Research Article Open Access
International Consensus: Paraneoplastic Neurological Antibodies - are we there yet?
Abid Karim1,3*, William Egner2, Dina Patel2, Alex Richter1,3 and Saiju Jacob1,3
1Neuroimmunology, Clinical Immunology Service, University of Birmingham, Birmingham, B15 2TT, UK
2UK NEQAS Immunology, Immunochemistry & Allergy, Northern General Hospital, Herries Road, Sheffield, S5 7AU, UK
3Queen Elizabeth Neurosciences Centre, University Hospital Birmingham NHS Foundation Trust, Birmingham, B15 2TH, UK
- *Corresponding Author:
- Abid Karim
Neuroimmunology, Clinical Immunology Service
University of Birmingham, Birmingham, B15 2TT, UK
Tel: +44 (0)121 415
E-mail: a.r.karim@bham.ac.uk
Received date: Jan 08, 2016; Accepted date: Mar 18, 2016; Published date: Mar 22, 2016
Citation: Karim A, Egner W, Patel D, Richter A, Jacob S (2016) International Consensus: Paraneoplastic Neurological Antibodies - are we there yet?. J Clin Exp Neuroimmunol 1:105. doi: 10.4172/jceni.1000105
Copyright: © 2016 Karim A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Clinical & Experimental Neuroimmunology
Abstract
A survey amongst the participants of the Paraneoplastic Neurological Antibody Scheme, registered with United Kingdom National External Quality Assessment Service (UK NEQAS), across the UK, continental Europe and non- European countries examined various factors involved in the laboratory diagnostic methodologies, timely provision of results and a snapshot of External Quality Assessment (EQA) performance to ascertain the level of harmonisation amongst participating laboratories. Despite variations in some aspects of the analytical methods there appears to be a good agreement in the outcome of the results as demonstrated by the EQA performance.
Introduction
Laboratory contribution to clinical diagnosis is an essential part of patient care. In order to accurately diagnose, treat and advise patients, physicians rely on timely laboratory data that remains consistent regardless of its origin and most will take the quality (accuracy, reproducibility, clinical relevance) of the result for granted. Most physicians rightly assume that quality is assured as a routine part of the work of the laboratory and would not expect that different versions of tests would produce different results on the same sample, or that exactly the same test on the same sample might produce different results in different places. Laboratories and their suppliers strive to achieve this by monitoring and standardizing test methodologies with the aid of robust internal and external quality control. Where standardization (same result in same units on the same sample, everywhere) is not possible, we aim for harmonization of reporting outcomes (all positive and negative results match, irrespective of units). Despite such an ethos, laboratory results on the same patient sample can vary due to rapid development in the diagnostic service or methodology, or the pressures of increasing workload. From time to time, in order to continue with the ethos of improving patient care and outcome, it is essential to examine procedures; where a problem is intractable and important we may need to develop guidelines/best practice advice to attain harmonization.
We sought to examine how effectively we had achieved the above goals in a specialized neurological test for the Paraneoplastic neurological syndromes (PNS) that are associated with paraneoplastic neurological anti-neuronal antibodies (PNA). Paraneoplastic neurological syndromes are autoimmune disorders where the remote immunological effects are triggered by the presence of a (often occult) tumor. This autoimmune response results in neurological signs due to neuronal damage or dysfunction. The first credible evidence for such malignancy-associated autoimmunity, misdirected against neurological tissue, was provided by Posner in 1985 [1]. PNA are an invaluable early and precise diagnostic marker of rare debilitating neurological disorders. Furthermore, these antibodies do alert the clinicians of possible existence and location of underlying malignancy. Consequently, the early diagnosis of PNS can often lead to the discovery and effective treatment of the underlying malignancy, and is also a crucial step in the management of the PNS [2].
