Research Article Open Access
Interactions between Lead on Nutrients (Ca2+, K+, N), and their Consequences on Growth in Sesuvium portulacastrum and Brassica juncea
| Hanen Zaier*#, Tahar Ghnaya# and Chedly Abdelly | ||
| Plants Extremophiles Laboratory, Centre of Biotechnology of Borj-Cédria, Tunisia | ||
| #These authors contributed equally | ||
| Corresponding Author : | Hanen Zaier Plants Extremophiles Laboratory Centre of Biotechnology of Borj-Cédria, Tunisia Tel: +216 71 430 215 Fax: +216 71 430934 E-mail: hanen_zaer@yahoo.fr |
|
| Received December 11, 2013; Accepted August 28, 2014; Published August 30, 2014 | ||
| Citation: Zaier H, Ghnaya T, Abdelly C (2014) Interactions between Lead on Nutrients (Ca2+, K+, N), and their Consequences on Growth in Sesuvium portulacastrum and Brassica juncea. J Bioremed Biodeg 5:243. doi:10.4172/2155-6199.1000243 | ||
| Copyright: © 2014 Zaier H, et al. This is an open-a ccess article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | ||
Related article at Pubmed Pubmed  Scholar Google Scholar Google |
||
Visit for more related articles at Journal of Bioremediation & Biodegradation
Abstract
Lead is one of the most toxic metals in the environment and causes drastic morphological and physiological deformities in plants. Growth restriction, chlorosis and necrosis are usually accompanied with a large disturbance of the uptake of essential elements. The aim of this work is to study the effects of lead on mineral nutrient acquisition (Ca2+, K+, N), and their consequences on growth in two different species: halophytic (Sesuvium portulacastrum) and glycophytic (Brassica juncea). Seedlings were grown for 21 days in split-root conditions. One half of the root system was immersed in complete nutrient solution supplemented with 400 μM Pb(NO2)3, and the other half was immersed in a Pb2+-free medium, containing all nutrients or deprived of potassium or calcium or nitrogen. Using this approach, we demonstrated that lead interfere mainly with the absorption of Ca2+ by the roots, thus limiting the growth of plants and their ability to accumulate this metal. We propose that the increase of Ca2+ availability in soils could improve the growth of both species in the presence of lead. This would be essential for improving their utility for phytoremediation of this metal in contaminated soils.
| Keywords |
| Halophytes; Glycophyte; Lead accumulation; Nutritional disturbance; Phytoremediation |
| Introduction |
| Soil pollution by metals has attracted considerable public attention in recent decades [1]. So, human activities have continuously increased the level of heavy metals circulating in the environment. This pollution by heavy metal in the biosphere has accelerated rapidly since the onset of the industrial revolution posing major environmental problems [2]. Lead contamination has resulted from mining, smelting activities, Pbcontaining paints, gasoline, explosives, as well as from the disposal of municipal sewage sludge enriched in Pb [3,4]. The toxic effects on human populations and potential health hazard induced by the consumption of Pb-contaminated food have been extensively studied [5,6]. Results of these studies demonstrated that Pb could be implied in several diseases such liver and kidney alterations [7]. In the environment, heavy metals cannot be degraded neither by microbial nor by chemical process, and tend to accumulate in soils or to be transported by streaming water and contaminate surface water and ground water [8]. For these reasons, it would be interesting to develop techniques for heavy metal extraction from soils. Much research has been conducted on remediation of metalcontaminated soils by employing chemical and physical techniques [9,10]. However, environmentalists and governments do not encourage their use because they are expensive, soils disturbing and applicable only to small areas. More