Interleukin-18 has an Important Role in Differentiation and Maturation of Mucosal Mast Cells
Received: 30-May-2018 / Accepted Date: 25-Jun-2018 / Published Date: 02-Jul-2018
Abstract
A significant amount of correlational evidence has linked increased levels of IL-18 with allergic diseases in both human and animal models, and, as mast cells are major mediators of allergies, we hypothesized that IL-18 may have a role in mast cell biology. Rationale for our hypothesis is based on the evidence that IL-3 deficient mice are not devoid of mast cells, even though IL-3 is a major differentiation and growth factor for mast cells. Accordingly, we cultured IL-18 responsive bone marrow CD34+ cells in vitro under a variety of conditions and cytokine combinations to examine mast cell differentiation and maturation using flow cytometry, quantitative PCR,and immunostaining techniques. Additionally, in vivo mast cell transformation and maturation were also analysed using endogenous IL-18 gene-deficient or Fabpi-IL-18 overexpressed mice. Our data indicate that both IL-3 and IL-18 exposed CD34+ bone marrow precursors differentiate and mature into mast cells. Further, we observed that IL-18 differentiates mast cells independent of IL-3, as pharmacologic blockade of IL-3 does not prevent in vitro IL-18-driven mast cell differentiation. Further, we found that endogenous IL-18 deficiency restricts maturation of IL-3 generated mast cells and IL-18 derived mast cells require IL-3 for their survival. Additionally, we observed IL-18 intestinal overexpression promotes tissue mast cell proliferation and mucosal mast cell development. Taken together, we provide the evidence that IL-18 has an important contributory role in mast cell differentiation, maturation and in vivo development of mucosal mast cells. Therefore, IL-18 may represent a future pharmacologic target for treating mast cell-mediated allergic diseases.
Keywords: Allergy; Interleukin-18; Mast cell; Mucosal immunity
Introduction
Interleukin (IL)-18 was first known as interferon (IFN)-γ inducing factor due to its ability to induce Type 1 helper T (Th1) cells to release IFN-γ [1]. IL-18 is an IL-1 family cytokine implicated in various aspects of the innate and adaptive immune system, with some analogy to IL-1β. While IL-18 was first demonstrated to induce a Th1 response, it is now appreciated that whether a Th1 or Th2 response is induced depends on the local cytokine milieu and other experimental conditions [2]. Induced IL-18 is now identified in a number of disorders, such as autoimmunity, atopic and allergen-induced allergic responses, and defence against pathogens, most notably helminths. IL-3 has long been known to be a major differentiation factor for mast cells [3]. However, the exact lineage from hematopoietic stem cells (HSCs) to mast cells has remained unclear until recently. HSCs give rise to multipotent progenitors, and these can become common myeloid progenitors and subsequently granulocyte/monocyte progenitors (GMPs) [4]. However, the mast cell lineage was the subject of significant debate and research. IL-3 is now appreciated to induce the development of basophil-restricted and basophil/mast cell progenitors from GMPs [5]. Notably, several studies have demonstrated correlation between increased serum IgE and IL-18 levels [6-8], and increased IgE levels increase expression of FcεRI on mast cells [9], so it stands to reason that IL-18 may correlate with mast cell function. There is also a significant amount of correlational evidence linking increased levels of IL-18 with allergic diseases in both human and animal models such as rhinitis, dermatitis, and asthma, as well as eosinophilia gastrointestinal disorders [10]. Recent research has found IL-18 to induce the release of a variety of molecules from mast cells, such as the Th2 cytokines IL-4 and IL-13, and found that IL-18 works in synergy with IL-3 to increase this release [11]. IL-18 is reported to correlate with mast cell accumulation in human eosinophilia esophagitis (EoE) and induce accumulation in mouse models of asthma and EoE [12]. The role of IL-18 on the in vivo accumulation and maturation of mast cells is unclear, as there is conflicting evidence in the literature. Most studies to date have utilized a model of intestinal mastocytosis induced by intestinal nematodes, with several reporting increased mast cell accumulation with faster parasite expulsion by IL-18 [13], while other studies observed this same result upon endogenous knockout of IL-18 and found decreased mast cell accumulation upon rIL-18 treatment [14]. A mouse model of atopic dermatitis also suggested that IL-18-dependent IL-3 production contributes to the development of cutaneous mastocytosis [15]. The lack of evidence regarding the direct effects of IL-18 on mast cell differentiation and maturation and the conflicting results regarding the effects of IL-18 on mucosal mast cells led us to hypothesize that IL-18 may have a contributory role in their differentiation, maturation, and development. Herein, we show that indeed IL-18 has a significant role in mast cell differentiation and in vivo maturation of mucosal mast cells.
Methods
Cell cultures
Bone marrow was isolated from the tibia and femur of wild-type (Balb/c) mice or IL-18 endogenous knockout (IL-18 KO) mice and grown in RPMI 1640 media supplemented with 20% fatal bovine serum (FBS), 2 mM glutamine, 25 mM HEPES, 0.1 mM non-essential amino acids, 1 mM sodium pyruvate, 50 μM β-mercaptoethanol, 100 U/mL penicillin, and 100 μg/mL streptomycin at a concentration of approximately 1 x 106 cells/mL.The media of all cultures was changed three times per week. To these cultures were added stem cell factor (SCF) with IL-3 and/or IL-18, or SCF alone all at a concentration of 20 ng/mL. The IL-3 cultures were maintained in SCF and IL-3 throughout the experiment, the IL-18 cultures were maintained only in SCF and IL-18 for the first two weeks followed by addition of IL-3 for the second two weeks, and the culture labeled IL-3+IL-18 was exposed to SCF with both IL-3 and IL-18 throughout the experiment. The kinetic experiment used SCF and IL-3 (20 ng/mL) with varying concentrations of IL-18 (0-20 ng/mL). All cytokines were purchased from PeproTech (Rocky Hill, NJ).