In the early years, detection of PNA suffered from variability due to non-standard procedures, often developed and validated in-house by research groups [3-7]. As these become adopted widely, or were translated into commercial versions of the original assay or new lookalike variants, we saw increasing disparities in inter-laboratory comparisons, thus prompting the development of the first guideline for detection and classification of paraneoplastic anti-neuronal specific antibodies [8]. This guideline was supposed to provide greater harmonization of use and reporting, but covered only three antibodies (Hu (ANNA1), Ri (ANNA2) and Yo (PCA1)). However, following Posner’s publication, the numbers of recognised PNS-associated antineuronal antibody specificities have expanded rapidly.
The current repertoire of autoantibody targets associated with PNS can be divided into fully or partially characterised antigens. These can be further subdivided into; those which are easily identifiable (Hu, Ri, and Yo), and the more challenging types (CV2/CRMP5, Ma2, amphiphysin and Tr). These require expertise and experience in analysis together with harmonisation and EQA (see Table 1 for a list of the characterised antibodies).
| Identified with ease antibodies | ||
|---|---|---|
| Antibody | Neurological disorder(s) | Most frequent tumour(s) |
| Hu (ANNA1) | Paraneoplasticcerebellar degeneration, paraneoplastic encephalomyelitis, sensory neuropathy | Small cell lung carcinoma |
| Yo (PCA-1) | paraneoplastic cerebellar degeneration | Ovary, breast |
| Ri (ANNA2) | opsoclonus/myclonus, paraneoplastic cerebellar degeneration, brainstem encephalomyelitis | Breast, small cell lung carcinoma, gynaecologicaltumours |
| Difficult specificities | ||
| Ma2 (Ta) | brainstem encephalomyelitis, limbic encephalopathy | Testicular cancer |
| CV2/CRMP5 | paraneoplastic encephalomyelitis / sensory neuropathy | Small cell lung carcinoma, thymoma |
| Amphiphysin | Stiff person syndrome, paraneoplastic encephalomyelitis | Breast cancer, small cell lung carcinoma |
| Tr (PCA-Tr) | Paraneoplastic cerebellar degeneration | Hodgkin’s lymphoma |
Table 1: Characterised paraneoplastic neurological antibodies with their most commonly associated malignancies. These have been divided into ease of their identification. Note this list is not exhaustive and only includes some of the most commonly quoted antibodies.
As a result of the proliferation of new specificities and an extended understanding of new disease associations, interpretation has become very complex; not only can single antibody specificity be found in more than one neurological disorder, but individual syndromes can be associated with different antibody specificities. A further confounding factor is that less than 50% of patients with PNS will harbour PNAs and in 30%, more than one PNA is likely to be detected [9].
Furthermore, detection of antibody does not necessarily mean manifestation of clinical disease. PNA can be found in up to 16% of cancer patients who are neurologically asymptomatic, whilst up to 11% of subjects with PNA and neurological symptoms may not have a detectable neoplasm.
These anomalies created a need for further standardization of diagnostic criteria and classification of PNS. This was addressed by a study supported by the European Union to define standards for the diagnosis and classification of PNS [10]. However, the authors noted and raised concerns that detection methodologies for neuronal antibodies were not widely standardised and to our knowledge there has been no significant improvement since then. As a pre-requisite to achieve clinically useful standardization, it is essential to have interlaboratory monitoring via independent EQA Schemes.
Due to diversity in the clinical syndromes and autoimmune neurological response, screening for a range of neuronal antibodies is now thought more effective than testing for specific PNA individually. However, there are considerable difficulties in obtaining enough positive control material to cover all the rare specificities. There was a clear need and role for an EQA scheme in this area.
In 2010, the challenge of developing external quality assessment was taken on by United Kingdom National External Quality Assessment Service for Immunology, Immunochemistry & Allergy (UK NEQAS IIA) at the behest of its independent steering committee. UK NEQAS IIA introduced a pilot scheme for paraneoplastic neurological antibodies, sending out two samples every two months. Five years later, this scheme has grown to over a 100 international participants.