recently, increasing attention has been given to the development of a plant-based technology (phytoremediation) to remediate heavy metal contaminated soils [11,12]. In the phytoremediation process, several sequential crops of selected plant species can be cultivated to reduce the concentrations of heavy metals in upper layers of contaminated soils to environmentally acceptable levels [13,14]. After a period of metals accumulation in shoots, the metal-rich plant material may be safely harvested and removed from the site without extensive excavation and then incinerated or stocked in controlled areas [15,16]. However, due to the high toxicity to plants, rare are the species able to accumulate largely these pollutants. Hence, Pb which considered as non essential metal for plants is known to cause adverse physiological and biochemical deleterious effects. The main toxic effects of Pb include interference with other nutrients uptake and translocation [17], growth retardation [18], decrease in dry weight of different plant parts [19], disturbed respiration [20] and a decline in the total chlorophyll and photosynthetic activity [21- 23]. Hence, it is known also, that lead reduces the uptake of essential macro and micronutrients. Ca, K, N, P, Mg, Fe and Zn absorption and translocation in several plant species are affected by the presence of Pb in the culture substrate leading to mineral deficiency in tissues [24,25]. This indirect toxic effect of Pb in plant could be associated to direct toxic effect governed by the interaction of ions Pb2+ with several enzymes, metabolic processes and nucleic acids alteration [26]. Both direct and indirect effects are responsible to the global effect of lead on plant development such chlorosis and growth reduction. The research of new plant species having a high potential of metals tolerance and accumulation is highly encouraged by botanists and environmentalists. In this context, it has been suggested that salt-tolerant plants would be better adapted to coping with environmental constraints, including heavy metals [27-31] than salt-sensitive (glycophytic) crop plants (Zea. mays L., Brassica. Juncea L., Pisum.sativum L.) commonly chosen for metals phytoextraction studies. In a previous study, we showed that the halophyte specie S. portulacastrum is more tolerant to lead than the glycophyte one B. juncea. When cultivated under the same conditions on Pb contaminated nutrient solution, S. portulacastrum accumulated more lead in the shoot than B. juncea. Hence, the amounts of Pb2+ translocated at 1000 μM Pb2+ were 3400 μg g-1 DW and 2200 μg g-1 DW in S. portulacastrum and B. juncea, respectively [30]. However, this accumulation was accompanied by a growth reduction and mineral nutrition disturbances essentially in B. juncea. This study aims at determining whether Pb2+ limits the growth of these species through impairment of the acquisition of some essential nutrients (Ca2+, K+ and N) or through toxic effect related to an excessive metal accumulation in the shoots. Thus, we conducted a split root experiment to separate the two factors. |
| Materials and Methods |
| Plant material and growth conditions |
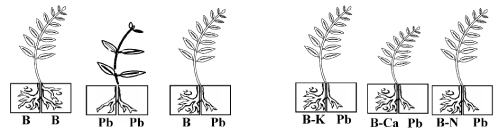
| S. portulacastrum L. (Aizoaceae), a dicotyledonous halophyte commonly known as sea purslane, was propagated by cutting. Three cm long-stem segments with one node and two opposite leaves were taken from mother plants cultivated in greenhouse, on a mixture of sandy soil and organic matter, and irrigated with tap water. Cuttings were disinfected for 5min in saturated calcium hypochlorite solution, thoroughly washed with distilled water, and placed for 7 days in an aerated solution diluted 10 times, supplemented with Fe EDTA and micronutrients [32,33]. Rhizogenesis took place after 1 week. Seeds of B. juncea L. (Acc PI 173874) were kindly provided by the North Central Regional Plant Introduction