Flow cytometer analysis
Several combinations of fluorochromes were utilized for analysis based on the combination required for the experiments. One staining combination used was fluorescein isothiocyanate (FITC)-conjugated anti-CD49b (DX5), phycoerythrin (PE)-conjugated anti-c-kit (CD117), 7-aminoactinomycin D (7-AAD), and allophycocyanin (APC)-labeled anti-FcεRIα. Another staining combination utilized FITC-conjugated anti-FcεRIα, PE-conjugated anti-CD49b, 7-AAD, and APC-conjugated anti-c-kit. A third combination utilized FITCconjugated anti-c-kit, PE-conjugated anti-CD49b, 7-AAD, and APCconjugated anti-FcεRIα. In experiments to examine basophil/mast cell precursors and CD34 expression by mast cells, the following combination was used: FITC-conjugated anti-FcεRIα, either PEconjugated anti-CD34 or PE-conjugated anti-CD49b, and PECyanine7- conjugated anti-c-kit. In all experiments, cells were collected, washed, and incubated with 3% normal goat serum at 4˚C for 20 m and then re-suspended in 1% BSA and stained at 4°C for 40 m. Following staining, cells were washed once in 1% BSA and once in PBS before being re-suspended in PBS. 7-AAD stain was utilized to assess viability, and 7-AAD was added to the cells immediately prior to flow analysis. Flow cytometer analysis was performed using a BD Accuri C6 and analysis was accomplished using FlowJo for Windows Version 10. In all experiments, differentiated basophils were defined as FcεRI+c-kit-CD49b+ while mast cells were defined as FcεRI+c-kit +CD49b-.
RNA analysis
Mouse mast cell proteases (mMCPs) show differential regulation based on the stage of development of the mast cell and which mature phenotype it has developed into. mMCP-1 and -2 are expressed in mucosal mast cells while mMCP-4, -5, -6, and -7 are expressed in connective tissue mast cells. The mMCPs used (mMCP-1 and -4) for qPCR were chosen as they are exclusive to mucosal and connectivetissue phenotypes, respectively, and represent beta-chymases. Four weeks cultures were isolated and the RNA was extracted using either Trizol Reagent (Thermo Fischer Scientific; Waltham, MA) according to the manufacturer’s protocol or the QIAamp RNA Blood Mini Kit (Qiagen; Germantown, MD) according to the manufacturer’s protocol. RNA was stored in RNAse-free water at -80˚C, DNAse treatment was performed prior to cDNA synthesis using DNA-free Kit (Ambion; Carlsbad, CA). cDNA was made from the RNA samples using iScript cDNA Synthesis Kit (Bio-Rad; Hercules, CA) according to the manufacturer’s protocol. Quantitative PCR was performed using Sybr Green and primers for b-actin [16], mMCP-1 [17], mMCP-4 [18], FcεRIα [19], FcεRIβ/MS4A2 [19], IL-18 receptor [20], and histidine decarboxylase (HDC) [21] as described previously. B-actin was used for RNA quantification, and Cq values for genes of interest and b-actin were used to create expression values. All the values reported in the results were normalized to the lowest value for ease of comparison.
Cytospin preparation and staining
Approximately 50,000 cells were collected and re-suspended in 1 mL of PBS. Cells were spun with a cytocentrifuge at 850 rpm for 4 m. Cytospin preparations were fixed in either ice-cold Carnoy’s solution or ice-cold methanol. Fixed slides were then stained for mast cells using the alcian blue-safranin O procedure or the toluidine blue procedure and examined by light microscopy. For alcian blue-safranin red (AB-S) staining, fixed slides were rehydrated in DI H20 and then covered in 1% alcian blue in 3% acetic acid (pH 2.5) for 30 m followed by 0.25% safranin O for 5 m. For toluidine blue staining, a working solution was created by mixing one part 1% toluidine blue in 70% EtOH to nine parts 1% NaCl in DI H20 at pH 2.3, and cells were stained for two minutes. The slides for modified Giemsa were stained for one hour. The slides for chloroacetate esterase staining were stained for 45 minutes in a buffered mixture of Napthol AS-D chloroacetate in N, N-dimethyl formamide and Hexazotized New Fuschin, and all slides were counterstained with hematoxylin.
Immunofluorescence staining
Approximately 50,000 cells were collected and fixed in 4% paraformaldehyde and cytospin cells slides prepared as before. Cytospin preparations were fixed in ice-cold methanol followed by immunofluorescent staining. Preparations were blocked in 3% normal goat serum for one hour followed by application of a 1:200 dilution of mouse anti-human tryptase primary antibody and overnight incubation at 4˚C. A FITC-conjugated rat anti-mouse IgG secondary antibody was used followed by 4', 6-diamidino-2-phenylindole dihydrochloride (DAPI) mounting. Images were collected using a confocal microscope. FITC was excited using an argon laser at 488 nm, and DAPI was excited using a 405-nm diode laser. Images were collected and analysed using NIS-Elements software (Nikon).