Many lessons have been learned. It is evident that there is a reluctance to confidently report negative samples for paraneoplastic neurological antibodies utilising normal clinical screening practice. This was highlighted by the fact that the majority of the participants would carry out unnecessary secondary testing on negative samples. This is human nature, but something that UK NEQAS IIA attempts to discourage. The purpose of EQA is to determine performance in routine practice. Nothing over and above this should be done to the samples, to ensure that the inter-laboratory comparisons are actually relevant to patient care.
In view of the lack of published guidelines for detecting neurological antibodies, the Steering group and UK NEQAS IIA resolved to disseminate information on best practice amongst the participants together with a snapshot of the performance. First we needed to obtain general consensus amongst the expert participants of the paraneoplastic neurological scheme registered with UK NEQAS IIA, with the aim to establish a general guideline for the detection process.
Method
A detailed questionnaire was prepared and distributed by Survey- Monkey™ to all137 participants in the UK NEQAS Paraneoplastic Neurological Antibody Scheme EQA across the UK, continental Europe and non-European countries. We surveyed the location, methods, specificities and turnaround time. We also surveyed screening policy; confirmation policy, reporting and examined the EQA performance of the group that coincided with the survey, but the focus of this report will be the eleven points that are relevant to the provision of diagnostic service and are outlined in Table 2.
| Screening Policy |
|---|
| Laboratory location – Sample stability during transportation |
| Level of Testing – complete or partial |
| Repertoire of antibodies |
| Source and type of tissue used |
| Type and sample dilution |
| Screening strategy – single or combined sample |
| Type of detection system – Immunofluorescence etc. |
| Confirmation Policy |
| Supporting evidence for the specificity of the positive pattern |
| Commercial (C.E marked) or in house componentof the assay |
| Reporting |
| Quoted turnaround time |
| EQA |
| Performance of the group |
Table 2: List of questions pertaining to the PNA service.
Results
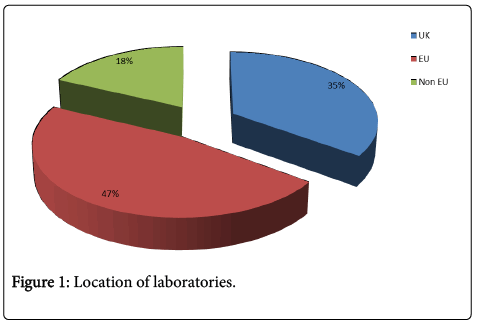
66 of 137 participants responded (48.2%). 54 (82%) were from the European Union (EU) (including 35% from the UK) and 18% non-EU (Figure 1). Forty eight (74%) responded to questions about their paraneoplastic neurological antibody service.
Repertoire
Most services offered the entire major antibody profile (Hu, Ri, Yo, CV2, Ma2 and amphiphysin) associated with PNS (between 89 to 96% for each specificity).
96% offered the 3 easily identifiable antibodies (Hu, Ri and Yo) and 89% also offered (CV2, Ma2 and amphiphysin).
Specialised and antibody detection of poorly characterised specificities, i.e. anti-Tr, appears to be confined to fewer centres (63%; Table 3a).
| % (n) | |
| Fully characterized | |
| Hu (ANNA1), Ri (ANNA2) &Yo (PCA1) | 96 (44) |
| CV2 (CRMP5), Ma2 &Amphiphysin | 89 (41) |
| Partially characterized | |
| Tr | 63 (46) |
Table 3a: Repertoire of antibody specificities provided by labs (n=46).
Turnaround time
The turnaround time was variable: for negative screening 87% reported within14 days but positive identification was only achieved in 14 days for 66%. 23% of laboratories report a positive result between 15 to 28 days, but 11% take between 29 and 45 days (Table 3b).
| Turnaround time (days) | Percentage | |
|---|---|---|
| Negative | Positive | |
| 14-Jan | 87 | 66 |
| 15-28 | 13 | 23 |
| 29-45 | - | 11 |
Table 3b: Quoted turnaround time for sample by the laboratory (n=47).