Station (NCRPIS-USDAUSA). They were sterilized in a 10% H2O2 solution during 20 min, washed with distilled water, sown on perlite imbibed with distilled water and incubated in the dark at 25°C for 5 days. The rooted cuttings (S. portulacastrum) and the seedlings (B. juncea) were transferred for 21 days to aerated Hoagland’s nutrient solution [34] containing different treatments. The Hoagland’s solution consisted of 5 mM Ca(NO3)2, 5 mM KNO3, 1 mM KH2PO4, 50 μM H3BO3, 1 mM MgSO4, 4.5 μM MnCl2, 3.8 μM ZnSO4, 0.3 μM CuSO4 and 0.1 mM (NH4)6Mo7O24 and 10 μM Fe-EDTA; pH was adjusted to pH 4.8 adjusted with HCl. For split-root experiments, each plant was placed between two 750 mL plastic containers filled with aerated solutions, with one half of the root system plunging in each container. Split-root experiment aimed at determining the implication of nutritional disruptions in growth inhibition under Pb2+ stress, and to compare the effects of Pb2+ on K+, Ca2+ and N acquisition by plants and their corresponding consequences on growth. Six treatments were applied (Figure 1). In the first and second treatments, the two halves of root system were immersed either in basal medium free of Pb2+ (B/B) or in basal medium added with 400 μM Pb(NO3)2(Pb/Pb). In the third treatment, one half of the roots was immersed in basal medium, and the other half in the same medium supplemented with 400 μM Pb(NO3)2 (B/Pb treatment). For the three other treatments, one half of the root system was immersed in basal medium supplemented with 400 μM Pb(NO3)2 and the other part in basal medium deprived of potassium ((B-K)/Pb) or of calcium ((B-Ca)/Pb) or of nitrogen ((B-N)/Pb). The culture solutions were renewed every 3 days. Eight plants grown individually were used for each treatment. Two harvests were made, at the beginning of treatment and 21 days later. At the harvests, shoots and roots developed in free-Pb2+ medium were successively rinsed three times in cold water and blotted between two layers of filter-paper. Treated roots were dipped in a 0.01M HCl cold solution to eliminate external heavy metal adsorbed at the root surface according to Aldrich et al. [35] then rinsed three times with cold distilled water and blotted with filter-paper. The fresh weight (FW) was measured immediately, and the dry weight (DW) after 48 h of desiccation in an oven at 60°C. |
| Water content |
| The tissue water content (TWC) was determined as TWC (ml g-1 DW) = (FW−DW)/DW. |
| Cations concentration |
| Dried samples (c.a. 100 mg) were grounded to a fine powder using a porcelain mortar and a pestle and digested in 4/1 (v/v) HNO3/HClO4 (20 ml) mixture at 100°C. After total evaporation, 30 ml of HNO3 0.5% were added and Pb2+ and Ca2+ concentrations were determined by atomic absorption spectrometry (Spectra AA 220 FS). Potassium and nitrogen contents were determined in the same homogenate by flame spectrometry (Corning photometer). Reduced nitrogen was measured according to Kjeldahl method. |
| Analysis of results |
| For the period between the initial and final harvests the following indexes were calculated. |
| The relative growth rate (RGR) based on whole-plant dry weight production, as RGR= lnW2 −lnW1/(t2 −t1), whereW1 and W2 were the dry matter at the beginning and the end of the treatment period and (t2−t1) was the duration of the period [36]. |
| Statistical analysis |
| Analyses of variance (ANOVA) with orthogonal contrasts and mean comparison procedures were used to detect differences between treatments. Mean separation procedures were conducted using the multiple range tests with Fisher’s least significant difference (LSD) (P<0.05). |
| Results |
| Plant morphology and growth |
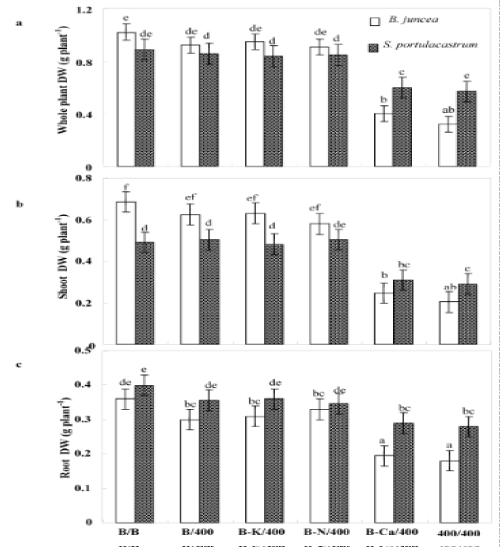
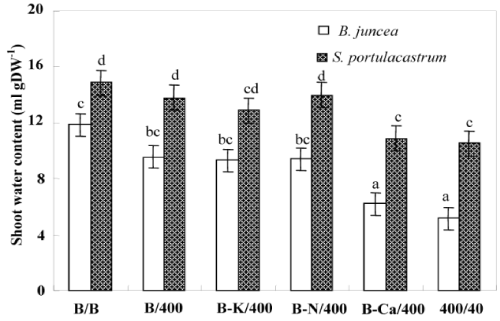