IL-18 genetic overexpression and IL-18 gene-deficiency
Experiments were conducted to examine the effects of IL-18 on mast cell development in vivo . Wild-type, IL-18 gene-deficient, and intestinal (iFABP)-IL-18 transgenic mice that genetically overexpress IL-18 only in the small intestine were sacrificed at 16 weeks and the ear, distal oesophagus, jejunum, and colon examined for mast cells. Wild type and IL-18 gene-deficient mice were obtained from the Jackson Laboratory (Bar Harbor, ME) and intestinal (FABPi)-IL-18 transgenic mice were developed in our laboratory using the intestinal specific fatty acid binding protein (FABPi) promoter and IL-18 cDNA as per the method described earlier [22]. The Tulane Institutional Animal Care and Use Committee (IACUC) approved the animal protocols that were employed in accordance with National Institute of Health (NIH) guidelines.
rIL-18 treatment
Balb/c mice were injected intraperitoneally three times per week with either saline or 5 μg rIL-18 for two weeks then sacrificed and the ear, stomach, and jejunum examined. In these mice, spleenocytes and bone marrow cells were isolated and a wash of the peritoneum was collected by injecting 5-10 mL of PBS into the peritoneal cavity followed by aspiration of the fluid. Spleenocytes, bone marrow cells, and peritoneal cells were examined by flow cytometry using the same protocol as mentioned previously. All tissue samples collected were fixed in 10% formalin followed by processing and embedding in paraffin. 5 μm sections were taken for staining with either modified Giemsa or chloroacetate esterase.
Statistical analysis
GraphPad Prism Version 5.01 was utilized for all statistical calculations. A one-way analysis of variance (ANOVA) with Tukey post-tests using an alpha of 0.05 was utilized to compare data with more than two groups. When a significant difference was identified between two means by the Tukey post-test, an unpaired, two-tailed ttest with an alpha of 0.05 was used to generate an exact p-value.
Results
IL-3 and IL-18 differentiate mast cells and basophils in culture
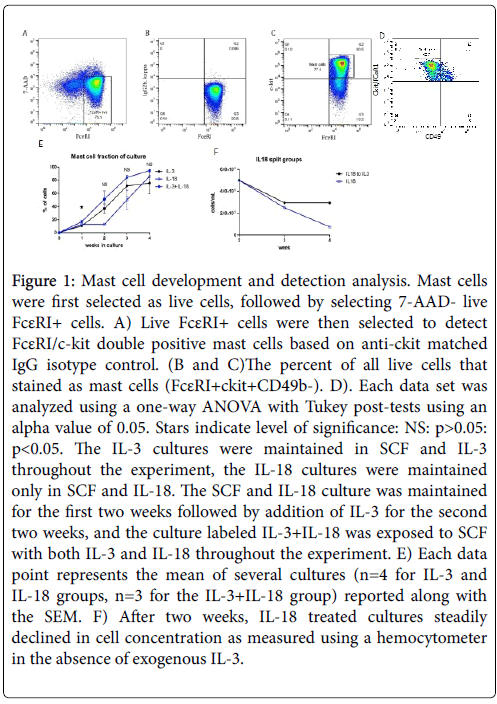
The role of IL-18 was examined for in vitro differentiation and development of mast cells by exposing mouse CD34+ bone marrow cells to rIL-18 for 4 weeks at 37˚C in a 5% CO2 cell culture incubator. Bone marrow cells were also exposed to the well-established mast cell differentiation cytokine rIL-3 for comparison and morphological analysis. Further, we also exposed cells to the combination of IL-3 and IL-18.The concentration of IL-18 exposed cells steadily decreased in vitro ; therefore, after two weeks IL-3 was added to the IL-18 exposed cells to maintain survival of differentiated mast cells. Therefore, only effects seen in the first two weeks can be attributed solely to exogenous IL-18 and the role of IL-3 cannot be ruled out at weeks 3 and 4. The in vitro developed mast cells were identified by flow cytometry by selecting live 7-AAD-FcεRI+ cells followed by selection of FcεRI/c-kit double positive cells (Figure 1A-C). Furthermore, the IL-3 or IL-18 derived mast cells were confirmed anti-CD49b negative cells (data not shown in dot blot flow cytometer analysis). FcεRI+ckit+CD49b- cells were identified as mast cells (Figure 1D) and FcεRI+ckit-CD49b+ cells were identified as basophils (data not shown). All cytokine exposed groups of bone marrow cells differentiated mast cells and basophils and produced a near pure mast cell culture by four weeks. The combined group had a significantly greater percentage of mast cells at week 1, and the mast cell fraction remained non-significantly larger in week 2 to week 4. The per cent of mast cells differentiated following week 1 of IL-3, IL-18 and IL-3+IL-18 cultures was ~10%, 12% and 16, respectively. This mast cell fraction increased in 4 weeks to ~92% with IL-3, ~94% with IL-18, and ~97% with the combination of IL-3 and IL-18. All groups of mast cells were confirmed to express IL-18 receptor mRNA.