Screening methodology
There is a clear consensus (90%) for the use of primate cerebellum as an initial screen for the detection of PNA (Table 4a). A few labs still use rat tissue (6%) or other assays (such as immunoblot) for primary screening (4%). Most screening tissue substrates were supplied by commercial manufacturers and were C.E marked.
| % (n) | ||
| (a) Cerebellum –100% commercial sourced material | Primate | 89.6 (43) |
| Rat | 6.3 (3) | |
| Immunoblot | 4.1 (2) | |
| % (n) | ||
| (b) Serum dilution | 1/10 | 27 (13) |
| 1/50 | 40 (19) | |
| 1/100 | 16.6 (8) | |
| 1/20, 1/40 and combination of dilution | 16.6(8) | |
| % (n) | ||
| (c) CSF dilution | Neat | 50 (25) |
| Not done | 36 (18) | |
| 1/5, 1/10 and 1/20 | 14 (7) | |
| % (n) | ||
| (d) Combining several different samples for screening | Combining samples | 21 (10) |
| Separate | 79 (38) | |
| % (n) | ||
| (e) Detection system | Immunofluorescence | 95.8 (46) |
| Immunohistochemistry | 4 (2) | |
| % (n) | ||
| (f) Confirmatory test - | Commercial immunoblots | 40 (85) |
| In house | 1 (2) | |
| Referral | 6 (13) | |
Table 4: Data is presented in order of the steps in the detection procedure. The majority of the laboratories are using a commercially available product (C.E marked*) for detection of paraneoplastic neurological antibodies. (n) = number of participants providing information on (a) source of tissue; (b) serum and (c) CSF dilution; (d) combining samples for screening; (e) detection system and (f) confirmatory test. * C.E is abbreviation for “Conformité Européene”, meaning “European Conformity”.
Screening dilution
Blood: These are variable, and require local validation of sensitivity. They ranged from 1/10 to 1/100 but 40% used 1/50, and 27%; 1/10 (Table 4b).
CSF: Only 64% of participants offered CSF screening. 50% advocate testing neat CSF but a few (14%) utilise a range of dilutions (from 1/5 to 1/20). The remaining 36% had no provision for CSF analysis for PNA (Table 4c).
However, it was very interesting and surprising to learn that 21% (10/48) of the contributors combined different samples together for screening purpose (Table 4d).
Confirmation of positive screens
Following the first serum or CSF incubation step, the bound human immunoglobulins were detected by fluorescence labelled anti-human immunoglobulin (96%) and only two participants resort to other means (Table 4e). In the event the screen was positive; the identity of the specific antigen was confirmed by commercial immunoblotting procedures by most (85%; Table 4f).
EQA performance
Two samples were sent by UK NEQAS IIA to 130 recipients and 85% responded. The results of which shows that 79% and 94% identified correctly the two samples as Yo (PCA1) and Hu (ANNA1) respectively (Table 5).
| PNA | % Correct identification | % Referral to other labs |
|---|---|---|
| Yo (PCA1) | 79 * | 11 |
| Hu (ANNA1 | 94* | 9 |
Table 5: Shows the performance of the 109 participants that coincided with the survey. Two positive samples were distributed by UK NEQAS. *This value includes 4.5% of the referral result.
Discussion
By 2015, the membership of the UK NEQAS IIA Para neoplastic neurological EQA scheme had expanded from just fewer than 30 in the pilot phase to over 100 worldwide - indicative of the high number of centers engaged in the provision of this specialized diagnostic service.
Repertoire
This survey revealed that most of the centers were providing the majority of the well-recognised and characterized antibody repertoire of currently established clinical utility (there are many newly described specificities that may be useful in future): Hu (ANNA1), Ri (ANNA2), Yo (PCA1), CV2 (CRMP5), Ma2 and amphiphysin but the anti-Tr antibody appears to be confined to more specialist centers (63%). The rarer specificities (such as PCA2 and ANNA3) tend to be only offered by to about a fifth of the hubs.