| After 8 days of treatment, chlorosis was visible in the young leaves of Pb/Pb treated plants of B. juncea. One week later, chlorosis increased and necrosis appeared in oldest leaves, with a subsequent falling of these senescing leaves. In contrast, S. portulacastrum plant morphology was not significantly modified in the presence of Pb2+ (Pb/ Pb). Both species almost produced similar biomasses in the absence of Pb2+ (Figure 2a). However, Pb/Pb treatment reduced the biomass production as compared to control B/B (treatment). So, this effect is more pronounced in B. juncea than in S.portulacastrum. Nevertheless, lead significantly reduced the dry weight of Pb/Pb treated plants of B. juncea, this reduction reaches 69% compared to control (T/T) but does not exceed 35% in S. portulacastrum. With the exception of (B-Ca)/ Pb, all the other treatments: B/Pb, (B-K)/Pb, and (B-N)/Pb, produced plants without visual toxicity symptom on their leaves, and biomass comparable to that of control plants. For both species, (B-Ca)/Pb plants showed reduction of dry weight plants, without visual toxicity symptoms compared to the control plants. The change in the growth of different parts (roots and aerial parts) showed the same aspects as that of the whole plant (Figure 2b and 2c). Figure 2c showed that only the Pb/Pb and (B-Ca)/Pb treatments significantly limited the root system growth and this effect is more pronounced in B. juncea than S. portulacastrum. Furthermore, the roots were less sensitive to Pb2+ than the shoots. Thus, in Pb/Pb treatment the root-biomass was reduced by 30% and 50% of the control respectively in S. portulacastrum and B. juncea, whereas in the shoots, this decrease was 41% and 70%. Table 1 compares the relative growth rate (RGR) of S. portulacastrum and B. juncea species. In unstressed conditions, the perennial halophyte species have a low RGR (0.06 day-1) as compared with B. juncea (0.12 day-1). For both species, the RGR values in the Pb/Pb and (B-Ca)/Pb treatments significantly reduced and this effect are more pronounced in B. juncea than S. portulacastrum. Therefore, in Pb/Pb treatment the RGR value was reduced by 33% and 50% of the control respectively in S. portulacastrum and B. juncea, and for (B-Ca)/Pb treatment, this decrease was 17% and 41%. For all the other treatments: B/Pb, (B-K)/ Pb, and (B-N)/Pb), the RGR values does not show a significant difference compared to that of control plants and it even remains unchangeable for S. portulacastrum. For both species, lead significantly reduced the shoot water content of Pb/Pb and (B-Ca)/Pb treated plants, but this reduction is more significant in B. juncea than in S. portulacastrum (Figure 3). For Pb/Pb treated plants, this reduction reaches 57% compared to control (T/T) but does not exceed 30% in S. portulacastrum. The shoot water content for all the other treatments: B/Pb, (B-K)/ Pb, and (B-N)/Pb), does not show a difference compared to the control (T / T). |
| Lead accumulation |
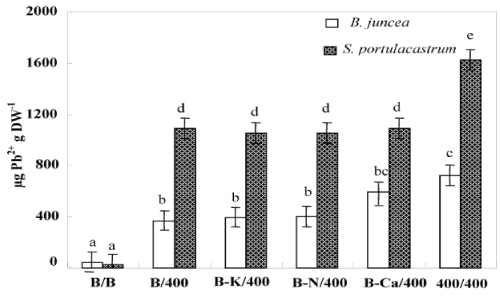
| Figure 4 showed that S. portulacastrum accumulated significantly more Pb2+ in the shoot than B. juncea. For all treatments, lead concentration in shoots was significantly higher in S. portulacastrum than in B.juncea (Figure 4). Hence, the concentration of sequestered Pb2+ in shoots of Pb/Pb plants were 1623 and 720 μg g-1 DW for S. portulacastrum and B. juncea respectively (Figure 4). For both species, the Pb/Pb treated have the higher Pb2+ concentration in their shoots than other treatments (B/Pb, (B-N)/Pb, (B-Ca)/Pb and (B-K)/ Pb). B/Pb, (B-N)/Pb, (B-Ca)/Pb and (B-K)/ Pb S. portulacastrum plants showed a similar Pb2+ concentration in their shoots. (B-Ca)/Pb and Pb/ Pb B. juncea plants accumulated significantly more Pb2+ in the shoots as compared to B/Pb, (B-N)/Pb and (B-K)/Pb plants. The phytoextraction potential of plants is estimated by the determination of the total amounts of metals accumulated in the shoots which represents the product of shoot biomass by its metal concentration. This