Figure 1: Mast cell development and detection analysis. Mast cells were first selected as live cells, followed by selecting 7-AAD- live FcεRI+ cells. A) Live FcεRI+ cells were then selected to detect FcεRI/c-kit double positive mast cells based on anti-ckit matched IgG isotype control. (B and C)The percent of all live cells that stained as mast cells (FcεRI+ckit+CD49b-). D). Each data set was analyzed using a one-way ANOVA with Tukey post-tests using an alpha value of 0.05. Stars indicate level of significance: NS: p>0.05: p<0.05. The IL-3 cultures were maintained in SCF and IL-3 throughout the experiment, the IL-18 cultures were maintained only in SCF and IL-18. The SCF and IL-18 culture was maintained for the first two weeks followed by addition of IL-3 for the second two weeks, and the culture labeled IL-3+IL-18 was exposed to SCF with both IL-3 and IL-18 throughout the experiment. E) Each data point represents the mean of several cultures (n=4 for IL-3 and IL-18 groups, n=3 for the IL-3+IL-18 group) reported along with the SEM. F) After two weeks, IL-18 treated cultures steadily declined in cell concentration as measured using a hemocytometer in the absence of exogenous IL-3.
IL-3 is a survival factor for in vitro IL-18 differentiated mast cells
Since IL-18 differentiated mast cells steadily decrease in number with the time in culture, we analysed differential survival after two weeks of IL-3-derived, IL-18-derived, and IL-3 with IL-18 combined derived mast cells. Each group of differentiated mast cells was split after two weeks into one of three cytokine conditions: IL-3, IL-18, or IL-3 with IL-18. Cell survival, as measured by total cell counts using a hemocytometer, was similar for IL-3 and IL-3 with IL-18 combined treatment. However, cells from each culture condition switched to grow in IL-18 alone steadily decreased in number each week. Figure 1E shows the total cell number in IL-18 derived mast cells in weeks 2-4. The decrease in cell number in the absence of IL-3 indicates IL-3 is a survival factor for mast cells.
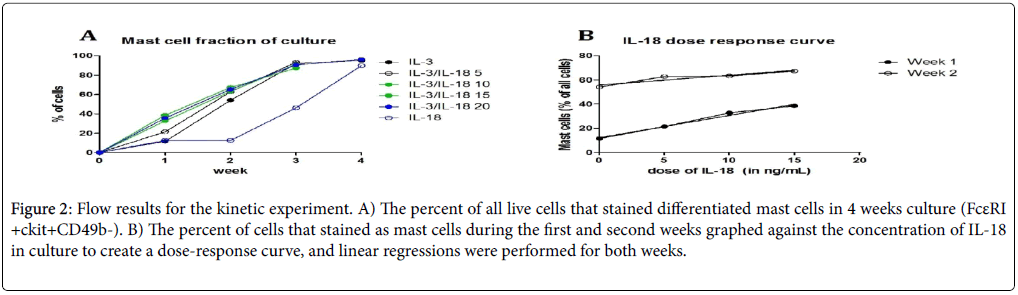
Further, to establish that synergy of IL-3 and IL-18 is important for mast cell survival and differentiation; varying doses of IL-18 were added in culture to IL-3. We observed that the percentage of mast cells increased with increasing doses of IL-18 with IL-3 (Figures 2A and 2B). The results in week one showed a strong positive correlation with IL-18 dose up to 15 ng/mL (r2=0.9848, p=0.0076).
Figure 2: Flow results for the kinetic experiment. A) The percent of all live cells that stained differentiated mast cells in 4 weeks culture (FcεRI +ckit+CD49b-). B) The percent of cells that stained as mast cells during the first and second weeks graphed against the concentration of IL-18 in culture to create a dose-response curve, and linear regressions were performed for both weeks.
Endogenous IL-18 is required for IL-18 driven mast cell differentiation
Next, we tested whether endogenous IL-18 deficiency affects mast cell differentiation. Accordingly, bone marrow of wild-type mice and IL-18 gene-deficient mice were exposed to IL-3 and/or IL-18 for four weeks in culture (data not shown). Differences in survival between wild-type and IL-18 gene-deficient cell cultures were minimal for IL-3 and IL-3+IL-18 exposed cells. However, the IL-18 gene-deficient cell culture treated with rIL-18 was almost completely dead by the end of two weeks, while wild-type cell cultures with rIL-18 alone survive through three weeks. This suggests that endogenous IL-18 is not required for the IL-3-driven differentiation of mast cells, but endogenous IL-18 may regulate IL-3 to produce synergy for mast cell development.
IL-18 promotes toluidine blue and alcian blue-safranin red stained mature mast cells
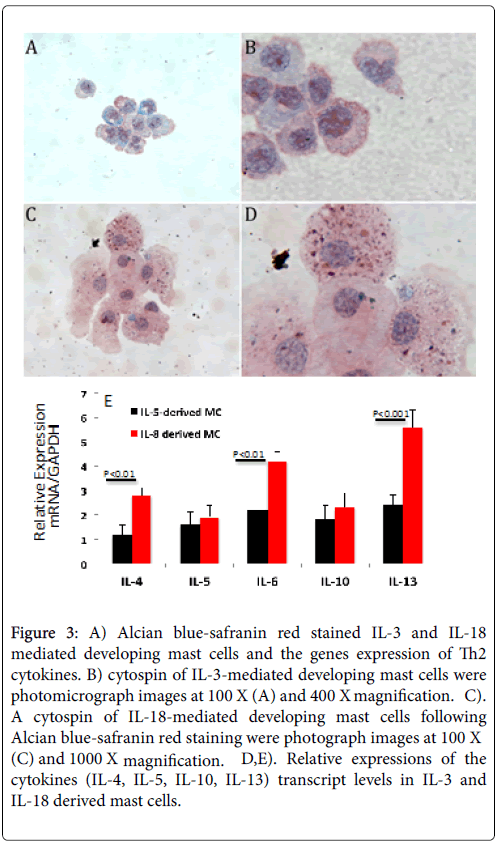
Cytospin preparations of IL-3, IL-18 and IL-3+IL-18 generated mast cells from the bone marrow cells of wild-type mice were stained with Toluidine blue (TB). Both IL-18 and the combination IL-3+IL-18 generated mast cells showed more TB stained granules compared to the IL-3 only generated mast cells. A similar observation was noticed with Alcian blue-safranin red (AB-S) staining of cytospin slides of mast cells, shown in Figure 3 for IL-3 generated mast cells (Figure 3A and 3B) and for IL-18 generated mast cells (Figure 3C and 3D). All groups of generated mast cells stained a mixture of red and blue, but only cells cultured in IL-18 showed mature alcian blue+(blue) and safranin+(red) granules.