Harmonization of primary screening method
In terms of the harmonization of the detection method of the neurological antibodies, 90% of the centers achieved this for primary screen by the use of commercially available C.E marked primate cerebellar tissue. This approach provides an opportunity to detect a whole range of antibodies rather than single or restricted number of specificities. The next phase is to determine if all of the tissues and different screening dilutions achieve the same performance characteristics in detecting antibody.
Determining the appropriate screening sensitivity and monitoring that through EQA and IAC will be important to ensure effective early detection of occult cancer. 4% of respondents were using antigenspecific alternative methods such as immunoblot for detecting para neoplastic antibodies. Immunoblotting a fixed panel may be satisfactory for the specificities it covers, but can lead to missing out diagnostically important reactivity’s if not included, and in such a rapidly developing field may prevent the development of new knowledge by observation of any unusual or new antibodies which may be observed by alternatives such as immunofluorescence [9]. This may be an important consideration for specialist laboratories who will be contributing to new discovery.
Heterogeneity of screening dilution is problematic for all assays, particularly for DIF and IIF assays, and will result in different diagnostic sensitivities. Many of these screening dilutions are dictated by the IVD declaration of a commercial kit. EQA data will be essential to monitor the relative sensitivity of methods across time, but laboratories will need to develop internal IQA and verification processes to ensure stability of their sensitivity across time. Dilutions for blood screening range from 1/10 to 1/100 with 40% operating at 1/50 (which is likely to be one manufacture, while another kit advises testing at two dilutions (1/10 and 1/100)). Despite the variation in the screening practice, the accompanying EQA data is very encouraging (Table 5) and demonstrates that the performance of the group is at an acceptable level with more than three quarters of the laboratories correctly identifying the two common antibodies (Hu and Yo).
This survey has further revealed what amounts to an apparently cost-cutting approach by 21% of the laboratories where they combine a number of samples in the same tube for screening purposes. This is a very unusual practice and raises numerous Quality Control issues not least the effects of mixing, matrix or other interferences which is presumably based on the fact that the majority of the samples are likely to be negative. You can wonder how this would comply with the new ISO 15189 standards and what approach is used with the UK NEQAS IIA samples. Clearly careful verification of this approach is needed.
CSF testing
CSF testing has become increasingly available and 64% of laboratories offer it currently. In 2011, McKeon [11] reported detecting para neoplastic antibodies in CSF when the blood test was seronegative. This report has generated a surge of interest in testing the CSF for these antibodies to the extent that now 63% of the participants have taken up testing neat CSF with about 13% of these are diluting the CSF between 1/5 to 1/20.
Early treatment of PNS is essential for two reasons, firstly to curtail the irreversible neuronal damage caused by the immune system and secondly to stabilize the syndrome in order to underscore the quality of life. The provision of timely reporting is essential: 66% of positive cases were reported within 14 days but in 11% up to 45 days (Table 3b). A snapshot of the actual time taken (i.e. receipt of sample to authorization of the result in working days) was obtained from one of the labs. Amongst 88 positive cases for Para neoplastic neurological antibodies, 66% of the results were dispatched within 7 days which reached 96% in 14 days (personal communication).
In view of the rapid rate of deterioration within days in some PNS cases, is this delay acceptable? Clearly, guidance is required regarding clinically appropriate TAT limits. It might be useful to do further studies in the future to identify the actual (rather than quoted) turnaround times for both negative and positive samples.
Guidance
There is clearly a need for guidance on best practice to assist harmonization. EQA performance data is helpful in advancing harmonization and standardization of the methodology and to formulate guidelines for detection of PNA.
We developed the following algorithm for the detection of PNAs: If the initial serum is positive with an identifiable pattern then it remains to be confirmed with a secondary method. However, where there is lack of clarity (non-recognisable pattern) due to high titre antibody or the presence of either non-neuronal antibodies such as mitochondria [5] or multiple PNAs [12] then further test is warranted which relies on a secondary method such as an immunoblot with several antigens (most economical) to resolve the pattern or eliminate PNA [5]. However, if the initial serum testing is negative but the clinical suspicion remains high then this may warrant repeating the tests in the CSF (preferably neat) [11].
