events of IVTMT versus intravenous thrombolysis and alone in patients who presented an anterior circulation stroke by LVO. A central medical ethics committee approved the THRACE trial protocol and consents form. Additional inclusion and exclusion criteria have been reported previously [7]. We included in this post- hoc study all patients randomized in THRACE undergoing a Magnetic Resonance Imaging (MRI) at admission, and we excluded 21 patients because their ADC map was too artefact and our software Strike® could not calculate a relevant infarct lesional volume and one patient because we didn’t have all data characteristics.
Relationship between ADC thresholds and obtained volumes
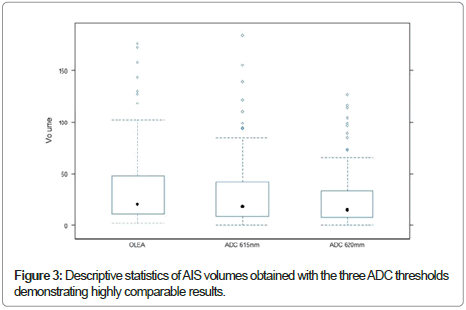
The median of ADC tissue volumes obtained were: Sphere® (600 × 10-6 mm /s)=37.4664 ml (3.4712 DS), Strike® (615 × 10-6 mm /s)=31.3464 ml (3.4712DS) and Strike® (620 × 10-6 mm /s)=26.4188 ml (3.5061DS). Figure 3 shows that these results are highly comparable. Bonferoni analysis revealed also that the ADC tissue volumes decreased slightly with increasing threshold (Table 2).
For the support of a therapeutic decision, the absolute ADC volume differences (95% CIs) for each threshold were: Sphere® (600 × 10-6 mm/s) and Strike® (615 × 10-6 mm /s)=6.1200 ml (p-value: 0.6405 [-5.6988, 17.9389]), Sphere® (600 × 10-6 mm /s) and Strike® (620 × 10-6 mm /s)=-11.0476 ml (p-value:0.0776 [-22.9260, 0.8308]) and Strike® (615 × 10-6 mm /s) and Strike® (620 × 10-6 mm /s)=-4.9276 ml (p-value: 0.9562 [16.8060, 6.9508]) (Table 3).
Predictive of the impact on clinical outcomes
From the multivariate logistic analyses, the observed association between ADC 600 mm /s (Olea®) and functional outcome remained significant (p=0.0448). ADC 615 mm /s and ADC 620 mm /s remained not statistically significant (Respectively p=0.0987 and p=0.0692).
Discussion
In our study, we didn’t find significant difference between mean AIS volumes using three different ADC thresholds and two software. However, a discrepancy on prognosis prediction using volumes computed by these three different thresholds has been highlighted.
MRI-based selection of patients for acute stroke interventions requires rapid accurate estimation of the infarct volume which is of great importance as it impacts on patient selection [2], eligibility for reperfusion procedures and in the evaluate of the risk of haemorrhagic transformation.
Two methods are typically used for identifying AIS volumes: the DWI stroke volume which corresponds to the abnormally hypersignal intensity areas on DWI sequence and the abnormal signal based on the threshold of the ADC computed map. About the latter, there are several currently available tools for the measurement of ischemic core using different diffusion coefficient ≤ 620 × 10-6 mm / (10)/s or 600 × 10-6 mm /s [11]. In some reports, patients with ADC infarct core volumes >70 cm , which depends of the ADC threshold used, or an infarct volume above a specified cut-off volume have been excluded from trials of endovascular treatment [12].
Frequent diverging thrombectomy decisions were observed when applying DAWN criteria, using 9 commonly used tools for the measurement of infarct volume, despite a high agreement [13,14].
Spatial estimation of penumbra tissue is also of great importance as it impacts on eligibility for reperfusion procedures and in prediction of complications, especially malignant infarct [15].
Recently, concerning RAPID® and Olea Sphere® at least, a study proves that thresholds used for ADC ischemic core volumes and diffusion/perfusion mismatch volume segmentation may lead to significantly different result in the individual patient and may thus seriously influence the decision for or against mechanical thrombectomy [16].
We hypothesize that the discrepancy in our finding on prediction of clinical outcomes using AIS volumes is due to the overestimation of the ischemic core using a low ADC threshold. The concept of ischemic core is now debated and the notion of “severely ischemic tissue with uncertain viability” seems more appropriate for the burden of death cell and because validated measures and irreversibility injured tissue imaging parameter are lacking.
In our study, some limitations have not allowed us to demonstrate statistically significant predictiveness of ADC volumes on functional prognosis.
Apart from technical concerns, which forced us to exclude 21 patients, the sample size was relatively small to demonstrate a predictability of the functional prognosis of the ischemic core volumes evaluate by ADC maps while several studies show it for all ADC thresholds.
Due to its retrospective design, it should be cautious about variability of institutional imaging protocols.