Figure 3: A) Alcian blue-safranin red stained IL-3 and IL-18 mediated developing mast cells and the genes expression of Th2 cytokines. B) cytospin of IL-3-mediated developing mast cells were photomicrograph images at 100 X (A) and 400 X magnification. C). A cytospin of IL-18-mediated developing mast cells following Alcian blue-safranin red staining were photograph images at 100 X (C) and 1000 X magnification. D,E). Relative expressions of the cytokines (IL-4, IL-5, IL-10, IL-13) transcript levels in IL-3 and IL-18 derived mast cells.
The alcian blue-safranin reaction distinguishes the level of sulfonation of mucopolysaccharides present in mast cells. As mast cell granules are formed, a heparin precursor that stains with alcian blue is synthesized and accumulates, and in connective-tissue type mast cells a highly N-sulfated heparin product that stains with safranin accumulates [23]. When IL-18 was withdrawn from the combined treatment after two weeks, granules were not observed to stain with AB-S, suggesting IL-18 plays an important role in maturing mast cells.
When IL-18 was added to mast cells that were differentiated in IL-3 alone, granules were observed, suggesting IL-18 can mature IL-3- derived immature mast cells. Further, we examine whether IL-3 and IL-18 differentiated mast cells have similar levels of cytokine genes expression. The real time PCR analysis indicated a significant increase of IL-4, IL-6 and Il-13 in Il-18 derived mast cells compare to the IL-3 derived mast cells (Figure 3E). The genes-expression level of IL-5, and IL-10 was found comparable in both Il-3 and IL-18 derived mast cells (Figure 3E).
IL-18-derived mast cells express chymase mRNA transcripts and tryptase protein, markers of maturation
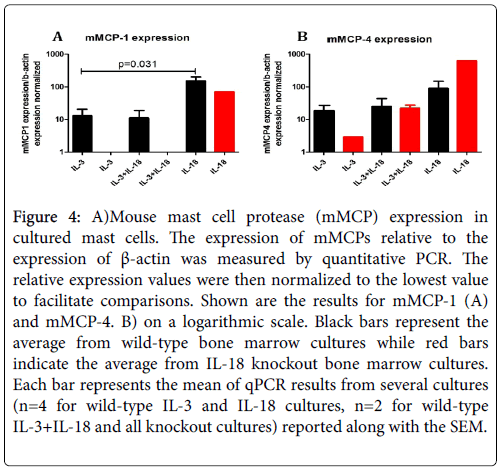
Several reports indicate that mucosal and connective tissue mast cells can be identified by differentially expressed mMCPs; therefore, we were interested to know whether the expression of mMCPs is different in IL-3 and IL-18 generated mast cells. Accordingly, we characterized IL-3 and IL-18 generated mast cells by the expression of mMCPs. The data obtained from quantitative PCR analysis are presented in Figure 4 for mMCP-1 (A) and mMCP-4 (B). The large variation in expression values limited the statistical power of the experiment; however, there was a significantly higher mMCP-1 expression in IL-18 derived versus IL-3 derived mast cells (p=0.031) from the bone marrow cells of wildtype mice. However, no mMCP-1 expression and low levels of mMCP-4 expression was observed in the mast cells generated from the bone marrow cells of IL-18 gene-deficient mice despite the exposure of IL-3 or IL-3 with IL-18 (Figure 4). Surprisingly, while IL-18 derived mast cells showed markedly different expression than IL-3 derived mast cells, the cells grown in both IL-3 and IL-18 from the beginning of the culture had an expression profile resembling that of IL-3. This may indicate that IL-3 supports proliferation of immature mast cells during the first two weeks of culture, and the large numbers of immature mast cells obscure the mature cells in total number. Therefore it is difficult to determine if IL-18 cooperates with IL-3 to mature mucosal mast cells. However, the lack of mMCP expression in the IL-18 gene deficient mast cells indicates IL-18 may be critical to maturation. The similarity in results between mMCP-1 and mMCP-4 expression indicate IL-18 may have similar roles in both mucosal and connective tissue-type beta-chymase expression. While IL-18 cooperates with IL-3 to induce chymase expression in connective tissue mast cells, IL-18 appears to be critical for chymase expression in mucosal mast cells.
Figure 4: A)Mouse mast cell protease (mMCP) expression in cultured mast cells. The expression of mMCPs relative to the expression of β-actin was measured by quantitative PCR. The relative expression values were then normalized to the lowest value to facilitate comparisons. Shown are the results for mMCP-1 (A) and mMCP-4. B) on a logarithmic scale. Black bars represent the average from wild-type bone marrow cultures while red bars indicate the average from IL-18 knockout bone marrow cultures. Each bar represents the mean of qPCR results from several cultures (n=4 for wild-type IL-3 and IL-18 cultures, n=2 for wild-type IL-3+IL-18 and all knockout cultures) reported along with the SEM.