parameter given in Table 2, demonstrates that S. portulacastrum extracted more Pb2+ than B.juncea. However, the Pb2+ amounts differed between the treatments within species: for S. portulacastrum, the highest extraction was obtained with Pb/Pb treatment. However, in B.juncea, B/Pb, (B-K)/ Pb and (B-N)/Pb plants extracted more Pb2+ as compared to Pb/Pb and B-Ca/Pb. The reduction of shoot amount of Pb2+ accumulated in the plants (Pb/Pb and (B-Ca)/Pb), in spite of the higher Pb2+ concentrations, was essentially the consequence of the low shoot biomass production showed in these plants under the effect of lead. |
| Calcium nutrition |
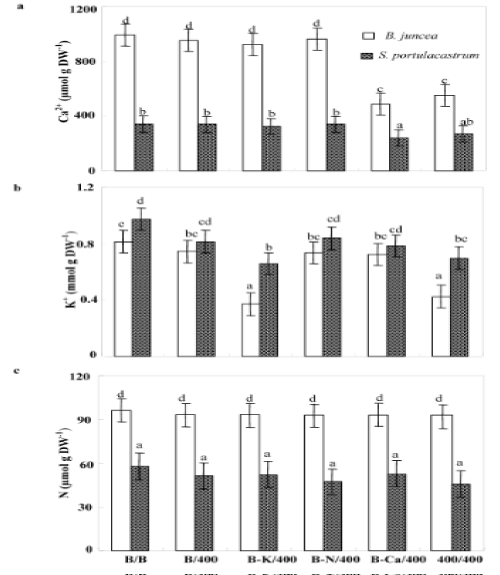
| Independently of treatments, the glycophyte species B. juncea showed higher Ca2+ tissue concentrations than S. portulacastrum. So, in both species, Ca2+ concentrations in the shoots of Pb/Pb plants decreased that of control (B/B) plants (Figure 5a), but this effect is more distinct in B. juncea than in S. portulacastrum. B/Pb, (B-K)/Pb and (BN)/ Pb treatments did not affect Ca2+ status. However, in (B-Ca)/Pb plants, Ca2+ concentrations were reduced in shoot tissues, this reduction reaches 51% and 29% of the control in B. juncea and S. portulacastrum respectively. In addition, for both treatments, Pb/Pb and (B-Ca)/Pb the reduction in Ca2+ shoot concentrations was accompanied by a growth inhibition, suggesting that Pb2+ impaired Ca2+ uptake by the roots. The shoot Ca2+ amounts were strongly diminished in plants subjected to Pb/ Pb and (B-Ca)/Pb in both species. However they remained unchanged in the shoots of other treated plants as compared to control plants (B/B) (Table 1). Hence, Ca2+ uptake in B/Pb, (B-K)/Pb and (B-N)/Pb plants was essentially assured by the part of root system developed in free Pb2+ basal medium containing Ca2+. |
| Potassium nutrition |
| For both species, the lowest shoot K+ concentrations were observed in (B-K)/Pb and Pb/Pb plants (Figure 5b), this effect is more pronounced in B.juncea than in S.portulacastrum. This data suggest that Pb2+ disturbed K+ uptake by the roots. Indeed, when a part of the root system was maintained in a medium containing K+ and free of Pb2+ (B/ Pb, (B-Ca)/Pb and (B-N)/Pb), K+ shoot concentration was not modified as compared to control (B/B). The amounts of K+ accumulated in the shoots of B. juncea, were decreased significantly in Pb/Pb (Table 1) as compared to S. portulacastrum. For both species, plants subjected to Pb/Pb and (B-Ca)/Pb accumulated the lowest amount of K+. These results were deeply related to a decrease in biomass concomitant to reduced K+ concentration in the shoots in Pb/Pb plants (Figure 2b and 5b). However in (B-Ca)/Pb plants of both species, the reduction of K+ shoot-amounts was essentially due to the decrease in the shoot biomass production. |
| Nitrogen nutrition |
| In both species, the reduced nitrogen concentration in the shoots was not modified by different treatments (Figure 5c). In the other hand, (B-N/Pb) plants accumulated more reduced N than Pb/Pb plants (Table 1) in spite of the similar shoot N concentration showed in these plants. This difference in N amounts was essentially due to the elevated biomass production in (B-N)/Pb plants. These data suggest that in (BN)/ Pb plants, the half of root system developed in medium containing Pb2+ is able to absorb NO-3. So, in Pb/Pb plants, growth was not limited through the effect of Pb2+ on N uptake by the roots. |
| Discussion |
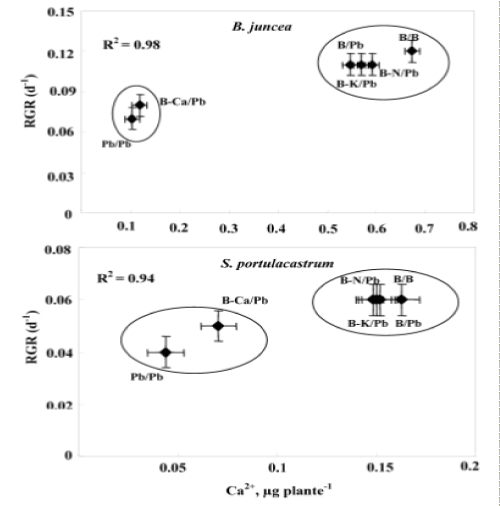