In addition, only two software packages were investigated and applied different segmentation algorithms for calculation of ADC maps.
Furthermore, additional calculation of the spatial overlap between lesions was not performed.
More studies will be necessary comparing more thresholds and automatic software AIS volumes to validate suggested predictive functional power.
Conclusion
ADC thresholds computed by two software, from 600 to 620 × 10-6 mm2, for the measurement of acute infarct volume are highly comparable. However, a predictability of the functional prognosis for patients with acute ischemic stroke treated by mechanical thrombectomy combined with intravenous thrombolysis was not demonstrated for all of three thresholds due to an overestimation induced by a low threshold.
Disclosures
Authors have nothing to disclose.
References
- Oppenheim C, Samson Y, R Manaï, T Lalam, X Vandamme (2000) Prediction of malignant middle cerebral artery infarction by diffusion-weighted imaging. Stroke 20: 2175-2181.
- Lansberg MG, Lee J, Christensen S, Straka M, De Silva DA (2011) RAPID automated patient selection for reperfusion therapy: A pooled analysis of the Echoplanar Imaging Thrombolytic Evaluation Trial (EPITHET) and the Diffusion and Perfusion Imaging Evaluation for Understanding Stroke Evolution (DEFUSE) Study. Stroke 42: 1608-1614.
- Lansberg M, Lee J, Christensen S, Straka M, De Silva DA (2011) Utility of automated MRI analysis software (RAPID) to select patients for reperfusion therapy: A Pooled analysis of the EPITHET and DEFUSE studies. Stroke J Cereb Circ 67: 1608-1614.
- Thomas RGR, Lymer GK, Armitage PA, Chappell FM, Carpenter T (2013) Apparent diffusion coefficient thresholds and diffusion lesion volume in acute stroke. J Stroke Cerebrovasc Dis Off J Natl Stroke Assoc 22: 906-909.
- Xie Y, Oppenheim C, Guillemin F, Gautheron V, Gory B (2018) Pretreatment lesional volume impacts clinical outcome and thrombectomy efficacy. Ann Neurol 79 :178-185.
- Bråtane BT, Bastan B, Fisher M, Bouley J, Henninger N (2009) Shemic lesion volume determination on diffusion weighted images vs. apparent diffusion coefficient maps. Brain Res 47:182-188.
- Purushotham A, Campbell BCV, Straka M, Mlynash M, Olivot J-M (2015) Apparent diffusion coefficient threshold for delineation of ischemic core. Int J Stroke Off J Int Stroke Soc 98: 348-353.
- Bracard S, Ducrocq X, Mas JL, Soudant M, Oppenheim C (2016) Mechanical thrombectomy after intravenous alteplase versus alteplase alone after stroke (THRACE): A randomised controlled trial. Lancet Neurol 38:1138-1147.
- Broderick JP, Palesch YY, Demchuk AM, Yeats S, Khatri P (2013) Endovascular therapy after intravenous t-PA vers t-PA alone for stroke. N Engl J Med 368:893-903.
- Han M, Wook Choi J, Rim N-J, Young Kim S, Sue H (2016) Cerebral infarct volume measurements to improve patient selection for endovascular treatment. Medicine (Baltimore) 95: 4702.
- Straka M, Albers GW, Bammer R (2010) Real-time diffusion-perfusion mismatch analysis in acute stroke. J Magn Reson Imaging 2010: 1024-1037.
- Jovin TG, Chamorro A, Cobo E, De Miquel M, Molina C (2015) Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med 49: 2296-2306.
- Khoury N, Dargazanli C, Zuber K, Smajda S, Bitar M (2020) Diffusion-weighted imaging infarct volume measurement tools show discrepancies leading to diverging thrombectomy decisions. Journal of Neuroradioly 11: 30128-30130.
- Goyal M, Ospel J, Menon B, Almekhlafli M, Jayaraman M (2020) Challenging the ischemic core concept in acture ischemic stroke imaging. Stroke 60: 3147-3155.
- Panni P, Gory B, Xie Y, Consoli A, Desilles J (2019) Acute stroke with large ischemic core treated by thrombectomy. Stroke 19: 1164-1171.
- Deutschmann H, Hinteregger N, Wiepener U, Kneihsl M, Fandler S (2020) Automated MRI perfusion-diffusion mismatch estimation may be significantly different in individual patients when using different software packages. Eur Radio 87: 569-570.
Citation: Bufacchi A, Zhu F, Gory B, Hossu G, Micard E et al. (2020) Inter- Threshold Agreement for Infarct Lesion Volume According to the Apparent Diffusion Coefficient. J Clin Exp Pathol 10: 385.
Copyright: © 2020 Bufacchi A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Usage
- Total views: 1862
- [From(publication date): 0-2020 - Apr 05, 2025]
- Breakdown by view type
- HTML page views: 1138
- PDF downloads: 724