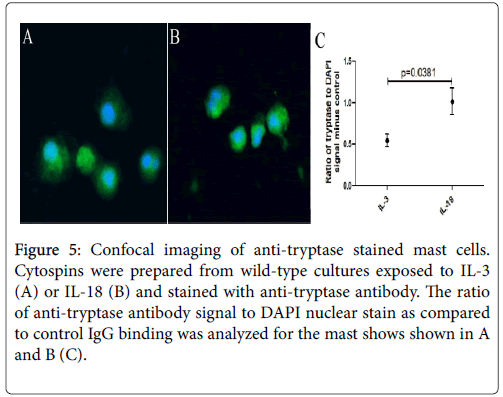
Next, we examined the mast cells by performing confocal microscopy following anti-tryptase staining of IL-3 and IL-18 generated mast cells. Images of both mast cells were taken with the same laser and acquisition settings to allow quantitative comparisons between two groups of mast cells. Tryptase staining was detected in both groups of mast cells. The ratio of anti-tryptase to DAPI signal was higher in the IL-18 generated mast cells (Figure 5B) compared to the IL-3 generated mast cells (Figure 5A).
Figure 5: Confocal imaging of anti-tryptase stained mast cells. Cytospins were prepared from wild-type cultures exposed to IL-3 (A) or IL-18 (B) and stained with anti-tryptase antibody. The ratio of anti-tryptase antibody signal to DAPI nuclear stain as compared to control IgG binding was analyzed for the mast shows shown in A and B (C).
Four mast cells from each group were analysed statistically for tryptase to DAPI signal and the antibody control binding subtracted out. The IL-18-derived mast cells showed a significantly greater signal than IL-3-derived mast cells (p=0.0381, Figure 5C). The analysis suggests that IL-18-derived mast cells have a higher concentration of tryptase granules in the cytoplasm compared to IL-3-derived mast cells.
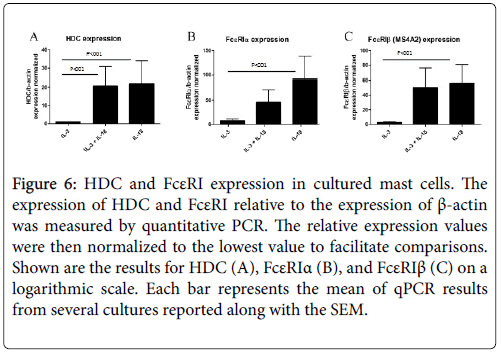
IL-18-derived mast cells express higher levels of HDC and FcεRI
In comparison to the mMCP genes that are specific for mast cell subtypes, HDC and FcεRI expression are more generally correlated with mast cell maturation and activation. Both the alpha and beta subunits of FcεRI were analysed, as some functional FcεRI trimer proteins contain the beta subunit (αβγ) and others do not (αγ2), and the beta subunit has been identified as an atopy gene. Surprisingly, expression of HDC and FcεRIβ was approximately equal for the IL-18 and combined IL-3 and IL-18-derived mast cells, and this was roughly 20 and 55 times greater than that of IL-3-derived mast cells for HDC and FcεRIβ, respectively. FcεRIα expression was greatest in IL-18- derived mast cells, which was twice as large as the combined cytokineexposed mast cells and nearly 100 times greater than IL-3-derived mast cells. This analysis further suggests that IL-18-derived mast cells have a more mature gene expression profile than IL-3-derived mast cells (Figure 6).
Figure 6: HDC and FcεRI expression in cultured mast cells. The expression of HDC and FcεRI relative to the expression of β-actin was measured by quantitative PCR. The relative expression values were then normalized to the lowest value to facilitate comparisons. Shown are the results for HDC (A), FcεRIα (B), and FcεRIβ (C) on a logarithmic scale. Each bar represents the mean of qPCR results from several cultures reported along with the SEM.
IL-18 in vivo increases mast cells in the intestinal mucosa
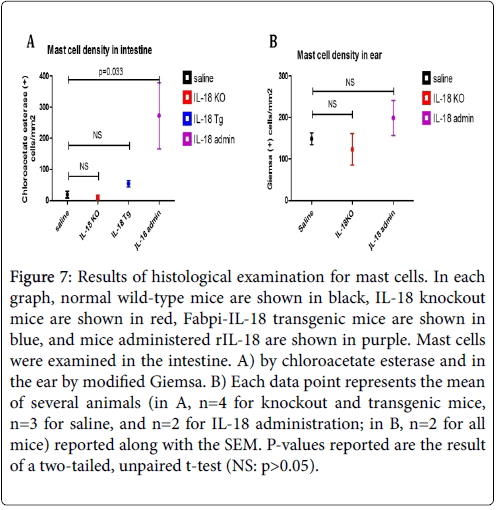
We examined tissues in both modified Giemsa and chloroacetate esterase to determine which yielded better visualization of mast cells. Our results indicated modified Giemsa stain was better for visualizing connective tissue mast cells and was thus used for counting in the ear, while mucosal mast cells stained better with chloroacetate esterase and thus this stain was used for counting in all gastrointestinal tissue. In the jejunum, significant differences were observed in both the intestinal villi and crypts, and the total number of mast cells in the jejunum is shown in Figure 7A. IL-18 gene-deficient (Knockout) mice had a slightly lower mast cell concentration and Fabpi-IL-18 transgenic mice had a higher concentration as compared to wild-type mice, but these changes were not statistically significant because only 2-3 mice of each group was analysed.