| Depending on their concentration in the environment, heavy metals, including micronutrients like Zn, Ni, Co, Cu necessary for plant growth and those such as Pb, Cd or Hg, for which no function in living organisms has yet been demonstrated, exert a toxic influence on plant metabolism. So, when present in excess within plant tissues, lead interferes with proper enzymatic functions and inhibits overall plant growth [16,30]. However, interspecific variability was showed in plants responses to this metal [37,38]. Based in a previous study, we showed that the halophyte specie S. portulacastrum is more tolerant and accumulated more lead in the shoot than glycophyte specie B. juncea [30,31]. However, this accumulation was accompanied by a growth reduction and mineral nutrition disturbances especially in B. juncea. The present study aims at determining whether Pb2+ limits the growth of these species through impairment of the acquisition of some essential nutrients (Ca2+, K+ and N) or through toxic effect related to an excessive metal accumulation in the shoots. Plant growth upon controlled conditions showed no statistical difference between the two tested species. However, Pb/Pb treatment induced growth inhibition particularly in B. juncea. On the other hand, for both species, (B/Pb), (B-K)/Pb and (B-N)/Pb plants showed no visual toxicity symptom and maintained normal growth in spite of the relatively high Pb2+ concentration in their shoots. In addition (B/Pb), (B-K)/Pb and (B-N)/ Pb plants does not show a significant difference in biomass production compared to that of control plants (B/B). Thus, large accumulation of lead in the shoots of both species is compatible with high growth when a part of the roots was maintained in the medium free of Pb2+. We suggest that the growth reduction induced by Pb2+ in Pb/Pb plants was partially due to Pb2+-induced nutritional disturbances, as has been described in different plant species [10,39,40]. In addition the (Pb/Pb) and (B-Ca)/Pb plants showed a significant reduction of their biomass production compared to that other treatments. We hypothesize therefore, that Pb2+ inhibited growth essentially through limitation of Ca2+ uptake by roots and/or transport to shoots. The total dry matter measured at the final harvest depended on the initial size of the plant (before the beginning of treatments) and on its growth during the treatment. Relative growth rate (RGR) is a recommended parameter to evaluate the specific effect of the constraints on the growth activity during the period of treatment [36]. Indeed, the rate of biomass production (RGR) was strongly correlated with the amount of Ca2+ accumulated in the shoots (Figure 6). This correlation presents two contrasting behaviors: Pb/Pb and (B-Ca)/Pb plants had the lowest RGR values and Ca2+ accumulation in their shoots, and the other plants [(B/B), (B/Pb), (B-K)/Pb and (B-N)/Pb] presented normal growth activity concomitantly with seemingly adequate Ca2+ amounts in shoots. The similar behavior of Pb/Pb and (B-Ca)/Pb plants suggests that the restriction of Ca2+ uptake in the presence of lead is an important factor that limiting plant growth. Based on the RGR values, Table 1 showed in the Pb/Pb and (B-Ca)/Pb treatments significantly reduced and this effect is more pronounced in B. juncea than S. portulacastrum. For all the other treatments: B/Pb, (B-K)/ Pb, and (B-N)/Pb), the RGR values does not show a significant difference compared to that of control plants and it even remains unchangeable for S. portulacastrum in spite of a large shoot Pb2+ accumulation. These data demonstrated that the halophyte species exhibited a higher tolerance to accumulated toxic ions as compared to B. juncea which have nevertheless been frequently used for metal phytoextraction [37,41]. Thus, due to the importance of Ca in many aspects of plant cell biology [42], tolerance to low Ca conditions could therefore represent an important aspect of tolerance to lead in plants, as is the case in the higher plant species [25,43]. Restriction of growth concomitant to a perturbation of Ca2+ uptake induced by lead has been reported in several plant species, such as rye, maize, tomato and mustard varieties [25] and could result from the inhibition of Ca transporters by toxic lead ions [43,44] and/or replacement of Ca ions with Pb ions due to the high affinity of the latter for Ca binding-sites on biological structures [42,45]. Numerous studies report an ameliorating effect of calcium on heavy-metal toxicity [46,47]. For example, a higher calcium concentration in a medium was also reported to abolish the toxic effects of both Cd2+ [48,49] and Pb2+ [50] on the activity of photosystem II. In addition, high Ca-status and a high level of tolerance to Ca-deficit accompanied enhanced Zn, Pb, Cu and Al tolerance [51-53]. Such a result points to new, interesting possibility, that calcium plays a role in the regulation of lead detoxification by influencing the formation of lead containing precipitates in cell walls. Antosiewicz and Hennig [44], suggest that in the physiological range of Ca2+ concentrations LCT1 could contribute to regulating the activity of the plant cadmium and lead detoxification system. Numerous authors have described the phenomenon of calcium mitigating heavy metal toxicity [47-49,54]. It is known that calcium is involved in the regulation/control of the secretion activity of Golgi apparatus vesicle movement and their fusion with the plasmalemma [55]. Since lead is to be extruded by Golgi vesicles out of the symplast to the cell wall, the theoretical hypothesis could be forwarded that under low calcium this process could be disturbed, which might cause less efficient lead immobilisation in the cell wall. In turn, this could lead to the formation of smaller precipitates and effectively to higher Pb2+ toxicity. Consequently, based on the study of Behling et al. [56] indicating higher level of soluble calcium in all parts of plants of the Ca-efficient tomato line relative to the Ca-inefficient, one may expect less disordered Ca-dependent Pb-detoxification in Ca-dT plants due to higher availability of Ca2+ for metabolic processes. The K+ shoot concentration was significantly reduced in Pb/Pb plants suggesting that Pb2+ impaired the uptake of K+ by roots (Figure 5b). This result was consistent with several previous studies [30,38,39]. Since Pb2+ has no chemical similarity with K+, we suggest that it exerts an indirect effect on K+-uptake, perhaps by complexing ATP and reducing energy availability [57]. (BK)/ Pb plants expressed a normal growth activity in spite of the reduced K+ shoot concentrations. It’s known that, potassium is essential for growth in higher plants. Its absorption is mainly by ATPases that hydrolyze ATP to provide energy for the transport of K+ in the interior of the cell root. The effect of Pb2+ on the uptake of K+ could not be due to direct competition at the sites of absorption for the lack of homology between these cations (Pb2+ on one hand and the other K+). So, in an indirect way that is the inhibition of the uptake of potassium, probably by the binding of metal cations on the ATP molecules thus preventing hydrolysis and consequently reducing the energy required for the absorption of K+ [57]. The uptake of K+ in the presence of heavy metals could also be decreased due to a restriction of passive absorption of this cation and an increase in efflux in the medium. The reduced N concentration was not significantly modified by Pb2+ treatments in the leaves of both species. The same result was found by Paivoke [39]. The different N amounts in shoots observed in response to different treatments (Table 2) were probably a consequence of treatment influence on biomass production. Thus, we conclude that Pb2+ did not limit growth through the restriction of N root uptake. On the other hand, (Pb/Pb) and (B-Ca)/Pb plants showed a significant decreased in root water absorption leading to significant shoot compared to that other treatments. This effect is more pronounced in B. juncea than in S. portulacastrum. In fact, several data demonstrated that heavy metal affect severely water status of sensitive-metal species