Based on this survey, the authors present a summary of the current state of practice by the UK NEQAS IIA paraneoplastic neurological EQA scheme participants (Table 6) with the performance that is between around 80 to 95% (Table 5). The practice used by the majority of laboratories includes an initial screening of PNA using 1/50 (second most common 1/10) dilution of the serum on primate cerebellum and the pattern read using indirect immunofluorescence (Table 6). An identifiable monospecific PNA pattern on the cerebellum is reported as the one which has been confirmed by the immunoblot.
| Source of tissue | Primate cerebellum |
|---|---|
| Sample dilution | |
| Serum Serum CSF |
1/50 (Manufacture 1) 1/10 (Manufacture 2) Neat |
| Detection system | Indirect immunofluorescence |
| Confirming the identity of antigen | Immunoblot |
Table 6: Based on the feedback from the centers around the world, the following summary statement is made based on widespread laboratory practice for the detection of paraneoplastic neurological antibodies
associated with PNS.
In conclusion, despite some variations there was a fairly good agreement in methods used and EQA performance.
Acknowledgements
The authors wish to acknowledge the support, comments and suggestions made by the committee member of the United Kingdom National External Quality Assessment Service for Immunology, Immunochemistry & Allergy. Furthermore our thanks go to Clinical Immunology, The Medical School; Birmingham for providing us with the actual turnaround time in working days.
References
- Graus F, Cordoncardo C, Posner JB (1985) Neuronal antineuronal antibody in sensory neuropathy from lung cancer. Neurology 35: 538-543.
- Karim AR, Jacob S (2012) Immunological markers in neurological disorders. Ann Clin Biochem 49: 29-43.
- Graus F, Dalmau R, Rene R,Tora M, Malats N et al. (1997) Anti-Hu antibodies in patients with small-cell lung cancer: association with complete response to therapy and improved survival. J ClinOncol 15: 2866-2872.
- Bradwell AR (2000) Paraneoplastic neurological syndromes associated with Yo, Hu, and Ri autoantibodies. Clin Rev Allergy Immunol19: 19-29.
- Karim AR, Hughes RG, Winer JB, Williams AC, Bradwell AR (2005) Paraneoplastic Neurological Antibodies: A Laboratory Experience. Ann NY AcadSci 1050: 274-285
- Bradwell AR, Hughes RG, Hunt MJ, Karim AR. Atlas of autoantibody patterns, 3rd edition, Printers KNP Group Ltd, UK, ISBN: 070442701X, 2008
- Probst C, Saschenbrecker S, Stoecker W, Komorowski L (2014) Anti-neuronal autoantibodies: Current diagnostic challenges. Mult Scler Relat Disord 3: 303-320.
- Moll JW, Antoine JC, Brashear HR, Delattre J, Drlicek M, et al. (1995) Guidelines on the detection of paraneoplastic anti-neuronal-specific antibodies. Neurology 45: 1937-1941.
- Pittock SJ, Kryzer TJ, Lennon VA (2004) Paraneoplastic antibodies coexist and predict cancer, not neurological syndrome. Ann Neurol 56: 715-719.
- Graus F, DelattreJY, Antoine JC, Dalmau J, Giometto B,et al.(2004) Recommended diagnostic criteria for paraneoplastic neurological syndromes. J NeurolNeurosurgPsychiat 75: 1135-1140.
- McKeon A, Pittock SJ, Lennon VA (2011) CSF complements serum for evaluating paraneoplastic antibodies and NMO-IgG. Neurology 76: 1108-1110.
- Karim AR, Jacob S (2010) Neuroimmunology. In: Hall A and Yates C (eds.) Immunology. Oxford University Press, 74- 209.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 11484
- [From(publication date):
March-2016 - Apr 04, 2025] - Breakdown by view type
- HTML page views : 10554
- PDF downloads : 930