Figure 7: Results of histological examination for mast cells. In each graph, normal wild-type mice are shown in black, IL-18 knockout mice are shown in red, Fabpi-IL-18 transgenic mice are shown in blue, and mice administered rIL-18 are shown in purple. Mast cells were examined in the intestine. A) by chloroacetate esterase and in the ear by modified Giemsa. B) Each data point represents the mean of several animals (in A, n=4 for knockout and transgenic mice, n=3 for saline, and n=2 for IL-18 administration; in B, n=2 for all mice) reported along with the SEM. P-values reported are the result of a two-tailed, unpaired t-test (NS: p>0.05).
The Fabpi-IL-18 transgenic mice, overexpressed IL-18 only in the enterocytes of jejunum region (local), not in all segment of gastrointestinal tract, or induce systemic IL-18. Notably, the group of mice administered rIL-18 had an almost 15-fold increase in mast cells in the jejunum (p=0.0327). There were no significant changes observed in the oesophagus, stomach, or colon of IL-18 knockout or Fabpi-IL-18 transgenic mice compared to wild-type mice (data not shown). Additionally, in the ear, a measure of connective tissue-type mast cells, IL-18 gene-deficient mice had a slightly lower mast cell concentration and mice administered rIL-18 had a higher concentration as compared to wild-type mice (123 and 199 versus 149 mast cells/mm2), although this was not statistically significant (Figure 7B).
Discussion
We demonstrated a role for IL-18 in the development and maturation of mast cells by conducting both in vitro and in vivo experiments. Our results indicate that IL-18 can differentiate mast cells independent of IL-3, and these mast cells have characteristics of mature mast cells, however these IL-18 derived mast cells require IL-3 for long-term survival. The more rapid increase in the mast fraction from IL-18 or IL+IL-18 cultures compare to IL-3 culture indicates that IL-18 facilitates IL-3-induced mechanisms. Importantly, we first time demonstrate that IL-18 promotes mast cells differentiation independent of IL-3. The granular content and mMCP transcript expression of IL-18-derived mast cells have characteristics of both mucosal and connective-tissue mast cells, and combined with results from confocal microscopy suggest that IL-18 is capable of maturing mast cells into both a mucosal-type and a connective tissue-type.
Endogenous IL-18 deficiency causes non-significant decreases in the number of both connective tissue and mucosal-type mast cells. Additionally, several of the IL-18 deficient mice had no identifiable mast cells in the small intestine or colon. This result, combined with the cell culture data demonstrating endogenous knockout of IL-18 prevented mMCP-1 expression in IL-3-derived cultures, suggests that: (1) there may be an IL-18 independent pathway to the development of mucosal mast cells that does not normally predominate and is induced by some stimulus, or (2) that the mast cells observed in the one IL-18 knockout mouse may be progenitors that have migrated but not matured. Further, we observed that the number of mast cells increases non-significantly in both the villi and the crypts of the small intestine of mice that overexpress IL-18 in the small intestine (iFABP promoterdriven IL-18). Notably, the induction of mast cells remained localized to the small intestine; there were no increases in the esophagus or the colon. The administration of recombinant IL-18 induces mast cells in both the ear and the intestine.
The non-significant change in mast cell concentration of the ear with IL-18 genetic knockout suggests that the differentiation of connective tissue mast cells is not dependent on IL-18. The mRNA results do indicate that connective tissue-type chymase production is decreased with endogenous IL-18 knockout and is increased with exogenous administration of IL-18 in vitro , indicating IL-18 may play a role in the connective tissue mast cell biology. IL-18 administration also caused non-significant increases in mast cell concentration in the ear, and this result may become significant if more subjects were added. A previously mentioned Terada et al investigation found that IL-18 is required for the development of pathologic changes consistent with atopic dermatitis such as mast cell recruitment in the ear [15]. Our present study indicates that cutaneous mast cells are indeed present in IL-18 knockout mice, but at a lower concentration. This may indicate that IL-18 is not necessary for the actual maturation of connective-tissue mast cells but is necessary for the inflammatory response that leads to the recruitment of mast cells to cutaneous tissue in large numbers.
Our findings that IL-18 can mature and recruit mucosal mast cells in the small intestine are consistent with several reports that speculated the possible therapeutic applications of monoclonal IL-18 antibodies or IL-18 binding protein, the endogenous antagonist of IL-18, to treat autoimmune diseases, boost immune function for chemotherapy, and for various other applications. [24,25]. Taken together, the current study indicates that anti-IL-18 therapy may be an effective treatment strategy for mast cell-mediated gastrointestinal allergic diseases.
Conflicts of interest
We declare that we have no professional or personal affiliations with any organization or entity with a financial or non-financial interest related to the topics presented in this manuscript that could influence the positions stated.
Acknowledgement
We thank Tulane University Honors Program and the Tulane Center for Engaged Learning and Teaching (CELT) student assistance to SN for the undergraduate independent study. Additionally, we recognize the partial support of NIH grant R01 AI080581 (AM) for completing this study. Dr. Mishra is the Endowed Schlieder Chair; therefore, we thank Edward G. Schlieder Educational Foundation for their support.
References
- Okamura H, Nagata K, Komatsu T (1995) A novel costimulatory factor for gamma interferon induction found in the livers of mice causes endotoxic shock. Infect Immun 63: 3966-3972.