by affecting transpiration, osmotic potential of cell sap, and water content [58,59]. For all treatments, lead concentration in shoots was significantly higher in S. portulacastrum than in B.juncea. Hence, the concentration of sequestered Pb2+ in shoots of Pb/Pb plants were 1623 and 720 μg g-1 DW for S. portulacastrum and B. juncea, respectively. For both species, the Pb/Pb treated have the higher Pb2+ concentration in their shoots than other treatments (B/Pb, (B-N)/Pb, (B-Ca)/Pb and (B-K)/ Pb). The evaluation of the Pb2+-phytoextraction capacity is based on the lead amount deposited in the shoots, which is the product of shoot biomass by shoot Pb2+ concentration. Based in this parameter, we demonstrate that S. portulacastrum extracted more Pb2+ than B. juncea. However, the Pb2+ amounts differed between the treatments within species: for S. portulacastrum, the highest extraction was obtained with Pb/Pb treatment. Nevertheless, in B.juncea, B/Pb, (B-K)/Pb and (B-N)/Pb plants extracted more Pb2+ as compared to Pb/Pb and (B-Ca)/Pb plants (Table 2). This behavior is explained by the production of biomass concomitant with a relatively elevated Pb2+ concentration in the shoots. The reduced biomass showed especially in B. juncea, (B-Ca/)Pb plants limited significantly their phytoextraction capacity in spite of the high Pb2+ concentration in shoots. The disturbances of Ca2+ uptake induced by lead contributed largely to the growth restriction and therefore reduced Pb2+-phytoextraction capacities in both species mainly in B. juncea. |
| Conclusion |
| Our results showed that the restriction of growth in plants grown in the presence of 400 μM Pb(NO3)2 related to nutritional disturbances rather than to toxic effects of lead. So, the reduction of nutrient uptake by Pb2+ contributes largely to the growth restriction. Neither K+ nor N seem to be limiting for growth when absorbed from Pb2+ contaminated solution, but the restriction of Ca2+ uptake by lead is an important factor of growth reduction, which limits Pb-phytoextraction capacities of these species. We propose that the increase of Ca2+ availability in soils could improve the growth of both species especially for B.juncea that is more sensitive than S. portulacastrum in the presence of lead. This would be essential for improving their utility for phytoremediation of this metal in contaminated soils. So, the supply of this nutriment might be cheaper, more feasible and more efficient than the addition of synthetic chelators, presenting risk of negative effects on plant growth. |
| Acknowledgement |
| This work was conducted in the Laboratory of Extremophile Plants in the Biotechnology Center of Borj Cedria Tunisia and supported by the Tunisian Ministry of Higher Education and Scientific Research (LR10CBBC02). Seeds of B. juncea (Acc PI 173874) were kindly provided by the North Central Regional Plant Introduction Station (NCRPIS), United States Department of Agriculture (USDA) from United States of America. |
References
|
Tables and Figures at a glance
| Table 1 | Table 2 |
Figures at a glance
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 |
 |
 |
 |
| Figure 4 | Figure 5 | Figure 6 |
Relevant Topics
- Anaerobic Biodegradation
- Biodegradable Balloons
- Biodegradable Confetti
- Biodegradable Diapers
- Biodegradable Plastics
- Biodegradable Sunscreen
- Biodegradation
- Bioremediation Bacteria
- Bioremediation Oil Spills
- Bioremediation Plants
- Bioremediation Products
- Ex Situ Bioremediation
- Heavy Metal Bioremediation
- In Situ Bioremediation
- Mycoremediation
- Non Biodegradable
- Phytoremediation
- Sewage Water Treatment
- Soil Bioremediation
- Types of Upwelling
- Waste Degredation
- Xenobiotics
Recommended Journals
Article Tools
Article Usage
- Total views: 13397
- [From(publication date):
November-2014 - Apr 03, 2025] - Breakdown by view type
- HTML page views : 8910
- PDF downloads : 4487