- Xu D, Trajkovic V, Hunter D (2000) IL-18 induces the differentiation of Th1 or Th2 cells depending upon cytokine milieu and genetic background. Eur J Immunol 30: 3147-3156.
- Razin E, Ihle JN, Seldin D (1984) Interleukin 3: A differentiation and growth factor for the mouse mast cell that contains chondroitin sulfate E proteoglycan. J Immunol 132: 1479-1486.
- Akashi K, Traver D, Miyamoto T, Weissman IL (2000) A clonogenic common myeloid progenitor that gives rise to all myeloid lineages. Nature 404: 193-197.
- Ohmori K, Luo Y, Jia Y (2009) IL-3 induces basophil expansion in vivo by directing granulocyte-monocyte progenitors to differentiate into basophil lineage-restricted progenitors in the bone marrow and by increasing the number of basophil/mast cell progenitors in the spleen. J Immunol 182: 2835-2841.
- Ando M, Shima M (2007) Serum interleukins 12 and 18 and immunoglobulin E concentrations and allergic symptoms in Japanese schoolchildren. J Investig Allergol Clin Immunol 17: 14-19.
- Aral M (2006) The Relationship Between Serum Levels of Total IgE, IL-18, IL-12. Mediators Inflamm 2006:1-4.
- Trzeciak M, Glen J, Bandurski T (2011) Relationship between serum levels of interleukin-18, IgE and disease severity in patients with atopic dermatitis. Clin Exp Dermatol 36: 728-732.
- Gould HJ, Sutton BJ, Beavil AJ (2003) The biology of IGE and the basis of allergic disease. Annu Rev Immunol 23: 579-628.
- Sanders NL, Mishra A (2016) Role of Interleukin-18 in the Pathophysiology of Allergic Diseases. Cytokine Growth Factor Rev 32: 31-39.
- Yoshimoto T, Tsutsui H, Tominaga K (1999) IL-18, although antiallergic when administered with IL-12, stimulates IL-4 and histamine release by basophils. Proc Natl Acad Sci USA 96: 13962-13966.
- Dutt P, Shukla JS, Ventateshaiah SU (2015) Allergen-induced interleukin-18 promotes experimental eosinophilic oesophagitis in mice. Immunol Cell Biol 93: 849-857.
- Sasaki Y, Yoshimoto T, Maruyama H (2005) IL-18 with IL-2 protects against Strongyloides venezuelensis infection by activating mucosal mast cell–dependent type 2 innate immunity. J Exp Med 202: 607-616.
- Helmby H, Grencis R (2002) IL-18 Regulates Intestinal Mastocytosis and Th2 Cytokine Production Independently of IFN-γ During Trichinella spiralis Infection. J Immunol 169: 2553-2560.
- Terada M, Tsutsui H, Imai Y (2006) Contribution of IL-18 to atopic-dermatitis-like skin inflammation induced by Staphylococcus aureus product in mice. Proc Natl Acad Sci USA 103: 8816-8821.
- Mavi P, Niranjan R, Dutt P (2014) Allergen-induced resistin-like molecule-α promotes esophageal epithelial cell hyperplasia in eosinophilic esophagitis. Am J Physiol Gastrointest Liver Physiol 307: G499-507.
- Wastling JM, Knight P, Ure J (1998) Histochemical and Ultrastructural Modification of Mucosal Mast Cell Granules in Parasitized Mice Lacking the β-Chymase, Mouse Mast Cell Protease-1. Am J Pathol 153: 491-504.
- Hunt JE, Stevens RL, Austen KF (1996) Natural Disruption of the Mouse Mast Cell Protease 7 Gene in the C57BL/6 Mouse. J Biol Chem 271: 2851-2855.
- Ryan JJ, Kinzer CA, Paul WE (1995) Mast Cells Lacking the High Affinity Immunoglobulin E Receptor are Deficient in FceRI-γ Messenger RNA. J Exp Med 182: 567-574.
- Yoshimoto T, Takeda K, Tanaka T (1998) IL-12 up-regulates IL-18 receptor expression on T cells, Th1 cells, and B cells: synergism with IL-18 for IFN-gamma production. J Immunol 161: 3400-3407.
- Ohtsu H, Kuramasu A, Suzuki S (1996) Histidine decarboxylase expression in mouse mast cell line P815 is induced by mouse peritoneal cavity incubation. J Biol Chem 271: 28439-28444.
- Mishra A, Hogan SP, Brandt EB (2002) Enterocyte expression of the eotaxin and interleukin-5 transgenes induces compartmentalized dysregulation of eosinophil trafficking. J Biol Chem 277: 4406-4412.
- Combs JW, Lagunoff D, Benditt EP (1965) Differentiation and proliferation of embryonic mast cells of the rat. J Cell Biol 25: 577-592.
- Dinarello CA, Novick D, Kim S, Kaplanski G (2013) Interleukin-18 and IL-18 binding protein. Front Immunol 4: 289.
- Alagkiozidis I, Facciabene A, Tsiatas M (2011) Time-dependent cytotoxic drugs selectively cooperate with IL-18 for cancer chemo-immunotherapy. J Transl Med 9: 77.
Share This Article
Recommended Journals
Open Access Journals
Article Usage
- Total views: 4694
- [From(publication date): 0-2018 - Apr 29, 2025]
- Breakdown by view type
- HTML page views: 3747
- PDF downloads: 947